Abstract
Macrophages play an important role in host-defense and inflammation. In response to an immune challenge, macrophages become activated and produce proinflammatory mediators that contribute to nonspecific immunity. The mediators released by activated macrophages include: superoxide anion; reactive nitrogen intermediates, such as nitric oxide and peroxynitrite; bioactive lipids; and cytokines. Although essential to the immune response, overproduction of certain macrophage-derived mediators during an immune challenge or inflammatory response can result in tissue injury and cellular death. The present report is focused on understanding some of the molecular mechanisms used by macrophages to produce reactive nitrogen intermediates in response to immunostimulatory agents such as heat shock protein 60 and bacterial lipopolysaccharide. The role of Toll-like receptors and transcription factors such as nuclear factor kappa B (NFκB) in the innate immune response is also described. A basic understanding of the underlying molecular mechanisms responsible for macrophage activation should serve as a foundation for novel drug development aimed at modulating macrophage activity.
Keywords: macrophages, nitric oxide, immunity, instructional methods
INTRODUCTION
The primary objective of the Introduction to Pharmacology course given at the College of Pharmacy and Allied Health Professions at St. John's University is to introduce students to the fundamental principles of pharmacology. This course is required for all students enrolled in toxicology, physician's assistant, and doctor of pharmacy (PharmD) programs, and it is also a prerequisite for advanced courses within the pharmacy curriculum. Our PharmD program is a 6-year program and the majority of matriculating students begin immediately after high school.
The Introduction to Pharmacology course is a 2-credit, half-semester, course that is offered during the spring semester of the third year of study toward the PharmD degree. This course serves as a steppingstone into advanced courses taken in the fourth and fifth years of the PharmD curriculum. The fourth- and fifth-year PharmD students take a series of integrated, team-taught, Drugs and Disease courses that examine the predominant human pathologies that students will encounter in their professional practice. The Drugs and Disease courses incorporate advanced pharmacology concepts as well as issues relating to molecular and cellular pathology, medicinal chemistry, and clinical therapeutics. Therefore, students must progress into the fourth year possessing a firm command of the basics of pharmacology. The Introduction to Pharmacology course is the major forum in which PharmD students have the opportunity to master basic pharmacological principles. All of the pharmacology topics encountered in subsequent courses build upon these key concepts. Failure of students to master the information in the Introduction to Pharmacology course puts them at a serious disadvantage and decreases the likelihood of understanding the advanced pharmacology concepts that they will later encounter.
The importance and relevance of pharmacology to pharmacy is emphasized in each lecture. The students are urged to study a minimum of 3 hours for every 1 hour of lecture. Most students who heed this advice perform well and usually find themselves in the upper third of their class with respect to the distribution of final grades for the course. Students who perform poorly are encouraged to make use of the instructor's office hours to identify learning problems and discuss study strategies.
The class meets twice weekly for 6 weeks. Unlike more advanced course offerings within the curriculum, the entire Introduction to Pharmacology course is taught by a single professor. Each lecture is 2 hours and the topics emphasized are pharmacokinetics, pharmacodynamics, and drug metabolism. The students' mastery of these key aspects of pharmacology is then assessed through a 2-hour written examination given after 6 lectures. Postmidterm lectures focus on the pharmacology of the autonomic nervous system and the immune system. The final examination is comprehensive and cumulative.
The subject of the present report forms part of a lecture relating to the pharmacology of innate immunity. It is not meant to serve as a comprehensive overview of what is presently known on the subject but rather to discuss several examples of key biological pathways and chemical mediators associated with macrophages. It is our belief that students need to be educated and prepared for a pharmacy career that will span a lifetime. Research that is presently being done in academia and at research centers to unravel complex molecular signaling pathways and to gain an understanding on how such pathways trigger the production of inflammatory mediators by macrophages will hopefully result in the therapeutic agents of tomorrow. Such novel drugs could provide the cure for sepsis or serve as better alternatives to NSAIDs and steroids in the treatment of chronic inflammatory diseases such as rheumatoid arthritis.
The material presented here examines the role of the macrophage in innate immunity. Although a great deal can be written on this subject, the lecture on this topic is aimed at providing a few examples of molecular mechanisms that are used by macrophages to defend the host against infection. After having attended the lecture and reviewed the material presented, it is expected that students will be able to answer the following questions:
What is immunity? What are the 2 major types of immunity in humans?
What are the 7 categories of innate immunity? What are the main features of each category?
What are macrophages and how do they contribute to innate immunity?
What is bacterial lipopolysaccharide (LPS)?
What is heat shock protein 60 (HSP60)? What is common to both HSP60 and LPS?
What are Toll-like receptors (TLRs)? How do they help macrophages to respond to LPS?
What is the role of the transcription factor NFκB in macrophage activation by LPS?
OBJECTIVES
Immunity
The term “immunity” refers to all of the mechanisms used by the body to obtain protection against pathogens such as opportunistic bacteria, fungi, and viruses, and against a wide variety of foreign substances. Foreign substances capable of inducing an immune response that culminates in the production of antibodies are called immunogens. Immunogens are capable of stimulating immune responses partly because of the presence of specific molecular structures on their surface, known as antigenic determinants or “epitopes.” Microbes, foreign substances, or foreign particles capable of interacting with components or products of the immune system are designated as “antigens.” Whereas all immunogens stimulate immune responses, antigens may or may not be immunogenic. The reason for this is many compounds exist that are capable of binding with components of the immune system but incapable of inducing immune responses. In the present article, our discussion of antigens will be limited to those that are also immunogenic.
There are 4 major types of antigen classes: carbohydrates, lipids, nucleic acids, and proteins. Antigens signal the immune system that something “foreign” is present in the body. When an immunogen such as a pathogenic bacterium gains access into the body of a healthy and immunocompetent individual, antigens present on the surface of the bacterium not only bind to antigen receptors on immune cells but also to circulating antibodies. The binding of antigens to these immune system components activates a cascade of events that results in immunity. Humans possess 2 broad types of immunity termed acquired immunity and innate immunity. The interaction of the cellular and molecular components of these types of immune defenses ensures adequate resistance to infection.1
Understanding the mechanisms whereby pathogens activate immune responses is an area of great scientific interest and of epidemiological concern. One could argue that of all the organ systems in the body, the immune system ranks second in complexity only to the nervous system. The past 10 years have uncovered many critical molecular aspects of immunity. When one considers that the system is involved in numerous human pathologies ranging from sepsis to autoimmune diseases, an understanding of the molecular mechanisms that drive immunity should serve as a platform for the development of useful therapeutic agents. The present report provides a concise overview of the 2 basic types of immunity, describes the role played by macrophages in the development of an innate immune response, and stresses the importance of the activation of Toll-like receptors by specific ligands to innate immunity.
Acquired Immunity
Acquired immunity is specific to and activated by particular antigens. In other words, acquired immunity may protect an individual against infection by one type of pathogen but not against infection by another type of pathogen. So what, then, determines whether a pathogen will be dealt with via an acquired immune response? The answer to this question depends on whether the immune system has previously been presented with that particular antigen. Acquired immunity develops after birth as the result of accidental or intentional exposure to pathogens. The B- and T-lymphocytes are the 2 major cell types involved in acquired immunity. In response to the binding of antigens to specific receptors on their surface, mature B-lymphocytes differentiate into plasma cells which produce and secrete antibodies into the bloodstream. In contrast, T-lymphocytes secrete cytokines and growth factors that are required for B-cell activation and also for the regulation of cell-mediated immunity and for the activation of cells involved in innate immunity, especially the macrophages.2
Innate Immunity
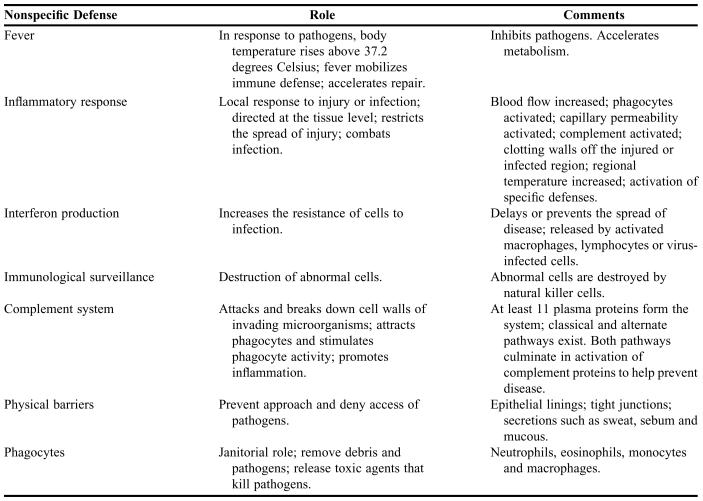
In contrast to antigen-mediated acquired immune responses, innate immune defenses are nonspecific in nature. The physiological purpose of innate defenses is to prevent the approach, deny the entrance, or destroy microorganisms or other foreign environmental agents without distinguishing among specific types of immunogens. Table 1 lists the 7 major responses and components of innate immunity, among which the phagocytes play a unique nonspecific role in host defense.1
Table 1.
Seven Major Responses and Components of Innate Immunity*
*Compiled from references 1 and 2.
Phagocytes serve as “janitors” of the innate immune system in that they remove entire pathogens and debris. The phagocytic cells of the human immune system include the microphages and the macrophages. Examples of microphages are the neutrophils and eosinophils, both of which are leukocytes that normally circulate in the blood and that will enter peripheral tissues when these tissues have been injured or infected. Macrophages are physically larger than microphages and are found both circulating in the blood and as fixed populations within certain body tissues. Microphages and macrophages represent the “first line” of defense to invading pathogens. In response to pathogens, these phagocytic cells engulf and destroy the invaders, sometimes even before the detection of the invaders by the lymphocytes involved in acquired immunity.1,2
THE ROLE OF MACROPHAGES IN INNATE IMMUNITY
Macrophages are derived from hematopoietic stem cells in the bone marrow. Following a maturation and proliferation process that is dependent upon hematopoietic factors such as macrophage colony stimulating factor (MCSF) and granulocyte-macrophage colony stimulating factor (GMCSF), monoblast precursor cells develop into monocytes (Figure 1). Monocytes then leave the bone marrow and enter the blood where they circulate throughout the body. They are estimated to comprise 5% of all circulating leukocytes. Approximately 24 hours after entering the systemic circulation, monocytes migrate into tissues where they differentiate into macrophages. During this maturation process, the cells become larger and the numbers of mitochondria and lysosomes within them increase. The relative amounts of lysosomal enzymes, including acid phosphatase, beta-glucuronidase, cathepsin, and aryl sulfatase are also notably higher in macrophages than in their precursors.3 The process of macrophage differentiation is regulated by their microenvironment and by the degree of hematopoietic stimulation within the tissue.4,5
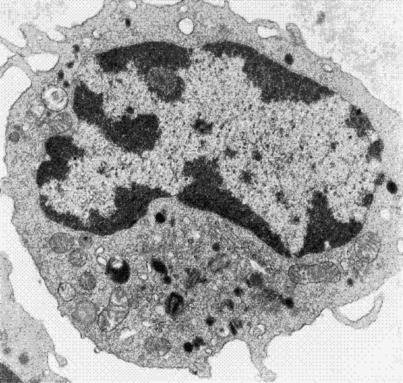
Figure 1.
Transmission electron micrograph of a circulating monocyte. Monocytes are phagocytic cells that migrate into tissues and differentiate into macrophages. Note that the nucleus occupies most of the volume of the cell. Organelles such as mitochondria and lysosomes and structures such as phagosomes are also observed in the cytoplasm. (Magnification 5000x).
Macrophages represent a functionally heterogeneous population of immune cells in the body. Despite tissue-specificity differences among them, macrophages share several common features. They are highly phagocytic cells, able to engulf not only bacteria but also foreign particulate and soluble substances through the process of endocytosis. Macrophages also exhibit several receptors on their surface for the recognition of other cells or antigens to be phagocytosed and for binding to complement fragments, mannose residues on bacteria, and the Fc region of immunoglobulin G. An additional role of macrophages is to participate in the activation of T lymphocytes by presenting certain antigens to them via surface molecules designated as major histocompatibility complexes (MHC).5
When a macrophage comes in contact with a foreign stimulus, it becomes “activated.” Activated macrophages respond to pathogens in 1 of 3 ways. They may engulf a pathogen or other foreign objects and destroy them within lysosomes via the action of lysosomal enzymes. (In Figure 1, the black spherical structures seen in the lower portion of the cytoplasm are the lysosomes.) In some instances, macrophages may bind or remove an invading pathogen from the interstitial fluid only when assisted by lymphocytes involved in acquired immunity. Thirdly, activated macrophages release a wide array of mediators including reactive oxygen and nitrogen species, hydrolytic enzymes, bioactive lipids, and cytokines such as tumor necrosis factor alpha (TNFα).1,6,7 These mediators are believed to confer bactericidal and cytostatic action to the macrophage as well as play a role in the recruitment and accessibility of other inflammatory cells to an area of injury. Overproduction of nitric oxide and other macrophage-derived mediators can also lead to tissue injury and toxicity in the host.8
Macrophages are found in all organs and connective tissues where they play an important role in host defense. Significant populations of macrophages are found in the liver, lung, spleen, kidney, and brain. Hepatic macrophages, also known as Kupffer cells, constitute approximately 30% of the non-parenchymal cells of the liver and account for 80%-90% of all the macrophages in the body.5,9,10 Under normal circumstances, these cells have relatively long lives and are localized in hepatic sinusoids where they participate in the clearance of pathogens and bacterial-derived toxins from the circulation. During inflammatory conditions of the liver, as observed following chemical insult with hepatotoxicants such as acetaminophen or carbon tetrachloride, there is an accumulation of macrophages and an increase in the production of cytotoxic mediators such as nitric oxide and TNFα at the area of injury. Blocking the activation of macrophages with macrophage-specific inhibitors such as gadolinium chloride results in decreased tissue injury following exposure to these hepatotoxicants.11
Nitric Oxide
Nitric oxide is a free radical membrane-permeable inorganic gas.12 The importance of nitric oxide in biological functioning is apparent from the numerous physiological processes regulated by this molecule. Aside from its role in smooth muscle relaxation and blood pressure regulation, nitric oxide is involved in renal function, neurotransmission, respiration, gastrointestinal function, wound healing, and nonspecific immunity.12,13 Nitric oxide is enzymatically generated by most cells of the body including the vascular endothelium, neurons of the brain and enteric nervous system, and cells of the immune system.14 Three unique isoforms of the enzyme nitric oxide synthase (NOS) are presently known to catalyze the 5-electron oxidation of L-arginine to L-citrulline and nitric oxide. These 3 enzymes are not alternately spliced variants of a single gene but rather are coded by unique genes. NOS1 was the first isoform to be identified and is often referred to as neuronal NOS (nNOS), as it was first characterized in neurons. NOS2 is an inducible isoform that was originally identified in macrophages and is sometimes referred to as macrophage NOS or iNOS. NOS3 was characterized in endothelial cells and is often referred to as endothelial NOS (eNOS).15
The nitric oxide synthases can be categorized as either calcium/calmodulin-dependent (NOS1 and NOS3) or calcium/calmodulin-independent (NOS2). Whereas activated calmodulin binds to NOS1 and NOS3 in a calcium-dependent manner, its binding to NOS2 takes place in a tight manner in the absence of calcium.16-18 In the presence of the essential cofactors heme, tetrahydrobiopterin, FAD, FMN, NADPH and molecular oxygen, nitric oxide synthases convert L-arginine into nitric oxide and L-citrulline in a 1:1 stoichiometric ratio. The NOS1 and NOS3 isoforms are constitutively expressed and continuously release low levels of nitric oxide. Since the induction of NOS2 by cytokines and proinflammatory agents such as lipopolysaccharide (LPS) results in high levels of nitric oxide for extended periods of time, it is designated as the high throughput isoform.19
Nitric oxide is a key mediator of nonspecific immunity. Evidence in support of this role stems from several reports describing the production of nitric oxide by macrophages stimulated by antigens such as bacteria, viruses, and parasites.19-21 Mice deficient in the NOS2 gene were more susceptible to infection than their wild-type counterparts.22 The toxicity of nitric oxide to the invading pathogen is related, at least, to its ability to inhibit ribonucleotide reductase and to interfere with DNA synthesis. Nitric oxide also inactivates iron-sulfur clusters of such vital enzymes as NADH:ubiquinone oxidoreductase.23 The removal of excess nitric oxide from the blood is still not well understood. Its clearance in vivo is postulated to occur through reaction with oxyhemoglobin to form methemoglobin and nitrate.24
Though nitric oxide itself is quite toxic, its reactive nature leads to the formation of other toxic reactive nitrogen intermediates.19,23 Nitric oxide reacts with molecular oxygen in solution to produce both stable and relatively nontoxic species, such as nitrite and nitrate, and toxic and reactive species such as nitrogen dioxide. Nitrogen dioxide can further react with nitric oxide to yield dinitrogen trioxide, a potent nitrosating agent. Nitrogen oxides have been shown to increase lipid peroxidation and decrease antioxidant defense systems.25 Exposed thiol groups can react with nitric oxide and then later liberate the extremely reactive nitrosonium ion. The reaction of nitric oxide and superoxide results in the formation of peroxynitrite, a powerful oxidant. This reaction is rapid, approximately threefold faster than the reaction of superoxide with superoxide dismutase. As activated macrophages produce both nitric oxide and superoxide, the formation of peroxynitrite is likely in these cells.8,26 In turn, nitration of exposed tyrosine residues by peroxynitrite also contributes to cellular toxicity.
Although necessary for adequate immunity, the production of nitric oxide and other reactive nitrogen intermediates for the purpose of preventing the spread of infections can also have toxic effects on the host. Indeed, since nitric oxide reacts with most host biomolecules including DNA, proteins, lipids, thiols and metals, overproduction of this and other reactive nitrogen intermediates can have deleterious effects.23 The formation of nitrotyrosine residues has been observed in atherosclerotic plaques, synovial fluid of arthritic patients, lung tissue of animals exposed to LPS, and in several other human and animal disease states.27 Overproduction of nitric oxide has also been implicated in sepsis, neurodegenerative disorders, inflammation, and colon cancer.28-30
Lipopolysaccharide and Toll-Like Receptors
Lipopolysaccharide (LPS) is a structural component of the outer membrane of gram-negative bacteria which contributes to the structural integrity of the organism and confers toxicity against the susceptible host. The molecule has a lipid domain (Lipid A) which anchors it into the outer membrane, a core polysaccharide domain attached to Lipid A, and a polysaccharide (O antigen) that extends to the external environment from the core polysaccharide. After lysis of gram-negative bacteria by the complement system, LPS is released into the circulation where it becomes bound to plasma proteins designated as LPS-binding proteins. In this conjugated form, LPS is presented to specific receptors on monocytes and macrophages. Understanding the mechanisms that underlie LPS signaling should provide a foundation for novel drug development aimed at preventing or decreasing some of the pathophysiological effects of LPS such as fever, hypotension, shock, and possibly death.2,6
LPS stimulates macrophages to produce cytotoxic and proinflammatory mediators such as nitric oxide, TNFα, bioactive lipids, and cytokines.6 When LPS binds and activates receptors on the surface of macrophages, that signal is propagated across the membrane through the cytoplasm and eventually brought to the nucleus where gene transcription of the proinflammatory mediators is stimulated. For example, LPS stimulates gene transcription of NOS2 in macrophages, in part, by stimulating NFκB transcription factors to leave the cytoplasm and enter the nucleus. Once inside the nucleus, the NFκB factors will drive the transcription of proinflammatory genes like NOS2.6 The mechanism by which extracellular LPS is able to stimulate gene activation via this and other transcription factors in macrophages has only recently been elucidated.6
Scientists have known since the early 1990s that the effects of LPS on macrophages are mediated by the activation of the CD14 receptor.6 The CD14 receptor is a glycophosphatidylinositol-linked outer membrane protein that neither traverses the membrane nor has an intracellular effector domain.31 Because this cell surface receptor lacks an intracellular effector domain, it was formerly believed that a second molecular component of the receptor was present to enable the LPS-mediated signaling to be transduced across the membrane, and ultimately, to effect gene activation.32 The existence of this additional LPS-receptor component, designated as the “Toll-like” receptor 4 (TLR4), has recently been confirmed and found to be itself a transmembrane receptor with an extracellular LPS-binding domain and a cytoplasmic domain that serves as a platform for the recruitment of protein kinases involved in cell signaling. In humans, TLRs participate in the innate immune response.32,33 Several in vitro studies support a role for TLR4 in LPS signaling.34-36 LPS was also recently reported to stimulate a physical association between CD14 and TLR4.37 Animals resistant to toxic doses of LPS have a single point mutation in the coding region of the TLR4 gene. The net effect of this point mutation is that the TLR4 gene subsequently codes for a mutated and nonfunctional TLR4 receptor, thus demonstrating the receptor-conferred sensitivity to LPS.32,36
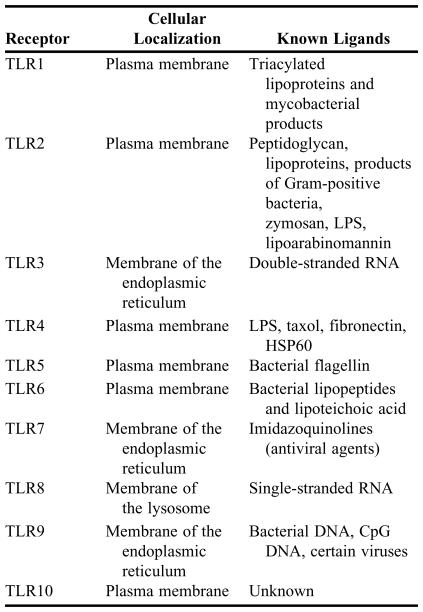
The 10 TLRs thus far identified in humans are listed in Table 2. Each TLR is a transmembrane-spanning protein with common leucine-rich repeat (LRR) on the outside of the membrane and a highly conserved domain that shares homology with the intracellular domain of the interleukin-1 receptor on the inside of the membrane.38 Known ligands of TLRs include LPS, peptidoglycan, lipoteichoic acid, and stress proteins.32,33
Table 2.
Toll-like Receptors (TLR) Involved in Innate Immunity in Humans.*
*Compiled from references 32, 33, 39 and 51
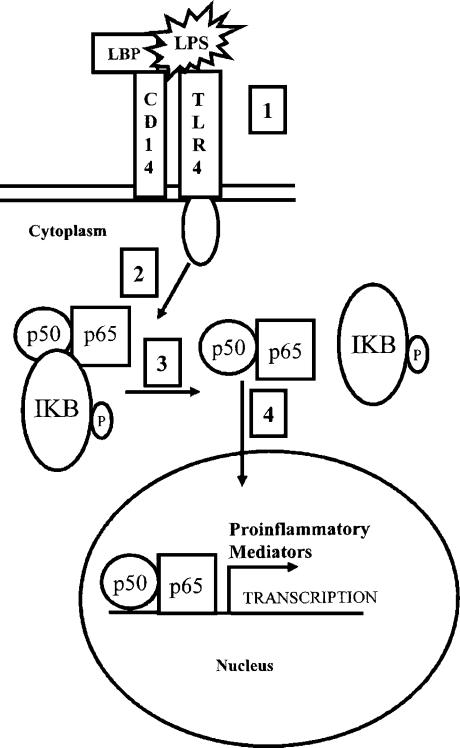
LPS stimulates the activity of NFκB.6 The mechanism leading to this activation involves a “signaling” process that starts when LPS binds to the CD14/TLR4 complex on macrophages. Binding of LPS to this receptor complex stimulates a phosphorylation signaling cascade which culminates in the translocation of NFκB transcription factors into the nucleus.39 Once inside the nucleus, these factors bind to specific promoters to stimulate gene transcription (Figure 2).
Figure 2.
Activation of NFκB in macrophages by LPS. Under normal circumstances, the NFκB transcription factor, a dimer made up of two subunits called p50 and p65, is observed in the cytoplasm complexed to an inhibitor protein called IκB. During a microbial infection, LPS-binding protein (LBP) binds to circulating LPS and presents it to the CD14 and TLR4 receptors (Step 1). The TLR4 is then activated, initiating a phosphorylation cascade that culminates in the phosphorylation of IκB (Step 2). Phosphorylation of IκB results in the dissociation of IκB from the complex (Step 3). Interestingly, the phosphorylated IκB peptide is degraded by the 26S proteasome. The p50/p65 dimer translocates into the nucleus where it binds to specific DNA sequences called kappa B sites (κB) and promotes gene transcription (Step 4). Proinflammatory proteins such as NOS2 and TNFα are coded for by genes that are transcriptionally regulated by NFκB. (Adapted from references 6, 32, 39 and 49).
Heat Shock Protein 60 and TLR4
Heat shock proteins (HSPs) are a family of highly conserved proteins that assist in protein folding and transport and are constitutively present in cells.40 HSPs can be further induced in cells by both physical and chemical stresses including exposure to heat, heavy metals, and ultraviolet light.41,42 Human and microbial HSP60 homologs share considerable similarity at the amino acid level. Interestingly, in addition to its known role as a chaperonin, it has recently been demonstrated that this 60 kilodalton protein can induce macrophages to produce proinflammatory mediators such as nitric oxide, TNFα, and interleukins 6, 12, and 15.43 HSP60 has also been reported to alter the expression of cellular adhesion molecules, to modulate signal transduction pathways involving the ERK and p38 kinases, and to induce transcription factors such as NFκB.44-47 HSP60 mediates its effects, in part, by binding to surface CD14 and Toll-like receptors, which are also utilized in LPS-mediated signaling and may, therein, serve as the endogenous ligand of the LPS receptor.44-48 Taken together, these data suggest that HSP60 may be involved in pathologies involving inflammation.
Nuclear Factor Kappa B
NFκB is a dimeric transcription factor comprised of 2 dissimilar subunits (p50 and p65) and first identified as a regulatory factor involved in the gene expression of the immunoglobulin kappa light chain in B cells.49 Due to the κB consensus sequences that are observed in the promoters of several genes that are upregulated during inflammation, the role of NFκB in inflammation is diverse. Some of the proinflammatory genes regulated by NFκB include NOS2, TNFα, IL-1, COX-2, and several chemokines, all of which can enhance inflammation by attracting additional inflammatory cells to the site of injury. NFκB also regulates the transcription of its inhibitor IκB.49 Some of the known inducers of NFκB are TNFα, IL-1, LPS, double stranded RNA, viral proteins, ionizing radiation, ultraviolet light, and reactive oxygen species such as superoxide anion and hydrogen peroxide. HSP60 has also been shown to induce NFκB activation in macrophages and other cells of innate immunity.43-47 Antioxidants such as pyrrolidine dithiocarbamate are known to inhibit p50/p65 activation and may play a role in decreasing the tissue injury associated with an inflammatory response.50
Although proinflammatory mediators are required to prevent the spread of invading pathogens, their excessive production by phagocytes involved in innate immunity can lead to tissue injury.5,11,26 Knowledge of the underlying mechanisms of LPS-mediated signal transduction in macrophages establishes a foundation for developing an understanding of the need for pharmacologic intervention at one of several levels. For example, a new drug against septic shock could be developed to specifically antagonize the TLR4 by competing with LPS for binding. The net effect would be to decrease both nitric oxide production and the symptoms of sepsis. A new drug could be developed to block the recruitment of kinases to the cytoplasmic domain of TLR4 that are needed to phosphorylate downstream targets. Preventing such action would in essence also shut off the pathway and prevent the formation of potentially damaging proinflammatory mediators. Drugs that specifically inhibit NOS2 or NFκB might also serve as clinically useful agents, especially in conditions of chronic inflammation where overproduction of nitric oxide contributes to tissue injury. Understanding mechanisms of receptor-mediated signaling helps the pharmacologist to design new drugs with potential utility in a clinical setting.
INSTRUCTIONAL METHODS AND CONTENT
Among the several types of undergraduate and graduate degrees offered by the College of Pharmacy and Allied Health Professions at St. John's University, the PharmD is achieved at the end of a 6-year curriculum. The material discussing the activation of macrophages by agonists such as bacterial LPS or HSP60 is taught in a single 2-hour lecture in the Introduction to Pharmacology Course. The course material is presented in a lecture-based format using Microsoft PowerPoint. Both an outline of each lecture and the lecture slides are made available to students 24 hours prior to each class. These materials are posted on the St. John's University intranet and are available only to matriculated students. Students are assigned readings from a commonly used textbook and are also given homework questions based on reading assignments derived from pharmacology papers found in recognized journals. The pace of the lecture is set in such a way as to allow students to take detailed notes. A 5-minute break is taken at the end of the first hour of lecture. Although students can ask questions at any point during the lecture, the final 15 minutes of the class are specifically devoted to answering questions. In addition, students have the option to ask further questions during office hours. Regular office hours are often extended, especially during examination time, to ensure that the students can have their questions on the lecture material answered.
The class size of Introduction to Pharmacology ranges from 40-50 students. Two written examinations (midterm and final) are given using a written, multiple-choice format. In addition to the 2 examinations, 4 unannounced quizzes are given in short-answer and essay format. These unannounced quizzes, though unpopular with students, help them to stay on top of their assigned readings and to better focus their study efforts in preparation for the examinations. Examination grades are not curved and no extra-credit points are offered. Multiple-choice examinations alone may not be the strongest assessment tools of a student's knowledge base. However, the examinations are constructed in a straightforward and factual manner, and students generally appreciate the rapid grading this testing format permits. Grades are posted within 48 hours of an examination. Throughout the entire course, a web log (blog) is maintained and is accessible to all students. The blog is not only used to post relevant information such as supplemental readings and study outlines, but also to inform the students about the availability of online pharmacology resources that could be used to enhance their learning experience. (The blog site used in this course is available for inspection at: http://pharmakologika.blogspot.com.).
Given the limited timeframe for this course (6 weeks), there is no substitute for being prepared both as a student and as a lecturer. The instructor makes every effort to ensure that students are receiving accurate and enriching information concerning key pharmacological principles that will serve as a solid foundation in future courses. The instructor also expects that students will put in solid study efforts on a weekly basis that go beyond simply “showing up for class.” Although most students agree that the workload is heavy, those who put in the effort are quite successful in the course.
Acknowledgments
Many thanks to Dr. Louis Trombetta for providing the transmission electron micrograph of the monocyte found in Figure 1. Sincere thanks are also extended to Dr. Cesar Lau-Cam and Dr. Diane Hardej for their critical evaluation of the manuscript and valuable suggestions.
REFERENCES
- 1.Martini FA. Fundamentals of Anatomy and Physiology. 6th ed. New York: Benjamin Cummings; 2004. pp. 791–812. [Google Scholar]
- 2.Benjamini E, Coico R, Sunshine G. Immunology: A Short Course. 4th ed. New York: A John Wiley & Sons, Inc.; 2000. pp. 17–38. [Google Scholar]
- 3.Zeligs BJ, Nerurkar LS, Bellanti JA. Maturation of the rabbit alveolar macrophage during animal development. III. Phagocytic and bactericidal functions. Pediatr Res. 1977;11:1208–11. doi: 10.1203/00006450-197712000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Rutherford MS, Witsell A, Schook LB. Mechanisms generating functionally heterogeneous macrophages: chaos revisited. J Leukoc Biol. 1993;53:602–18. doi: 10.1002/jlb.53.5.602. [DOI] [PubMed] [Google Scholar]
- 5.Morio LA, Chiu H, Sprowles KA, Laskin DL. Functional heterogeneity of rat hepatic and alveolar macrophages: effects of chronic ethanol administration. J Leukoc Biol. 2000;68:614–20. [PubMed] [Google Scholar]
- 6.Janeway CA,, Jr., Medzhitov R. Innate immune recognition. Ann Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 7.Nathan CF. Secretory products of macrophages. J Clin Invest. 1987;79:319–26. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laskin DL, Heck DE, Punjabi CJ, Laskin JD. Role of nitric oxide in hematosuppression and benzene-induced toxicity. Environ Health Perspect. 1996;104(Suppl 6):1283–7. doi: 10.1289/ehp.961041283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouwens L, Baekeland M, De Zanger R, Wisse E. Quantitation, tissue distribution and proliferation kinetics of Kupffer cells in normal rat liver. Hepatology. 1986;6:718–22. doi: 10.1002/hep.1840060430. [DOI] [PubMed] [Google Scholar]
- 10.Blouin A, Bolender RP, Weibel ER. Distribution of organelles and membranes between hepatocytes and nonhepatocytes in the rat liver parenchyma. A stereological study. J Cell Biol. 1977;72:441–55. doi: 10.1083/jcb.72.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laskin DL, Heck DE, Laskin JD. Role of inflammatory cytokines and nitric oxide in hepatic and pulmonary toxicity. Toxicol Lett. 1998;102-103:289–93. doi: 10.1016/s0378-4274(98)00316-6. [DOI] [PubMed] [Google Scholar]
- 12.Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992;6:3051–64. [PubMed] [Google Scholar]
- 13.Moncada S. Nitric oxide: discovery and impact on clinical medicine. J R Soc Med. 1999;92:164–9. doi: 10.1177/014107689909200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nathan C, Xie QW. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994;78:915–8. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 15.Cho HJ, Martin E, Xie QW, Sassa S, Nathan C. Inducible nitric oxide synthase: identification of amino acid residues essential for dimerization and binding of tetrahydrobiopterin. Proc Natl Acad Sci U S A. 1995;92:11514–8. doi: 10.1073/pnas.92.25.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snyder SH, Bredt DS. Nitric oxide as a neuronal messenger. Trends Pharmacol Sci. 1991;12:125–8. doi: 10.1016/0165-6147(91)90526-x. [DOI] [PubMed] [Google Scholar]
- 17.Cho HJ, Xie QW, Calaycay J, Mumford RA, Swiderek KM, Lee TD, Nathan C. Calmodulin is a subunit of nitric oxide synthase from macrophages. J Exp Med. 1992;176:599–604. doi: 10.1084/jem.176.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie QW, Cho HJ, Calaycay J, et al. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992;256:225–8. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]
- 19.Nathan C. Inducible nitric oxide synthase: what difference does it make? J Clin Invest. 1997;100:2417–23. doi: 10.1172/JCI119782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green SJ, Mellouk S, Hoffman SL, Meltzer MS, Nacy CA. Cellular mechanisms of nonspecific immunity to intracellular infection: cytokine-induced synthesis of toxic nitrogen oxides from L-arginine by macrophages and hepatocytes. Immunol Lett. 1990;25:15–9. doi: 10.1016/0165-2478(90)90083-3. [DOI] [PubMed] [Google Scholar]
- 21.Kremsner PG, Nussler A, Neifer S, Chaves MF, Bienzle U, Senaldi G, Grau GE. Malaria antigen and cytokine-induced production of reactive nitrogen intermediates by murine macrophages: no relevance to the development of experimental cerebral malaria. Immunology. 1993;78:286–90. [PMC free article] [PubMed] [Google Scholar]
- 22.MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Ann Rev Immunol. 1997;15:323–50. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- 23.Eiserich JP, Patel RP, O'Donnell VB. Pathophysiology of nitric oxide and related species: free radical reactions and modification of biomolecules. Mol Aspects Med. 1998;19:221–357. doi: 10.1016/s0098-2997(99)00002-3. [DOI] [PubMed] [Google Scholar]
- 24.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr., Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–83. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Hu ML, Louie S, Duvall TR, Tarkington BK, Motchnik P, Cross CE. Interaction of nitrogen dioxide with human plasma. Antioxidant depletion and oxidative damage. FEBS Lett. 1992;313:62–6. doi: 10.1016/0014-5793(92)81185-o. [DOI] [PubMed] [Google Scholar]
- 26.Laskin DL, Fakhrzadeh L, Laskin JD. Nitric oxide and peroxynitrite in ozone-induced lung injury. Adv Exp Med Biol. 2001;500:183–90. doi: 10.1007/978-1-4615-0667-6_24. [DOI] [PubMed] [Google Scholar]
- 27.Greenacre SA, Ischiropoulos H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic Res. 2001;34:541–81. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- 28.Hobbs AJ, Higgs A, Moncada S. Inhibition of nitric oxide synthase as a potential therapeutic target. Annu Rev Pharmacol Toxicol. 1999;39:191–220. doi: 10.1146/annurev.pharmtox.39.1.191. [DOI] [PubMed] [Google Scholar]
- 29.Payne CM, Bernstein C, Bernstein H, Gerner EW, Garewal H. Reactive nitrogen species in colon carcinogenesis. Antioxid Redox Signal. 1999;1:449–67. doi: 10.1089/ars.1999.1.4-449. [DOI] [PubMed] [Google Scholar]
- 30.Hao XP, Pretlow TG, Rao JS, Pretlow TP. Inducible nitric oxide synthase (iNOS) is expressed similarly in multiple aberrant crypt foci and colorectal tumors from the same patients. Cancer Res. 2001;61:419–22. [PubMed] [Google Scholar]
- 31.Means TK, Lien E, Yoshimura A, Wang S, Golenbock DT, Fenton MJ. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–55. [PubMed] [Google Scholar]
- 32.Beutler B. The Toll-like receptors: analysis by forward genetic methods. Immunogenetics. 2005;57:385–92. doi: 10.1007/s00251-005-0011-3. [DOI] [PubMed] [Google Scholar]
- 33.Blach-Olszewska Z. Innate immunity: cells, receptors, and signaling pathways. Arch Immunol Ther Exp. 2005;53:245–53. [PubMed] [Google Scholar]
- 34.Rhee SH, Hwang D. Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase. J Biol Chem. 2000;275:34035–40. doi: 10.1074/jbc.M007386200. [DOI] [PubMed] [Google Scholar]
- 35.Tapping RI, Akashi S, Miyake K, Godowski PJ, Tobias PS. Toll-like receptor 4, but not toll-like receptor 2, is a signaling receptor for Escherichia and Salmonella lipopolysaccharides. J Immunol. 2000;165:5780–7. doi: 10.4049/jimmunol.165.10.5780. [DOI] [PubMed] [Google Scholar]
- 36.Lien E, Means TK, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton MJ, Oikawa M, Qureshi N, Monks B, Finberg RW, Ingalls RR, Golenbock DT. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Invest. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Q, Akashi S, Miyake K, Petty HR. Lipopolysaccharide induces physical proximity between CD14 and toll-like receptor 4 (TLR4) prior to nuclear translocation of NF-kappa B. J Immunol. 2000;165:3541–4. doi: 10.4049/jimmunol.165.7.3541. [DOI] [PubMed] [Google Scholar]
- 38.Schuster JM, Nelson PS. Toll receptors: an expanding role in our understanding of human disease. J Leukoc Biol. 2000;67:767–73. [PubMed] [Google Scholar]
- 39.Takeuchi O, Akira S. Genetic approaches to the study of Toll-like receptor function. Microbes Infect. 2002;4:887–95. doi: 10.1016/s1286-4579(02)01615-5. [DOI] [PubMed] [Google Scholar]
- 40.Fink AL. Chaperone-mediated protein folding. Physiol Rev. 1999;79:425–49. doi: 10.1152/physrev.1999.79.2.425. [DOI] [PubMed] [Google Scholar]
- 41.Liu J, Kadiiska MB, Liu Y, Lu T, Qu W, Waalkes MP. Stress-related gene expression in mice treated with inorganic arsenicals. Toxicol Sci. 2001;61:314–20. doi: 10.1093/toxsci/61.2.314. [DOI] [PubMed] [Google Scholar]
- 42.Wagner M, Hermanns I, Bittinger F, Kirkpatrick CJ. Induction of stress proteins in human endothelial cells by heavy metal ions and heat shock. Am J Physiol. 1999;277:L1026–L1033. doi: 10.1152/ajplung.1999.277.5.L1026. [DOI] [PubMed] [Google Scholar]
- 43.Chen W, Syldath U, Bellmann K, Burkart V, Kolb H. Human 60-kDa heat-shock protein: a danger signal to the innate immune system. J Immunol. 1999;162:3212–9. [PubMed] [Google Scholar]
- 44.Kol A, Lichtman AH, Finberg RW, Libby P, Kurt-Jones EA. Cutting edge: heat shock protein (HSP) 60 activates the innate immune response: CD14 is an essential receptor for HSP60 activation of mononuclear cells. J Immunol. 2000;164:13–7. doi: 10.4049/jimmunol.164.1.13. [DOI] [PubMed] [Google Scholar]
- 45.Kol A, Bourcier T, Lichtman AH, Libby P. Chlamydial and human heat shock protein 60s activate human vascular endothelium, smooth muscle cells, and macrophages. J Clin Invest. 1999;103:571–7. doi: 10.1172/JCI5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Koivisto L, Heino J, Uitto VJ. Bacterial heat shock protein 60 may increase epithelial cell migration through activation of MAP kinases and inhibition of alpha6beta4 integrin expression. Biochem Biophys Res Comm. 2004;319:1088–95. doi: 10.1016/j.bbrc.2004.04.202. [DOI] [PubMed] [Google Scholar]
- 47.Billack B, Heck DE, Mariano TM, Gardner CR, Sur R, Laskin DL, Laskin JD. Induction of cyclooxygenase-2 by heat shock protein 60 in macrophages and endothelial cells. Am J Physiol Cell Physiol. 2002;283:C1267–77. doi: 10.1152/ajpcell.00609.2001. [DOI] [PubMed] [Google Scholar]
- 48.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–61. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 49.Karin M, Ben Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Ann Rev Immunol. 2000;18:621–63. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 50.Cuzzocrea S, Chatterjee PK, Mazzon E, et al. Pyrrolidine dithiocarbamate attenuates the development of acute and chronic inflammation. Br J Pharmacol. 2002;135:496–510. doi: 10.1038/sj.bjp.0704463. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–63. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]