Abstract
The development of protein subunit vaccines to combat some of the world's deadliest pathogens such as a malaria parasite, Plasmodium falciparum, is stalled, due in part to the inability to induce and sustain high-titer antibody responses. Here, we show the induction of persistent, high-titer antibody responses to recombinant Pfs25H, a human malarial transmission-blocking protein vaccine candidate, after chemical conjugation to the outer-membrane protein complex (OMPC) of Neisseria meningitidis serogroup B and adsorption to aluminum hydroxyphosphate. In mice, the Pfs25H-OMPC conjugate vaccine was >1,000 times more potent in generating anti-Pfs25H ELISA reactivity than a similar 0.5-μg dose of Pfs25H alone in Montanide ISA720, a water-in-oil adjuvant. The immune enhancement requires covalent conjugation between Pfs25H and the OMPC, given that physically mixed Pfs25H and OMPC on aluminum hydroxyphosphate failed to induce greater activity than the nonconjugated Pfs25H on aluminum hydroxyphosphate. The conjugate vaccine Pfs25H-OMPC also was highly immunogenic in rabbits and rhesus monkeys. In rhesus monkeys, the antibody responses were sustained over 18 months, at which time another vaccination with nonconjugated Pfs25H induced strong anamnestic responses. The vaccine-induced anti-Pfs25-specific antibodies in all animal species blocked the transmission of parasites to mosquitoes. Protein antigen conjugation to OMPC or other protein carrier may have general application to a spectrum of protein subunit vaccines to increase immunogenicity without the need for potentially reactogenic adjuvants.
Keywords: malaria, Pfs25, transmission-blocking vaccine
As reported by the World Health Organization in 2004, the worldwide incidence of malaria is ≈300 million clinical cases and 1.3 million deaths annually (1). Of the four species of malaria parasites that infect humans, Plasmodium falciparum is responsible for the majority of these deaths, and Plasmodium vivax accounts for >50% of all malarial infections outside Africa and 10% of those in Africa. Mounting drug resistance by the malaria parasite makes chemotherapy increasingly difficult. Three types of malarial vaccines are under research and development: (i) vaccines targeting the liver-stage parasite development for sterile immunity; (ii) vaccines targeting the blood-stage parasite to reduce disease burden; and (iii) vaccines targeting the parasite development in the mosquito stage to block transmission, called transmission-blocking vaccines (TBVs). For all three types of vaccines, antibody is important. For TBVs, antibody is the only mechanism for providing immune protection. TBV-elicited functional antibodies, ingested with the sexual stages of the parasite in a blood meal by a mosquito, will inhibit or block parasite development in the mosquito.
Pxs25 proteins encoded by orthologous genes and expressed on the surface of zygotes and ookinetes during the development of the malaria parasite P. falciparum (Pfs25) and P. vivax (Pvs25) are leading candidates for mosquito-stage transmission-blocking vaccines. Animal studies demonstrated that anti-Pfs25- and anti-Pvs25-specific antibodies have the ability to block parasite development in the mosquito in an ex vivo membrane-feeding assay (2). A Phase 1 human trial of Pvs25 adsorbed onto aluminum hydroxide (Alhydrogel) induced transmission-blocking activity (TBA) that correlated with the titer of the anti-Pvs25 antibodies, thus establishing the feasibility of inducing transmission-blocking immunity by using Pvs25-based vaccines (3). However, the Pvs25-specific antibody titers induced by Pvs25/Alhydrogel were low, indicating that a more potent formulation would be required for inducing higher antibody responses. Furthermore, because Pxs25 proteins are expressed only in the mosquito stage of the infection and not in the human host, the Pxs25-based vaccines will not be boosted by natural malaria infection. Thus, the vaccine must induce high-titer antibodies that are sustained throughout the transmission season.
In a commercial conjugate vaccine against invasive Haemophilus influenzae type b disease, PedvaxHiB, the outer-membrane protein complex (OMPC) of Neisseria meningitidis serogroup B, has been used as a carrier for the bacterial capsular polysaccharide polyribosylribitol phosphate (PRP) (24). OMPC provides cognate T cell help for production of PRP antibodies in infants when the PRP is conjugated to OMPC (4, 5). OMPC has also been conjugated to peptides to improve the immunogenicity of a peptide-based vaccine (6), but data on protein antigens conjugated to the carrier are lacking. To investigate whether OMPC can also enhance the immunogenicity of the otherwise poorly immunogenic parasite protein, Pfs25, we developed a method to conjugate the recombinant Pfs25H to OMPC. In preclinical animal studies, the antibody responses induced by the conjugate were >1,000 times higher than those induced by the nonconjugated Pfs25H formulated with Montanide ISA720. Antibodies induced by the conjugates were effective in blocking parasite infection in mosquitoes. In rhesus monkeys, the conjugate induced a high antibody response that was sustained for over 18 months, and a strong recall response was observed after these monkeys were given a booster dose of nonconjugated Pfs25H.
Results
OMPC Enhanced Immunogenicity of Pfs25H After Conjugation.
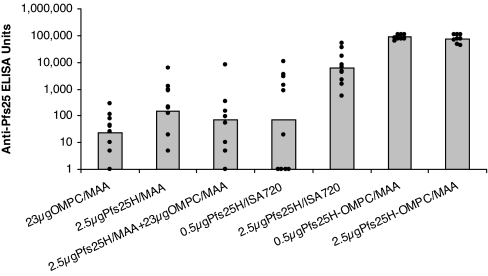
Our previous unpublished studies in mice immunized with Pfs25H formulated with many available adjuvants or immune stimulants indicated that Pfs25H was a poor immunogen with most formulations. The only exceptions were the water-in-oil emulsions containing Montanide ISA51 (ISA51) and Montanide ISA720 (ISA720), which induced adequate levels of anti-Pfs25 antibodies with TBA. We compared the immunogenicity of a Pfs25H-OMPC conjugate with Pfs25H alone formulated with ISA720 in mice (Fig. 1). The antibody responses induced by 0.5 μg of Pfs25H-OMPC conjugate were >1,000 times higher than those induced by 0.5 μg of Pfs25H/ISA720 and over 14 times higher than those induced by 2.5 μg of Pfs25H/ISA720. As a negative control, mice were immunized with activated/quenched but nonconjugated OMPC adsorbed onto Merck aluminum hydroxyphosphate adjuvant (MAA). Production of anti-OMPC antibodies in mice immunized with the conjugate was confirmed by ELISA using OMPC as the plating antigen. The anti-OMPC antibodies did not cross-react with Pfs25H antigen. Physical mixing of nonconjugated Pfs25H on MAA and OMPC on MAA in the same proportion as in the conjugate generated anti-Pfs25H responses comparable with those induced by Pfs25H alone on MAA, indicating that chemical conjugation is required for the OMPC immune enhancement.
Fig. 1.
Conjugation enhanced antibody responses of Pfs25H in mice. Seven groups of mice, 10 in each group, were immunized twice i.p. on days 0 and 28 with the indicated vaccines. Pfs25H-OMPC indicates the conjugate vaccine. Anti-Pfs25 antibody responses were evaluated in sera collected 2 weeks after the second immunization. The histogram bars indicate geometric mean antibody levels in these animal groups, with dots indicating the antibody level of each animal within each group.
Pfs25H-OMPC conjugate also induced strong antibody responses in rabbits (data not shown) and rhesus monkeys. In one rhesus study, a group of monkeys was immunized with 40 μg of the Pfs25H-OMPC on MAA, and another group was immunized with the same dose of a mixture of nonconjugated Pfs25H on MAA and OMPC on MAA in the same proportion as in the conjugate. Two weeks after the second immunization, the antibody levels in the conjugate group that received Pfs25-OMPC reached a geometric mean (with 95% confidence intervals) of 10,100 (7,230–14,180) ELISA units, whereas the group that received the Pfs25 and OMPC mixture had a near-background level of 61 (6–680) units (data not shown). This finding again demonstrated that chemical conjugation of Pfs25H and OMPC is required for immune enhancement.
Antibodies Induced by the Pfs25H-OMPC Conjugate Display TBA.
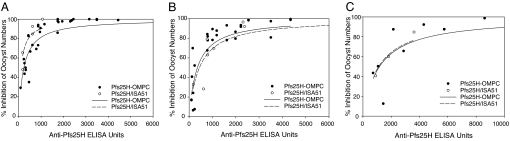
A critical question was whether the conjugation interfered with the epitopes that induce antibodies that possess TBA. To address this question, antisera obtained from animals immunized with Pfs25H-OMPC or Pfs25H/ISA51 were compared for their TBAs relative to the ELISA titers in membrane feeding assays. As shown in Fig. 2A, the IC50 ELISA titers (±SE) were 229 ± 29 units (R2 = 0.78) in mouse sera induced by Pfs25-OMPC/MAA and 109 ± 26 units (R2 = 0.80) in mouse sera immunized with Pfs25H/ISA51. Fig. 2B shows the comparison of the correlation between ELISA titer and TBA in rabbit sera, with IC50 values of 356 ± 65 units (R2 = 0.77) in rabbit anti-Pfs25-OMPC/MAA sera and 443 ± 110 units (R2 = 0.76) in the anti-Pfs25H/ISA51 sera. The TBAs of rhesus anti-Pfs25H-OMPC/MAA and anti-Pfs25H/ISA51 were also comparable, although the IC50 values were not calculated because of the limited data points available (Fig. 2C). These data indicate that the conjugation process did not compromise the functional epitopes in Pfs25H.
Fig. 2.
Conjugation process did not affect the functional epitopes for TBA. The mouse (A), rabbit (B), and rhesus (C) antisera obtained from animals immunized with Pfs25H-OMPC or Pfs25H/ISA51 were compared for their TBAs relative to the ELISA titers in membrane feeding assays. Mouse and rabbit sera used in the assays were obtained 2 weeks after the second immunization. Rhesus sera used in the assay were obtained on day 611, 2 weeks after the booster dose on day 596 (see also Fig. 3 and Table 1). Antisera and sexual-stage parasites were fed to mosquitoes together with the blood meals containing parasites, and the oocyst numbers were counted as an indication of the infectivity. The negative control samples were preimmune sera from the same species. Lines are the nonlinear regression fit of the data to a simple hyperbolic equation: % inhibition = 100·[C]/(IC50 + [C]), where [C] is the anti-Pfs25 ELISA units and IC50 is the anti-Pfs25 ELISA units giving 50% inhibition of oocyst number. Filled circles and solid lines indicate antisera induced by Pfs25H-OMPC conjugate, and open circles and dashed lines indicate antisera induced by Pfs25H/ISA51.
Because mice immunized with the conjugate also developed antibodies against OMPC, sera obtained from mice immunized with the nonconjugated OMPC control also were tested in membrane-feeding assays. Anti-OMPC sera had no effect on parasite infection in mosquitoes compared with naïve human sera (data not shown), thus attributing the TBA to the anti-Pfs25 antibodies in the anticonjugate sera.
Sustained Antibodies Induced by Pfs25H-OMPC Conjugate.
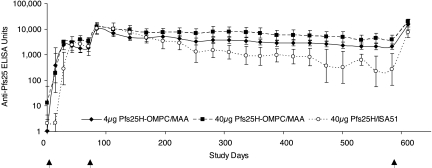
To test whether the conjugate could induce a lasting immune response, in one of the rhesus studies, monkeys were followed for 16 months after the second immunization (Fig. 3). At this time, the geometric mean of anti-Pfs25 levels in the group immunized with 40 μg of conjugated Pfs25H was >30% of their peak levels, significantly higher than the antibody levels in the 40 μg Pfs25H/ISA51 group (P < 0.02). The lasting antibody levels in monkeys that received 4 μg of conjugated Pfs25H also was significantly higher than those in the group that received 40 μg of Pfs25/ISA51 (P < 0.05). Mice and rabbits immunized with the conjugate were followed for 6 and 12 months, respectively. At the end of the studies, specific antibody levels in mouse sera were 50–70% of their peak levels, whereas in rabbit sera, those levels were 5–30% (data not shown).
Fig. 3.
Pfs25H-OMPC induced sustained antibody responses in rhesus monkeys. Two groups of monkeys were given two immunizations of 4 or 40 μg of Pfs25H conjugated to OMPC (diamond and square, respectively) on days 0 and 70. To compare the responses induced by the water-in-oil formulation, a third group of monkeys was given 40 μg of Pfs25H/ISA51 (circles) on days 0 and 70. On day 596, a dose of 40 μg of nonconjugated Pfs25H adsorbed to MAA was given to all three groups of monkeys. The antibody levels were assayed in sera by ELISA throughout the study. The data are expressed as the geometric mean ×/÷ geometric standard error. Arrows indicate the days of immunization.
Boosting with Nonconjugated Pfs25H/MAA.
To determine the ability of native protein to boost conjugate-induced immunity, all monkeys previously immunized with two doses of 4 μg of Pfs25H-OMPC/MAA, 40 μg of Pfs25H-OMPC/MAA, or 40 μg of Pfs25H/ISA51 were immunized with 40 μg of nonconjugated Pfs25H/MAA on Day 596 (Fig. 3). In each animal, Pfs25H/MAA immunization boosted a response (Table 1). Interestingly, after the booster immunization, eight of nine monkeys in the two Pfs25H-OMPC conjugate groups developed higher antibody titers than their previous peaks after the second immunization on day 70 (P = 0.01). In contrast, the antibody levels after boosts in all monkeys in the Pfs25H/ISA51 group were lower than their previous peaks after the second immunization (Table 1). Antibodies induced by the booster effectively blocked the oocyst development in mosquito midguts (Fig. 2C). These data indicate that nonconjugated Pfs25H/MAA can vigorously boost immunity induced by the conjugate vaccine.
Table 1.
Antibody levels in rhesus monkeys before and after immunizations
| 4 μg Pfs25H-OMPC |
40 μg Pfs25H-OMPC |
40 μg Pfs25H/ISA51 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D70 | D84* | D584 | D611* | D70 | D84* | D584 | D611* | D70 | D84* | D584 | D611* |
| Anti-Pfs25 ELISA units in individual monkeys | |||||||||||
| 1,080 | 4,780 | 1,970 | 8,040 | 3,370 | 16,620 | 5,450 | 30,670 | 110 | 1,740 | 1 | 1,380 |
| 870 | 5,570 | 1,290 | 9,950 | 5,040 | 11,830 | 9,720 | 23,680 | 7,730 | 21,120 | 810 | 9,510 |
| 1,970 | 17,080 | 1,580 | 13,990 | 4,120 | 16,650 | 3,220 | 18,590 | 3,260 | 15,980 | 780 | 15,360 |
| 6,070 | 48,360 | 5,500 | 91,780 | 2,130 | 6,990 | 1,440 | 12,380 | 9,050 | 32,650 | 2,780 | 19,280 |
| 2,000 | 5,650 | 1,820 | 7,560 | — | — | — | — | 1,660 | 6,900 | 760 | 6,210 |
| Geometric mean 95% confidence intervals | |||||||||||
| 1,860 | 10,450 | 2,090 | 15,068 | 3,490 | 12,297 | 3,960 | 20,220 | 2,090 | 10,580 | 270 | 7,520 |
| 960– | 4,360– | 1,270– | 6,060– | 2,440– | 8,230– | 1,780– | 13,860– | 430– | 3,830– | 20– | 3,010– |
| 3,610 | 25,020 | 3,430 | 37,440 | 5,010 | 18,370 | 8,770 | 29,510 | 10,160 | 29,180 | 4,280 | 18,800 |
| Probability | |||||||||||
| <0.03 | <0.03 | <0.04 | <0.04 | <0.03 | <0.03 | ||||||
The group names indicate the first two vaccinations on days 0 and 70. All three groups received 40 μg of Pfs25H/MAA as the third vaccination on day 596. Dn indicates the day number. Probability indicates the significance between antibody levels before and after the immunizations.
*Anti-Pfs25H ELISA units 2 weeks after the second immunization on day 70 or the third immunization on day 596.
Discussion
Despite the evidence that Pfs25 can potentially be effective as a vaccine to block malaria transmission by mosquitoes, the protein is a poor immunogen. Several methods have been used to increase the immunogenicity of protein antigens. One of the strategies is the formation of microparticles. A successful malaria vaccine example was the recombinant yeast coexpression of P. falciparum circumsporozoite protein (residues 207–395) fused to the N terminus of the hepatitis B surface antigen (HBsAg) and HBsAg to form virus-like particles. The vaccine induced high antibody titers to both circumsporozoite protein and HBsAg (7) and partial protective responses that lasted over 18 months in human trials (8). In a different approach, immunologists have known for many years that chemical cross-linking of poor protein immunogens to carriers or to themselves increases immunogenicity (9, 10). Increasing antigen molecular size or epitope density and specific epitope spatial arrangement may lead to enhanced immunity for a polymerized single-protein multimeric immunogen. For protein subunit-carrier chemical conjugates, additional factors, such as carrier heterologous T-helper epitopes or specific adjuvant properties, may also lead to immune enhancement. An influenza hemagglutinin-diphtheria toxoid conjugate vaccine was shown to be more immunogenic in institutionalized elderly people and produced greater protection from influenza infection (11).
In this article we have demonstrated that by chemical conjugation to OMPC, the poor immunogen Pfs25H can be converted to a potent immunogen that can induce high, persistent antibody levels. The antibody titers are similar to or higher than the Pfs25H in water-in-oil adjuvants such as ISA51 or ISA720. Unlike ISA51 or ISA720 formulations that are sometimes associated with local reactogenicity in rhesus monkeys and in humans (12), the Pfs25H-OMPC conjugate was well tolerated in rhesus monkeys. This finding is consistent with the fact that PedvaxHIB in which OMPC is used as carrier for the bacterial capsular polysaccharide is well tolerated and has been used in millions of people, including infants 2 months of age.
We also demonstrated that the specificity and efficacy of antibodies induced after conjugation are unaffected by the conjugation process. We showed that antibodies induced in mice and rabbits by conjugation have similar efficacy in blocking infection of mosquitoes to antibodies produced in water-in-oil adjuvants. The ELISA units required to produce a 50% reduction in mosquito infectivity are similar to the units for antibodies induced by water-in-oil adjuvants. Thus, the specificity of the blocking antibodies is likely to be similar to that induced with nonconjugated vaccines.
A unique feature of OMPC as a carrier for vaccines is that OMPC contains various outer membrane proteins, predominately PorB (serotype 2a) for strain B11 OMPC. These proteins are present in vesicle form containing lipids including reduced amounts of lipooligosaccharide. Unlike other PRP-conjugate vaccines that required multiple doses to induce protective responses, PRP-OMPC conjugate was able to induce protective responses to Haemophilus influenzae type b with a single dose (4, 13). The unique properties of OMPC are reflected in its ability to act as both a carrier and adjuvant (14), perhaps through engagement of Toll-Like Receptor 2 (15). For PRP-OMPC conjugate, the vaccine-induced protective immunity could persist for >10 months after vaccination (16). In this paper, we also found persistent antibody responses induced by the Pfs25-OMPC conjugate 16 months after last dose in rhesus monkeys.
There may be concern that antibodies induced by conjugation to heterologous T cell epitopes in OMPC, unrelated to the pathogen, may not be boosted through recall from memory B cells. Here, we demonstrated that rhesus monkeys primed with Pfs25H-OMPC can be vigorously boosted with nonconjugated Pfs25H. The peak titer levels after the booster dose were higher than the previous peak after the two primary immunizations. By contrast, in the Pfs25H/ISA51 group, the antibody levels after the booster dose were lower than their previous peaks. These data indicated that the conjugate might have induced a better memory response, which can be boosted by the poor immunogen alone. When memory B cells are induced, recall of humoral immunity is highly effective through B cell epitopes and possibly more effective through induced T memory cells directed against the original immunogen.
The goals for the Pfs25 vaccine development are to induce (i) high antibody levels conferring transmission blocking, (ii) sustained antibody levels throughout at least one transmission season, and (iii) a rapid recall response to a boost following the initial vaccination campaign. Thus, the immunogenicity of Pfs25H-OMPC in mice, rabbits, and rhesus monkeys; the longevity of the immune responses; the TBAs in these animal sera; and the rapid boost response, combined with the extensive safety record of OMPC as a carrier, provide strong justifications for advancing the development of this vaccine formulation toward a human trial. The protein subunit-carrier conjugate approach may be effective in developing vaccines targeting blood-stage malarial proteins to which immunity has been poor, and natural boosting by native antigens during malarial infection is possible.
Materials and Methods
Antigen and Carrier Preparation.
Pfs25H is a 20-kDa recombinant form of Pfs25 expressed in Pichia pastoris as a secreted protein. It was purified from the culture supernatant by microfiltration and ultrafiltration, affinity chromatography on nickel-trilotriacetic acid Superflow, hydrophobic interaction chromatography on a Phenyl Sepharose column, and size-exclusion chromatography with Superdex 75 (17). OMPC (nonproduct lots) was purified from a detergent extract of N. meningitidis strain B11 being isolated by ultracentrifugation, diafiltration, and sterile filtration (18).
Pfs25H-OMPC Conjugation, Formulation, and Characterization.
Pfs25H was activated with N-ε-[maleimidocaproyloxy]sulfosuccinimide ester (Pierce, Rockford, IL) by incubating 47 μM Pfs25H and 125 μM N-ε-[maleimidocaproyloxy]sulfosuccinimide ester in 25 mM Hepes/0.15 M NaCl, pH 7.3, buffer for 4.5 h in the dark at 4°C. The reaction mixture was desalted by dialysis or HiTrap desalting columns (GE Healthcare, Piscataway, NJ) using 25 mM 2-morpholinoethane sulfonic acid buffer, pH 6.1. Desalted, maleimide-activated Pfs25H was 0.2-μm filtered. Maleimide equivalents in an aliquot of the activated Pfs25H were measured with an N-acetylcysteine consumption assay using Ellman's reagent, 5,5′-dithionitrobenzoic acid (Pierce) to measure residual thiol (19). N-acetylhomocysteine thiolactone-activated OMPC was aseptically prepared with a 2-h aging time as described (20), except that potassium phosphate buffer was replaced with sterile water. Before use, N-acetylhomocysteinyl-OMPC was made to be 25 mM 2-morpholinoethane sulfonic acid buffer, pH 6.1. N-acetylhomocysteinyl-OMPC thiol equivalents were measured with 5,5′-dithionitrobenzoic acid. Malemide-activated Pfs25H was added aseptically to N-acetylhomocysteinyl-OMPC with gentle mixing to give a final maleimide/thiol (mol/mol) ratio of 0.075. The reaction mixture was aged in the dark at 4°C overnight and dialyzed in a sterile 300,000-molecular-weight cutoff dialysis bag against sterile 0.1 M Tris·HCl/10 mM EDTA/0.5% (wt/vol) sodium deoxycholate, pH 8.5, buffer at ambient temperature followed by dialysis against sterile 10 mM Hepes/0.15 M NaCl, pH 7.3, buffer at 4°C. The dialyzed Pfs25H-OMPC conjugate was centrifuged at 1,000 × g for 5 min to remove any large aggregates. For an OMPC control, N-acetylhomocysteinyl-OMPC was quenched with a 5-fold molar excess N-ethylmaleimide (Pierce) over the starting thiol and otherwise treated similarly to the conjugate sample. Activated Pfs25H, activated/quenched OMPC, and Pfs25H-OMPC conjugate were analyzed by SDS/PAGE/Coomassie blue-stained gels and by Western blotting with a monoclonal antibody recognizing Pfs25H as the primary antibody. Results showed no change in mobility of Pfs25H after activation, the presence of new Pfs25H bands with the expected decreased mobility (higher Mr) in the conjugate, and no free Pfs25H in the conjugate product. Pfs25H/OMPC loading was measured by amino acid analysis (21), total protein in the conjugate by a modified Lowry assay (22), and conjugate z-average particle size (in nanometers) by dynamic light scattering. Retention of OMPC vesicle structure in the conjugate was observed by phosphotungstic acid negative staining and transmission electron microscopy. Pfs25H-OMPC conjugate in 10 mM Hepes/0.15 M NaCl, pH 7.3, buffer was mixed with MAA (Merck Manufacturing Division, West Point, PA) to give the final desired concentration of conjugated Pfs25H and 0.45 mg/ml aluminum. Completeness of Pfs25H-OMPC conjugate adsorption was determined by near UV spectral analysis of the adsorbed vaccine supernatant and was ≥95%. Volume-weighted mean particle size (in micrometers) of the aluminum-adsorbed conjugate was determined by static light scattering. An equivalent amount of N-acetylhomocysteinyl-activated and N-ethylmaleimide-quenched OMPC as in the conjugate was adsorbed to MAA for the OMPC control and used for animal studies.
Four conjugate lots were produced from two different OMPC lots. For one conjugate lot, the Pfs25H/OMPC ratio (mg/mg) was 0.27, residual thiol content was <5%, conjugate z-average value was 148 nm, and mean particle size of MAA adsorbed conjugate was 34 μm. Similar values were obtained for other lots, which were prepared by the same process with only minor changes. Immunological potency of different conjugate lots was evaluated by immunizing mice with elevated doses within the linear range of the dose–response curve. The antibody responses induced by these lots were comparable (data not shown; P > 0.85). For Pfs25H-OMPC conjugate immunization, the microgram dose corresponds to the amount of the conjugated Pfs25H.
Formulation of Pfs25H with ISA51 and ISA720.
Pfs25H was aseptically homogenized at 8,000 rpm twice at room temperature at a designated final concentration in PBS and ISA720 (SEPPIC, Paris, France) (30:70 aqueous-to-oil based on volume) or in PBS and ISA51 (SEPPIC) (50:50 aqueous-to-oil based on volume) at room temperature for 3 min twice with a 50-ml stainless-steel sealed chamber assembly attached to an Omni Mixer-ES homogenizer (Omni International, Warrenton, VA). The range of volume-weighted mean emulsion droplet size was between 0.5 and 2.0 μm. By volume 90% of the droplets (D9v, 0.9) are within 0.6–2.6 μm as measured with a Mastersizer 2000 Particle Sizer Analyzer (Malvern Instruments, Malvern, United Kingdom).
Animal Immunizations and Serum Collection.
All animal studies were conducted in compliance with National Institutes of Health guidelines and under the auspices of an Animal Care and Use Committee-approved protocol.
Mouse studies.
Six- to 7-week-old female BALB/c mice were obtained from Taconic Farm (Germantown, MD) and were maintained in the National Institutes of Health animal facility. Mice were grouped and were given one or two immunizations, either i.p. or i.m., with different vaccine formulations. Sera were collected from tail vein bleeds at the designated time points.
Rabbit study.
Female New Zealand white rabbits of 2–3 kg were obtained from Covance (Germantown, MD) and were maintained in the National Institutes of Health animal facility. Rabbits, five in each group, were immunized i.m. with different vaccine formulations. At designated time points, blood was drawn from the rabbit ears, and the bleeds were processed for sera.
Monkey studies.
Naïve Macaca mulatta monkeys were obtained from Morgan Island, South Carolina, and were maintained in the National Institutes of Health animal facility. Monkeys were grouped randomly, five in each group. In one study, monkeys were immunized i.m. with designated vaccines on days 0 and 70; in another study, monkeys were immunized on days 0, 70, and 596. Sera were obtained from bleeds taken from the femoral region at designated time points.
ELISA.
Antibody levels were measured in serum by a standardized ELISA (3). ELISA plates (Immulon 4; Dynex Technology, Chantilly, VA) were coated with Pfs25H (100 ng per well) or OMPC (200 ng per well), stored at 4°C overnight, and then blocked with buffer containing 5% skim milk (Difco, Detroit, MI) in 1× Tris-buffered saline (BioFluids, Camarillo, CA) for 2 h at room temperature. Diluted sera were added in triplicate to antigen-coated wells and incubated for 2 h at room temperature. After washing with 0.1% Tween 20 in Tris-buffered saline, plates were incubated with alkaline phosphatase-labeled secondary antibodies (Kirkegaard and Perry, Gaithersburg, MD) for 2 h at room temperature. After adding phosphatase substrate solution (Sigma, St. Louis, MO), absorbance was read at 405 nm.
The ELISA was standardized by using reference antisera with an assigned unit value equivalent to the reciprocal of the dilution giving an OD405 of 1. In each ELISA plate, the absorbance values of a serially diluted reference standard were fitted to a four-parameter hyperbolic function to generate a standard curve. With this standard curve, the absorbance of an individual test serum was converted to an antibody unit value.
Transmission-Blocking Assay.
The TBA of the animal sera was tested by an ex vivo membrane feeding assay (23). Briefly, the test sera from animal studies were heat-inactivated and diluted with a naïve human serum pool to minimize nonspecific inhibitory effects on parasite development. The diluted test serum was mixed with an in vitro gametocyte culture of P. falciparum (NF54 line), and the mixture was fed to Anopheles stephensi (Nijmegen strain) mosquitoes through a membrane feeding apparatus. Mosquitoes were kept for 8 days after the feed to allow parasites to develop into oocysts. Infectivity was measured by dissecting at least 20 mosquitoes per serum sample, staining the midguts with 0.1% mercurochrome (Sigma), and counting the number of oocysts per midgut. The feeding experiment was not analyzed unless the average oocyst count in mosquitoes fed with naïve human serum pool was more than four. Percent inhibition of oocyst development per mosquito was determined by the formula: 100·(Mean Oocyst Numbernegative control − Mean Oocyst Numbertest)/Mean Oocyst Numbernegative control, where the negative control feed used sera from the bleeds before vaccination from the same animal. In one mouse experiment, the negative control feed also used sera from animals immunized with the nonconjugated OMPC control vaccine described above.
Statistics.
Statistical analyses were performed by using the UNISTAT software package (P-STAT, Hopewell, NJ). P values of ≤0.05 were considered significant. Antibody titers within a group were expressed as geometric means with 95% confidence intervals. Wilcoxon Signed Rank test was used for comparing antibody titers before and after immunizations. The antibody titer and the TBA expressed as percentage of inhibition of parasite development in mosquitoes. Nonlinear regressions using a simple hyperbola equation: % inhibition = 100·[C]/(IC50 + [C]) were performed to fit TBA vs. ELISA titer using Sigma Plot (SPSS, Chicago, IL). The resulting hyperbolic function was used to calculate IC50 values.
Acknowledgments
We thank A. Laughinghouse, K. Lee, S. Robinson, H. Zhou, B. Lucas, and Dr. G. Song for technical assistance; Dr. S. Pierce for critically reading the manuscript; Dr. T. L. Richie (Naval Medical Research Center, Silver Spring, MD) for the parasite line NF54; and Dr. M. Fay for advice on statistical analyses. This work was supported in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Abbreviations
- OMPC
outer-membrane protein complex
- MAA
Merck aluminum hydroxyphosphate adjuvant
- PRP
polyribosylribitol phosphate
- TBA
transmission-blocking activity.
Footnotes
The authors declare no conflict of interest.
References
- 1.World Health Organization. Malaria: Disease Information. Geneva: WHO; 2004. available at www.who.int/tdr/diseases/malaria/diseaseinfo.html, accessed. [Google Scholar]
- 2.Carter R, Mendis KN, Miller LH, Molineaux L, Saul A. Nat Med. 2000;6:241–244. doi: 10.1038/73062. [DOI] [PubMed] [Google Scholar]
- 3.Malkin EM, Durbin AP, Diemert DJ, Sattabongkot J, Wu Y, Miura K, Long CA, Lambert L, Miles AP, Wang J, et al. Vaccine. 2005;23:3131–3138. doi: 10.1016/j.vaccine.2004.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donnelly JJ, Deck RR, Liu MA. J Immunol. 1990;145:3071–3079. [PubMed] [Google Scholar]
- 5.Vella PP, Staub JM, Armstrong J, Dolan KT, Rusk CM, Szymanski S, Greer WE, Marburg S, Kniskern PJ, Schofield TL, et al. Pediatrics. 1990;85:668–675. [PubMed] [Google Scholar]
- 6.Fan J, Liang X, Horton MS, Perry HC, Citron MP, Heidecker GJ, Fu TM, Joyce J, Przysiecki CT, Keller PM, et al. Vaccine. 2004;22:2993–3003. doi: 10.1016/j.vaccine.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 7.Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, Desmons P, Wellde BT, Garcon N, Krzych U, Marchand M. N Engl J Med. 1997;336:86–91. doi: 10.1056/NEJM199701093360202. [DOI] [PubMed] [Google Scholar]
- 8.Alonso PL, Sacarlal J, Aponte JJ, Leach A, Macete E, Aide P, Sigauque B, Milman J, Mandomando I, Bassat Q, et al. Lancet. 2005;366:2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 9.Stanisic DI, Martin LB, Liu XQ, Jackson D, Cooper J, Good MF. Infect Immun. 2003;71:5700–5713. doi: 10.1128/IAI.71.10.5700-5713.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reichlin M, Nisonoff A, Margoliash E. J Biol Chem. 1970;245:947–954. [PubMed] [Google Scholar]
- 11.Gravenstein S, Drinka P, Duthie EH, Miller BA, Brown CS, Hensley M, Circo R, Langer E, Ershler WB. J Am Geriatr Soc. 1994;42:245–251. doi: 10.1111/j.1532-5415.1994.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 12.Miller LH, Saul A, Mahanty S. Trends Parasitol. 2005;21:412–414. doi: 10.1016/j.pt.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Granoff DM, Holmes SJ. Vaccine. 1991;9(Suppl):S30–S34. doi: 10.1016/0264-410x(91)90178-9. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Melgosa M, Ochs HD, Linsley PS, Laman JD, van Meurs M, Flavell RA, Ernst RK, Miller SI, Wilson CB. Eur J Immunol. 2001;31:2373–2381. doi: 10.1002/1521-4141(200108)31:8<2373::aid-immu2373>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 15.Latz E, Franko J, Golenbock DT, Schreiber JR. J Immunol. 2004;172:2431–2438. doi: 10.4049/jimmunol.172.4.2431. [DOI] [PubMed] [Google Scholar]
- 16.Lehmann D, Kakazo M, Yarsley S, Javati A, Taime J, Saleu G, Namuigi P, Alpers MP, Mendelman PM, Staub T. PNG Med J. 1998;41:102–111. [PubMed] [Google Scholar]
- 17.Zou L, Miles AP, Wang J, Stowers AW. Vaccine. 2003;21:1650–1657. doi: 10.1016/s0264-410x(02)00701-6. [DOI] [PubMed] [Google Scholar]
- 18.Fu J, Bailey FJ, King JJ, Parker CB, Robinett RS, Kolodin DG, George HA, Herber WK. Bio/Technology. 1995;13:170–174. doi: 10.1038/nbt0295-170. [DOI] [PubMed] [Google Scholar]
- 19.Ellman GL. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 20.Leanza WJ, Chupak LS, Tolman RL, Marburg S. Bioconjug Chem. 1992;3:514–518. doi: 10.1021/bc00018a009. [DOI] [PubMed] [Google Scholar]
- 21.Shuler KR, Dunham RG, Kanda P. J Immunol Methods. 1992;156:137–149. doi: 10.1016/0022-1759(92)90020-t. [DOI] [PubMed] [Google Scholar]
- 22.Markwell MA, Haas SM, Bieber LL, Tolbert NE. Anal Biochem. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- 23.Quakyi IA, Carter R, Rener J, Kumar N, Good MF, Miller LH. J Immunol. 1987;139:4213–4217. [PubMed] [Google Scholar]
- 24.Marburg S, Jom D, Tolman RL, Arisen B, McCauley J, Kniskern PJ, Hagopian A, Vella PP. J Am Chem Soc. 1986;108:2582–2587. [Google Scholar]