Abstract
Drug resistance to human immunodeficiency virus (HIV) is a major factor in the failure of antiretroviral therapy.1 In order for practitioners to provide effective pharmaceutical care to their HIV patients, it is essential that they understand the mechanisms of HIV drug resistance as well as the various factors that can contribute to its emergence. This article is based on didactic content from the infectious disease section of the Integrated Sequence II Course in the PharmD program at South University. In the course, students are first given an overview that includes key structural components of HIV and a discussion of the HIV life cycle. A detailed presentation on the pharmacology of the various classes of antiretroviral agents follows. The clinical impact and prevalence of HIV drug resistance is then discussed along with factors that might contribute to it. Mechanisms of drug resistance for each class of antiretroviral agents are presented in detail followed by a discussion of the basis and clinical utility of HIV drug resistance testing. Finally, new targets for HIV pharmacotherapy are presented along with an overview of new antiretroviral agents that are being developed. Content taught in lecture is reinforced by relevant case studies that students work on in small groups during the recitation period.
Keywords: human immunodeficiency virus, HIV pharmacotherapy, drug resistance, pharmacology
INTRODUCTION
Since its introduction, antiretroviral drug therapy has dramatically reduced morbidity and mortality associated with HIV infection. The use of combination drug therapies can significantly improve HIV patients’ chances for long-term survival. Unfortunately, the effectiveness of antiretroviral therapy can be markedly reduced by the emergence of drug resistance. A study by Richman2 in the journal AIDS reported that 76% of their population exhibited resistance to 1 or more antiretroviral drugs. While exposure to antiretroviral drugs can contribute to the development of resistance by HIV, even drug-naïve patients may be infected with strains of the virus that are resistant to drug therapy.3 The presence of antiretroviral drug resistance is an important cause of treatment failure in HIV patients. Drug resistant viruses are often resistant to multiple classes of antiretroviral drugs. This drug cross-resistance coupled with the often unpredictable development of drug resistance significantly complicates HIV therapy. Successful treatment of HIV requires a detailed knowledge of the various mechanisms by which resistance can arise as well as an understanding of strategies for overcoming resistance once it occurs. HIV drug resistance testing is proving to be a powerful tool that can help clinicians tailor their treatment regimen to the specific HIV strain(s) that infect their patients. In addition, numerous new agents and classes of antiretroviral drug are currently under development in an effort to keep pace with emerging HIV drug resistance.
INSTRUCTIONAL DESIGN
At South University the presentation on HIV drug resistance is given during our Integrated Sequence II (ISII) course which is an 8 quarter-hour block of integrated instruction in pharmacology, medicinal chemistry, and therapeutics. At this point in the program, our students have completed a course in Medical Microbiology and are familiar with the fundamental areas of pharmacology and medicinal chemistry taught in Integrated Sequence I. Within the ISII course itself, the material on HIV drug resistance is presented after students have received instruction on pharmacology and medicinal chemistry related to antiretroviral drugs therapy and before the clinical faculty member begins his/her presentation of antiretroviral therapeutics. Since students have just learned the mechanism of action for each of the antiretroviral drug classes it is a logical extrapolation to the mechanism of resistance for each class. When the clinical faculty member presents antiretroviral therapeutics, they can do so knowing that students have an understanding of drug resistance mechanisms, factors that contribute to the development of resistance, and the importance of HIV drug-resistance testing. Student knowledge related to antiretroviral drug pharmacology and resistance is reinforced by the presentation of relevant case studies during a weekly 3-hour recitation period. Students are given cases at the beginning of the period and then broken into small pre-assigned groups to work through the cases. At the end of each case is a series of questions addressing various aspects of therapeutics, pharmacology, medicinal chemistry, and pathophysiology related to HIV infection and treatment. At the end of 2 hours, each group is expected to write the case and submit it for review and grading. Each member of the group receives the same grade for the case write-up and presentation. Giving each student in the group the same grade stimulates active involvement in the group process. Students who do not contribute significantly during the group breakout sessions are often pressured by their peers in the group to increase their contribution. During the last hour of recitation, the class is brought back together and a member from each group (chosen at random by the faculty member) presents 1 of the case write-ups to the class and faculty member. Since students do not know ahead of time which of them will be presenting, they all need to be familiar with the content of the final case write-up. Students are encouraged to use PowerPoint slides or overhead transparencies for their presentation. During this presentation phase, input and questions from students and faculty members are likewise encouraged. Presentation of cases in front of students and faculty members also allows students to gain valuable experience in public speaking and case presentation.
Objectives
At the conclusion of the presentation, students should be able to:
Discuss various factors that contribute to the development of HIV drug resistance.
Explain the mechanism of action for each class of antiretroviral agents.
Detail the mechanism of HIV drug resistance to each class of antiretroviral agents.
Interpret data on the incidence and extent of HIV drug resistance.
Provide an overview of the utility of HIV drug-resistance testing including a contrast between phenotypic and genotypic testing.
Discuss new classes of anti-HIV drugs and potential new HIV targets that are under investigation.
Apply the material leaned in this presentation to clinically relevant case studies.
Factors Contributing to the Development of HIV Drug Resistance
Several factors related to the life cycle and replication of HIV are key contributors toward the rapid and widespread emergence of resistance that is seen with this organism. First the HIV reverse transcriptase (RT) enzyme is notoriously “low fidelity” (ie, the enzyme is somewhat nonselective during the copying process) and is prone to errors when copying viral RNA into DNA. By some estimates, HIV RT makes one error in each HIV genome per round of replication.4 This translates into roughly 1 mutation for every 2000 nucleosides. While most of these errors are base substitutions, other mutations such as insertions or duplications, can also occur. Second, HIV has an exceptionally high rate of replication; several billion new viral particles may be produced each day in the untreated patient. In most HIV-infected untreated adults, plasma HIV RNA levels range between 103 and 105 copies/ml, but can be greater than 106 copies/ml in acute infection or with advanced disease. Since the half-life of cells infected with HIV is generally 1-2 days,5 HIV must infect new cells at a very high rate to maintain the infection at a steady level. This high rate of replication coupled with the high rate of error for RT means that numerous HIV “variants” are rapidly formed and propagated. Patients who are infected with HIV can have multiple variants of the virus present in their system. These variants can have greatly different sensitivities to antiretroviral agents, a factor that can significantly complicate the selection of drugs and the course of therapy. Additional factors that may contribute to the development of HIV drug resistance include poor patient compliance, subtherapeutic blood levels of antiretroviral agents, and inappropriate choice of antiretroviral agent(s). Patients should be told to take their HIV medications as prescribed and not to miss any doses. Pharmacokinetic factors that can affect blood levels of antiretroviral agents include poor oral absorption and alteration of drug metabolizing enzymes by other agents, as well as various drug-drug interactions.
While some HIV variants may exhibit intrinsic or “primary” resistance to antiretroviral agents, most drug resistance develops as a result of exposure to these agents. Antiretroviral resistance can still occur even during successful therapy of HIV infection.6 Any mutations that confer a selective advantage to a particular viral variant will allow that particular viral variant to predominate. In a sense, the very use of antiviral agents exerts a “selective pressure” that favors propagation of resistant viruses. The use of multiple drugs in combination is one strategy of reducing the chance that a resistant viral variant will survive treatment.
Mechanism of Action and Resistance for Antiretroviral Drugs
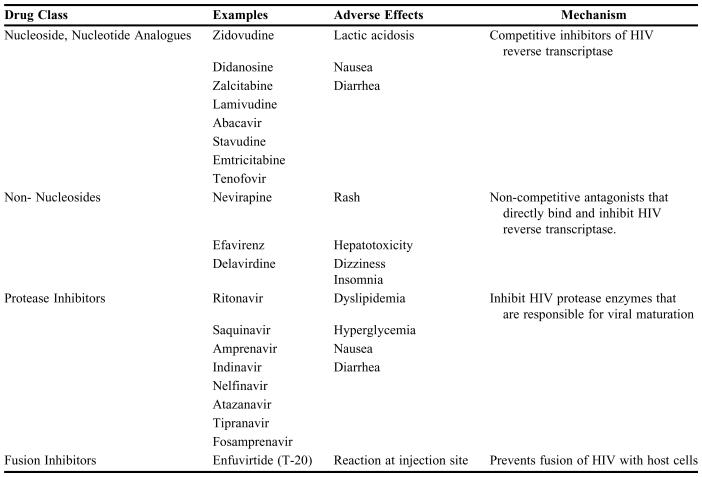
A listing of most commonly used anti-HIV drug classes can be found in Table 1 along with their class, mechanism of action, and major adverse effects. An excellent review of mechanisms of HIV drug resistance can be found in Clavel (2004).7
Table 1.
HIV Drug Classes and their Characteristics
All FDA approved antiretrovirals as of October 2005.
Anti-HIV Nucleoside and Nucleotide Analogues
HIV belongs to a class of viruses called retroviruses. These organisms are enveloped viruses that have RNA as their genetic material and utilize the enzyme reverse transcriptase. Reverse transcriptase is an RNA-directed DNA polymerase that copies the HIV RNA genome into a complimentary DNA strand to form a double-stranded DNA:RNA hybrid. The DNA/RNA hybrid that is formed is then copied into a double-stranded DNA copy of the HIV genome, which is incorporated into the human genome through the actions of HIV integrase enzyme. Once the HIV genome is integrated into the human cell, it may be transcribed and expressed into new viruses by the host cell machinery. Enzymatic cleavage of newly formed viral proteins must occur by HIV proteases in order for new HIV to be functional and infectious.
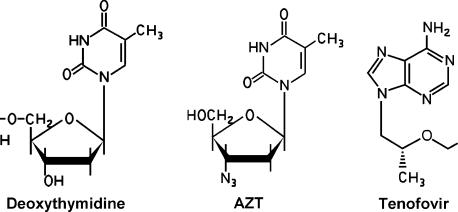
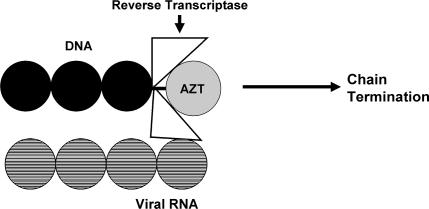
The nucleoside analogues such as zidovudine (azidothymidine, AZT) are comprised of a base (thymidine in the case of AZT) attached to a ribose sugar in which the normal 3’ hydroxyl has been replaced by an azido group (Figure 1). The presence of the 3’ OH is required for elongation of the growing DNA chain. Replacement of the OH at the 3’ position prevents bonds from being formed with this nucleoside. Incorporation of AZT into the growing DNA chain in place of the normal nucleoside leads to a “chain termination” that stops polymerization of the growing DNA molecule (Figure 2). Anti-HIV nucleoside inhibitors are also referred to as nucleoside reverse transcriptase inhibitors (NRTIs) since they are more potent inhibitors of HIV RT than human DNA polymerases. A new class of nucleotide analogues (eg, tenofovir) is also available for clinical use. The inhibitory mechanism of action for these agents is identical to that of the nucleoside analogues (Table 1), with the main difference being structural in that tenofovir is an acyclic deoxyadenosine (Figure 1). Both nucleoside and nucleotide RT inhibitors must enter the cell and become phosphorylated in order to act as synthetic substrates for RT. Both classes of agents can prevent infection of susceptible cells but will have no effect on cells that already harbor HIV. Likewise, both classes of agents target the active site on RT that is involved in DNA polymerization.
Figure 1.
Structure of a normal nucleoside, the nucleoside analogue AZT and the nucleotide analogue tenofovir.
Figure 2.
Use of AZT by RT causes DNA chain termination.7
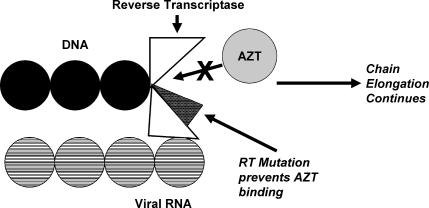
Resistance to NRTI's occurs through 2 mechanisms: the first is mutation of the residues that results in reduced incorporation of the NRTI into the growing DNA chain. While some of these mutations arise in the actual catalytic site of RT, a number of these mutations are actually proximal to the active catalytic site of RT but are still able to cause a conformational change in the enzyme that impairs binding of the drug to the active site (Figures 2 and 3). While thymidine analog mutations mainly affect AZT and stavudine, a number of other mutations have been observed for other analogs as well. Several of these mutations are can confer significant resistance to many or all nucleoside analogues. High levels of resistance to the cytosine analog lamivudine have been observed with the M184V mutation, while a high level of resistance to the guanosine analog abacavir appears to require at least 2 or 3 concomitant mutations (eg, M184V, L74V) to be present at the same time. The second mechanism of NRTI resistance is associated with enhanced removal of drug from its site of attachment at the end of the DNA chain. These RT mutations allow ATP or pyrophosphate (both of which are in high concentration within the cell) to bind at the active site adjacent to the bound nucleoside analog. The high energy ATP or pyrophosphate can then attack the bond that binds the drug to DNA, thereby liberating the drug and terminating its effect.
Figure 3.
RT mutation prevents AZT binding.7
Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
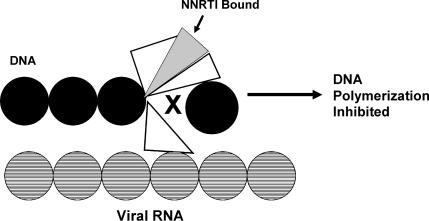
Drugs in this class are structurally different from the nucleoside RT inhibitors. The NNRTIs bind near the catalytic site of reverse transcriptase and alter the enzymes ability to change conformation. This increased enzyme rigidity prevents its normal polymerization function (Figures 4 and 5). The side effects of the NNRTIs are generally less than those of the nucleoside analogues; however, the main drawback of these agents is the rapid development of resistance. As a result, the NNRTIs are never used for monotherapy of HIV infection.
Figure 4.
Bound NNRTI inhibits RT polymerase activity.7
Figure 5.
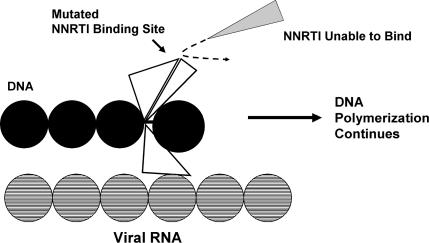
RT mutation prevents NNRTI binding.7
Resistance to this class of agents occurs mainly through mutation of hydrophobic RT residues within the binding pocket for the NNRTIs. Since all of the NNRTIs bind to essentially the same region of RT, mutations in this area will affect binding of all of the agents in this class to some extent. This may in part explain the high rates of HIV cross-resistance within this class of agents.8
Protease Inhibitors
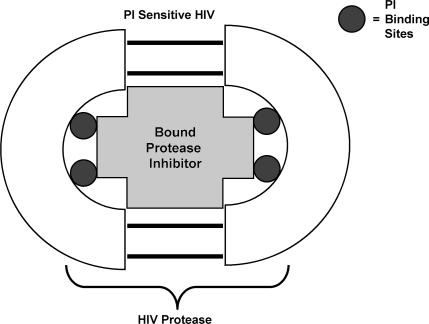
Newly assembled HIV particles are not fully functional or infectious until they have undergone a final “maturation.” This maturation involves cleavage of viral protein precursors by HIV protease enzymes. These enzymes are encoded by HIV and offer a unique and attractive target for preventing HIV maturation. HIV protease enzymes are symmetrical dimers with a central core that binds the peptides that are to be modified by the enzyme. Protease inhibitors are designed to fit and bind at the catalytic site of the enzyme with high affinity and thereby block its activity (Figure 6). Inhibition of HIV protease enzymes still allows viral particles to be formed and released from host cells; however, the particles released are immature and not infectious.
Figure 6.
Bound PI blocks protease activity.
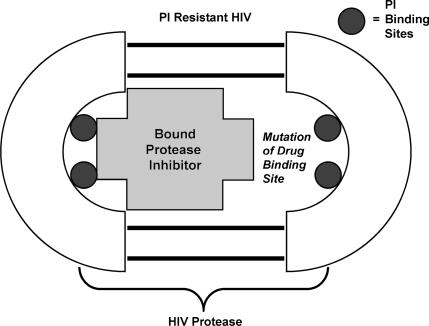
Resistance to protease inhibitors occurs primarily as a result of amino acid mutations that arise within or proximal to the catalytic binding site to the drug (Figure 7). Replacement of key amino acids within the protease enzyme can significantly alter the affinity of the enzyme for binding protease inhibitors. In addition, the geometry of the catalytic site is altered and enlarged by these mutations. Since the protease inhibitors bind the catalytic sites with significantly higher affinity than do the natural substrates, mutations in this region will have a greater impact on drug binding than on the endogenous peptides.
Figure 7.
Mutation in the protease enzyme reduces PI affinity and effect.
Fusion Inhibitors
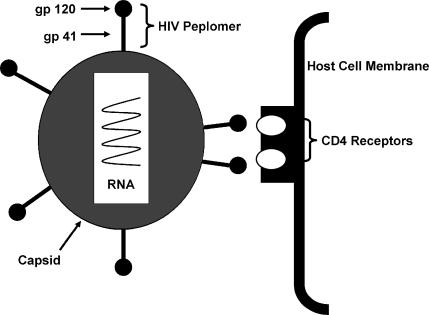
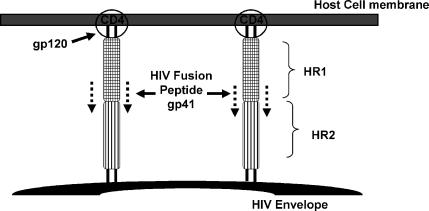
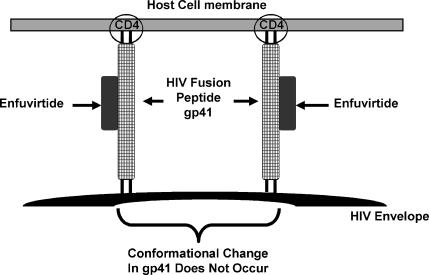
A drug that acts by blocking the fusion and entry of HIV into the host cells (enfuvirtide) has been used clinically. Fusion of HIV with the host cell membrane is an essential step in viral entry into the cell. HIV attaches specifically to CD4 (cluster of differentiation) molecules on the host cell membrane though glycoprotein (gp) 120 on the HIV peplomer (Figure 8). Once attachment to the host cell occurs, gp41, which constitutes the stalk of the HIV peplomer, embeds itself in the host cell membrane. The gp41 peplomer is comprised of 2 adjoining subunits, HR1 and HR2 (Figure 8). The embedding of the gp41 involves the HR1 subunit of gp41 “sliding” over the HR2 subunit to draw the HIV and host cell membranes closer together (Figure 9). The gp41 fusion peptide now undergoes a further conformational change that brings the HIV and host cell membranes in contact with one another. Fusion “pores” are formed that facilitate entry of the HIV nucleocapside (protein capsid + HIV genome) into the host cell. The drug enfuvirtide is a synthetic peptide that binds directly to the HIV gp41 and prevents it from undergoing the conformational change that leads fusion of the HIV and host cell membrane (Figure 10).
Figure 8.
HIV binding to a host cell.
Figure 9.
Fusion of HIV peplomer with host cell membrane.12
Figure 10.
Enfuvirtide binds to the HIV peplomer and blocks it's movement.12
Although just introduced to clinical practice, varying susceptibility of different HIV strains to enfuvirtide has already been documented.9 While clinical resistance to enfuvirtide has not yet been observed, amino acid mutations between residues 36 and 45, which are part of the binding site for enfuvirtide, have been identified which confer some acquired resistance to the drug. However, since the region in which the mutations occur is required for viral function, enfuvirtide-resistant mutants still replicate poorly and revert back to full-drug susceptibility once the agent is stopped.
Prevalence of HIV Drug Resistance
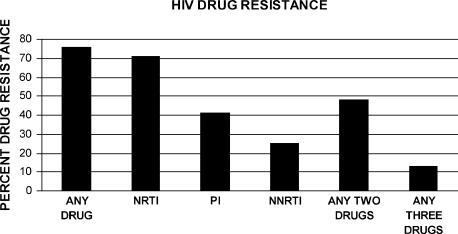
Investigators have attempted to determine the prevalence of HIV drug resistance to various classes of agents. A large scale study published in 2004 looked at rates of antiretroviral drug resistance in patients receiving HAART (highly active antiretroviral therapy).2 Of the population studied, 72%-80% exhibited resistance to 1 or more antiretroviral classes based on drug sensitivity assays (Figure 11). Resistance to any 2 classes of agents was 48% while resistance to 3 classes of agents was seen in 13% of patients. A second large-scale study by Weinstock3 reported RT or protease mutations in 90% of the patients enrolled in their study. This translated into reduced susceptibility to these classes of agents in 39% of patients. In a study of HIV-infected patients who failed therapy despite receiving a combination of 3 different classes of antiretroviral agents, the prevalence drug resistant HIV genotypes was 38%.10
Figure 11.
Prevalence of HIV drug resistance to various drug classes.2
Cross resistance of HIV is also of great concern. This phenomenon involves development of HIV resistance to agents within a particular class or with similar mechanism of action, but to which the virus has not been exposed. A study presented at the Fourth International AIDS Workshop reported that all patients who failed to respond to a regiment with the NNRTI efavirenz had mutations that conferred cross-resistance to the NNRTI nevirapine.11 Patients who failed a therapeutic regimen that included nevirapine and efavirenz proved to be cross-resistant to all available NNRTIs. Similar findings have also been reported with protease inhibitors. Half of HIV-infected patients treated with protease inhibitor-based combinations still fail therapy due to multiple PI resistance.12
Health officials in New York City expressed great concern recently when a patient infected with a highly drug-resistance variant of HIV progressed to full-blown AIDS in a matter of months.13 Although he was a newly diagnosed patient who had never received antiretroviral drugs, the strain of HIV he was infected with was resistant to 3 of the 4 available classes of anti-HIV drugs.
HIV Drug Resistance Testing
HIV drug resistance is 1 of the major limiting factors in the successful treatment of HIV infection.1 Resistance testing assays have become available that allow clinicians to obtain drug susceptibility profiles in their HIV-infected patients prior to initiating drug therapy. While HIV drug resistance testing was originally used as a research tool in clinical trials to explain treatment failure, it has rapidly moved into the mainstream of clinical HIV therapy. The goal of HIV drug resistance testing is to aid the physician in choosing the drug(s) that would be most effective against the particular HIV strains that infect their patient. The first attempt at controlling HIV replication is the most important because the patient gains the most benefit from minimizing viral replication and maximizing immune function.14
HIV drug resistance assays fall into 2 categories: genotypic assays and phenotypic assays. Genotypic assays analyze the HIV genome in order to detect specific mutations that can confer drug resistance. Interpretation of genotypic assay results is done by matching the results from the individual virus against lists of frequently updated HIV mutations that are known to confer drug resistance. These genotypic assays are relatively inexpensive and may now be performed rapidly on site with commercially available assay kits. However, genotypic testing can only identify documented HIV mutations and may not detect new mutations that arise in a particular HIV variant. In addition, since different mutations confer different degrees of drug resistance, it is often difficult to predict the actual degree of clinical drug resistance in a virus with multiple mutations.
In contrast, phenotypic resistance assays examine the actual drug susceptibility of a particular HIV variant. HIV genes for reverse transcriptase and protease enzymes are amplified and inserted into a recombinant virus which is then exposed to various anti-HIV drugs. Phenotypic testing provides information on the sensitivity of a particular HIV variant in comparison to a control isolate with full drug sensitivity. One practical difficulty associated with phenotypic testing is translating observed decreases in viral drug sensitivity in the assay into actual decreases in clinical sensitivity. What degree of phenotypic resistance needs to be present for each drug in order to see actual decreases in clinical effectiveness for that drug? It is only through large-scale clinical trials that an actual correlation might be made between changes in phenotypic sensitivity and actual drug resistance. So far these clinical/phenotypic correlations have only been done for a few anti-HIV drugs.15 The nature of phenotypic testing makes assays more technically difficult and expensive, thus such testing is only carried out at dedicated commercial facilities.
Investigators have looked retrospectively at the effectiveness of HIV drug resistance testing in improving clinical response to pharmacotherapy. Key trials such as the GART16 study, VIRADAPT study17 and ARGENTA study18 reported that HIV patients whose drug selection was based on genotypic resistance testing had significantly lower viral loads than patients who did not receive resistance testing prior to starting therapy. Reduced viral loads in these patients translated into reduced morbidity and mortality.17 A study by Sax19 found that the monetary cost of HIV drug resistance testing was more than offset by the long-term cost benefits of reduced morbidity and improved quality of life. While there are fewer studies examining the potential clinical benefit of phenotypic drug testing, several trials have likewise reported significantly reduced viral loads in patients whose therapy was guided by phenotypic testing.20 As a result of mounting evidence regarding the role of HIV drug resistance testing in improving the pharmacotherapy of HIV infection,21 resistance testing has become a standard of care for HIV patients, a position supported by the International AIDS Society and numerous prominent clinicians in the field. Current guidelines recommend that all recently infected HIV patients undergo resistance testing before the initiation of therapy. Resistance testing is also indicated in patients with an established HIV infection who have had 1 or more treatment failures or who are pregnant.
Strategies for Preventing or Overcoming HIV Drug Resistance
Pharmacists can play an important role in preventing the emergence of HIV drug resistance by stressing to their patients the need for strict adherence to their drug regimen. Many antiretroviral agents have relatively short half-lives and missed doses can reduce blood levels of drug and allow for viral proliferation and the development of resistance. Numerous studies have correlated good adherence to drug regimens with improved virologic response.22-24
Resistance testing is also a key factor in limiting the impact of HIV drug resistance. Testing of HIV strains for drug sensitivity allows the clinician to choose the drug or drug combination that will have the greatest chance for success, thus reducing viral loads in the shortest possible time. The use of drug combinations is another important strategy for preventing or treating HIV drug resistance. Highly active antiretroviral therapy (HAART) is a very effective strategy that involves the simultaneous use of several antiretroviral agents from different drug classes. The use of multiple drug classes in conjunction with resistance testing, significantly increases the likeliness that a particular strain of HIV will be effectively kept in check. Using HAART it is now possible to reduce viral loads to nearly undetectable levels in certain patients.
In light of the global nature of the HIV epidemic, the World Health Organization (WHO) has endorsed a multi-faceted approach to preventing the emergence and transmission of HIV drug resistance.25 This approach includes the use of standardized antiretroviral therapy based on drugs that are highly active and specific to a particular geographic region. Active adherence monitoring is also stressed. The WHO recommends implementation of an “early warning” system for detecting the emergence and spread of HIV drug resistance. Anti-HIV drugs must be quality controlled/assured and be made available to infected patients on an adequate and continuous basis. Finally, prevention programs can not be ignored. The patient discussed earlier who had multidrug-resistant HIV reported having multiple male sex partners and unprotected sex while using methamphetamines. This individual may represent a growing population who are abandoning safe-sex practices now that highly effective antiretroviral therapies are available. An additional factor of concern in this case is the increased use of methamphetamines which can heighten sexual sensation but impair good judgment.
New HIV Drugs and Targets
Emerging HIV drug resistance has driven the search for new classes of drugs that target different components of HIV and its life cycle.26 While a number of new NRTIs, NNRTIs, and PIs are in development, their mechanism is identical to currently available agents. The major advantage of these new drugs is improved potency and effectiveness against resistant HIV strains. New formulations of currently available agents that allow for less frequent dosing are also an important advance in terms of improving compliance and maintaining therapeutic blood levels. However, if effective HIV therapy is to stay ahead of emerging HIV resistance, new drug targets and drug classes will need to become a reality. Several new potential targets and drugs are currently under investigation. One new avenue of attack on HIV involves the pharmacological blockade of HIV integrase enzyme. This enzyme is required for integration of the HIV genome into the host cell genome. Blockade of this enzyme will prevent incorporation of the HIV genetic code into human cells and thus its subsequent expression. Merck Pharmaceuticals currently has an agent (MK-0518) in phase II clinical trials that is directed at this target.
A second new strategy for treating HIV infection centers on agents designed to block reading of the HIV genome. Such agents would be “anti-sense” drugs that bind to a complimentary segment of the HIV genome and block its activity.27,28 Phase I trials are being completed for one such agent (HGTV43, Enzo Therapeutics). Another agent under study (BI-201, BioInvent) is an antibody that binds to and blocks the HIV tat gene. This gene is essential for viral activity and its blockade may effectively block HIV replication. Phase II trials are currently underway for this agent as well. A third novel target, HIV vpr protein is blocked by the experimental drug VGX-410 (Viral Genomix, currently in phase II trials). This HIV functional protein appears to play an essential role in survival and replication of the virus in human cells.
The first HIV “maturation” inhibitors are also currently undergoing phase II trials (PA-457, Panacos Pharmaceuticals). These agents are designed to block processing of viral proteins (in this case the capsid protein) that are essential for production of mature and fully functional HIV.29 Another interesting class of novel anti-HIV agents is directed at disrupting the structural integrity of the viral capsid. Proteins making up the capsid are stabilized by the presence of “zinc fingers” and disrupting these entities appears to prevent the formation of functional viruses. On agent azodicarbonamide did undergo human clinical testing but was associated with a number of significant side effects.
Scientists made an interesting discovery that patients with a mutated cell surface receptor (CCR5) were resistant to HIV infection. Despite repeated sexual exposure to HIV with high-risk partners, a small percentage of individuals (2% of Caucasians carry this gene), did not get HIV30. It has been hypothesized that HIV might use CCR5 as a cellular “doorway” during the early stages of infection and that blocking it might prevent HIV entry.30,31 Though in the early stages, a number of CCR5 inhibitors are under development and preliminary studies in monkeys indicate they may be highly effective.31
SUMMARY
In order for practicing pharmacists to provide effective pharmaceutical care for their HIV-infected patients they must have a detailed knowledge of the pharmacology of antiretroviral drugs. Since the effectiveness of these agents can be greatly affected by HIV drug resistance, it is vital that students and practicing pharmacists understand mechanisms of HIV drug resistance, as well as factors that contribute to the emergence of resistance and ways to overcome it. HIV drug resistance is a complicated and dynamic topic. New information regarding mechanisms and prevalence of HIV drug resistance is appearing almost daily in the literature. The ever-changing nature of this subject requires that students, practitioners, and faculty members stay continuously abreast of the latest clinical and scientific developments in this area.
REFERENCES
- 1.Tobin N, FrenkelL Human immunodeficiency virus drug susceptibility and resistance testing. Pediatr Infect Dis J. 2002;21:668–83. doi: 10.1097/00006454-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 2.Richman DR, Morton SC, Wrin T, Hellman N, Berry S, Shapiro MF, Bozette S. The prevalence of antiretroviral drug resistance in the United States. AIDS. 2004;18:1393–1402. doi: 10.1097/01.aids.0000131310.52526.c7. [DOI] [PubMed] [Google Scholar]
- 3.Weinstock HS, Zaidi I, Heneine W, et al. The epidemiology of antiretroviral drug resistance among drug-naïve HIV-1-infected persons in 10 U.S. cities. J Infect Dis. 2004;189:2174–80. doi: 10.1086/420789. [DOI] [PubMed] [Google Scholar]
- 4.Preston BD, Poiesz BJ, Loeb LA. Fidelity of HIV-1 reverse transcriptase. Science. 1988;242:1168–71. doi: 10.1126/science.2460924. [DOI] [PubMed] [Google Scholar]
- 5.Perelson AS, Neumann AU, Markowitz M, Leonard JM. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–6. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 6.Marinez-Picardo J, DePasquale MP, Kartsonis N, et al. Antiretroviral resistance during successful therapy of HIV type I infection. Proc Natl Acad Sci. 2000;97:10948–53. doi: 10.1073/pnas.97.20.10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavel F, Hance AJ. HIV drug resistance. N Engl J Med. 2004;350:1023–35. doi: 10.1056/NEJMra025195. [DOI] [PubMed] [Google Scholar]
- 8.Brenner B, Turner D, Oliveira M, et al. A V106M mutation in HIV-1 clade C viruses exposed to efavirenz confers cross-resistance to non-nucleoside reverse transcriptase inhibitors. AIDS. 2003;17:F1–F5. doi: 10.1097/00002030-200301030-00001. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg ML, Cammack N. Resistance to enfuvirtide, the first HIV fusion inhibitor. J Antimicrob Chemother. 2004;54:333–40. doi: 10.1093/jac/dkh330. [DOI] [PubMed] [Google Scholar]
- 10.Gallego O, de Mendoza C, Perez-Elias MJ, et al. Drug resistance in patients experiencing early virological failure under a triple combination including indinavir. AIDS. 2001;15:1701–06. doi: 10.1097/00002030-200109070-00014. [DOI] [PubMed] [Google Scholar]
- 11.Delaugerre C, Rohban R, Simon A, et al. Resistance profile and cross-resistance of HIV-1 among patients failing a non-nucleoside reverse transcriptase inhibitor-containing regimen. J Med Virology. 2001;65:445–8. [PubMed] [Google Scholar]
- 12.Yeh RW, Coen DM. Pharmacology of viral replication. In: Golan DE, editor. Principles of Pharmacology. Vol. 545. New York: Lippincott Williams & Wilkins; 2005. 64 pp. [Google Scholar]
- 13. CNN Health. Drug-resistant HIV strain alarms officials. Available at http://edition.cnn.com/2005/HEALTH/conditions/02/11/drug.resistant.aids/. Accessed January 10, 2006.
- 14.Funesti J, Stacy F. HIV drug resistance and nursing practice. Am J Nursing. 2001;101:30–4. doi: 10.1097/00000446-200106000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch MS, Brun-Vezinet F, Clotet B, et al. Antiretroviral drug resistance testing in adults infected with human immunodeficiency virus type I: 2003 recommendations of an International AIDS Society-USA panel. Clin Infect Dis. 2003;37:113–28. doi: 10.1086/375597. [DOI] [PubMed] [Google Scholar]
- 16.Baxter JD, Mayers DL, Wentworth DN, et al. A randomized study of antiretroviral management based on plasma genotypic antiretroviral resistance testing in patients failing therapy. AIDS. 2000;14:F83–93. doi: 10.1097/00002030-200006160-00001. [DOI] [PubMed] [Google Scholar]
- 17.Durant J, Clevenbergh P, Halfon P, et al. Drug-resistance genotyping in HIV-1 therapy: The VIRADAPT randomized controlled trial. Lancet. 1999;353:2195–99. doi: 10.1016/s0140-6736(98)12291-2. [DOI] [PubMed] [Google Scholar]
- 18.Cingolani A, Antinori A, Rizzo MG, et al. Usefulness of monitoring HIV drug resistance and adherence in individuals failing highly active antiretroviral therapy: a randomized study (ARGENTA) AIDS. 2002;16:369–79. doi: 10.1097/00002030-200202150-00008. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein MC, Goldie SJ, Losina E, et al. Use of genotypic resistance testing to guide HIV therapy: clinical impact and cost-effectiveness. Ann Intern Med. 2001;134:440–50. doi: 10.7326/0003-4819-134-6-200103200-00008. [DOI] [PubMed] [Google Scholar]
- 20.Sax PE, Islam R, Walenasky RP, et al. Should resistance testing be performed for treatment-naïve HIV infected patients? A cost-effectiveness analysis. Clin Infect Dis. 2005;41:1316–23. doi: 10.1086/496984. [DOI] [PubMed] [Google Scholar]
- 21.Cohen CJ, Hunt S, Sension M, et al. A randomized trial assessing the impact of phenotypic resistance testing on antiretroviral therapy. AIDS. 2002;16:579–88. doi: 10.1097/00002030-200203080-00009. [DOI] [PubMed] [Google Scholar]
- 22.Hofer CB, Schechter M, Harrison LH. Effectiveness of antiretroviral therapy among patients who attend public HIV clinics in Rio de Janeiro, Brazil. J Acquir Immune Defic Syndr. 2004;36:967–71. doi: 10.1097/00126334-200408010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Cahn P, Vibhagool A, Schecter M, et al. Predictors of adherence and virologic outcome in HIV-infected patients treated with abacavir- or indinavir-based triple combination HAART also containing lamivudine/zidovudine. Curr Med Res Opin. 2004;20:1115–23. doi: 10.1185/030079904125004051. [DOI] [PubMed] [Google Scholar]
- 24.Liu H, Miller LG, Hays RD, Golin CE, Wu T, Wenger NS, Kaplan AH. Repeated measures longitudinal analyses of HIV virologic response as a function of percent adherence, dose timing, genotypic sensitivity, and other factors. J Acquir Immune Defic Syndr. 2006;41:315–22. doi: 10.1097/01.qai.0000197071.77482.6e. [DOI] [PubMed] [Google Scholar]
- 25. World Health Organization. Drug resistance, HIV/ AIDS. Available at http://www.who.int/drugresistance/hivaids/en/. Accessed January 11, 2006.
- 26.Marks K, Gulick RM. New antiretroviral agents for the treatment of HIV infection. HIV/AIDS Rep. 2004;1:82–8. doi: 10.1007/s11904-004-0012-0. [DOI] [PubMed] [Google Scholar]
- 27.Manoharan M. Oligonucleotide conjugates as potential antisense drugs with improved uptake, biodistribution, targeted delivery and mechanism of action. Antisense Nucleic Acid Drug Dev. 2002;12:103–28. doi: 10.1089/108729002760070849. [DOI] [PubMed] [Google Scholar]
- 28.Kurreck J. Antisense technologies. Eur J Biochem. 2003;270:1628–44. doi: 10.1046/j.1432-1033.2003.03555.x. [DOI] [PubMed] [Google Scholar]
- 29.Schubert U, Ott DE, Chertova EN, et al. PNAS. Proteasome inhibition interferes with Gag polyprotein processing, release and maturation of HIV-1 and HIV-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quillent C, Oberlin E, Braun J, et al. HIV-1-resistance conferred by combination of two separate inherited mutations of CCR5 gene. Lancet. 1998;351:14–8. doi: 10.1016/S0140-6736(97)09185-X. [DOI] [PubMed] [Google Scholar]
- 31.Veazey RS, Klasse PJ, Ketas TJ. Use of a small molecule CCR5 inhibitor in macaques to treat simian immunodeficiency virus infection or prevent simian-human immunodeficiency infection. J Exp Med. 2003;17:1551–62. doi: 10.1084/jem.20031266. [DOI] [PMC free article] [PubMed] [Google Scholar]