Abstract
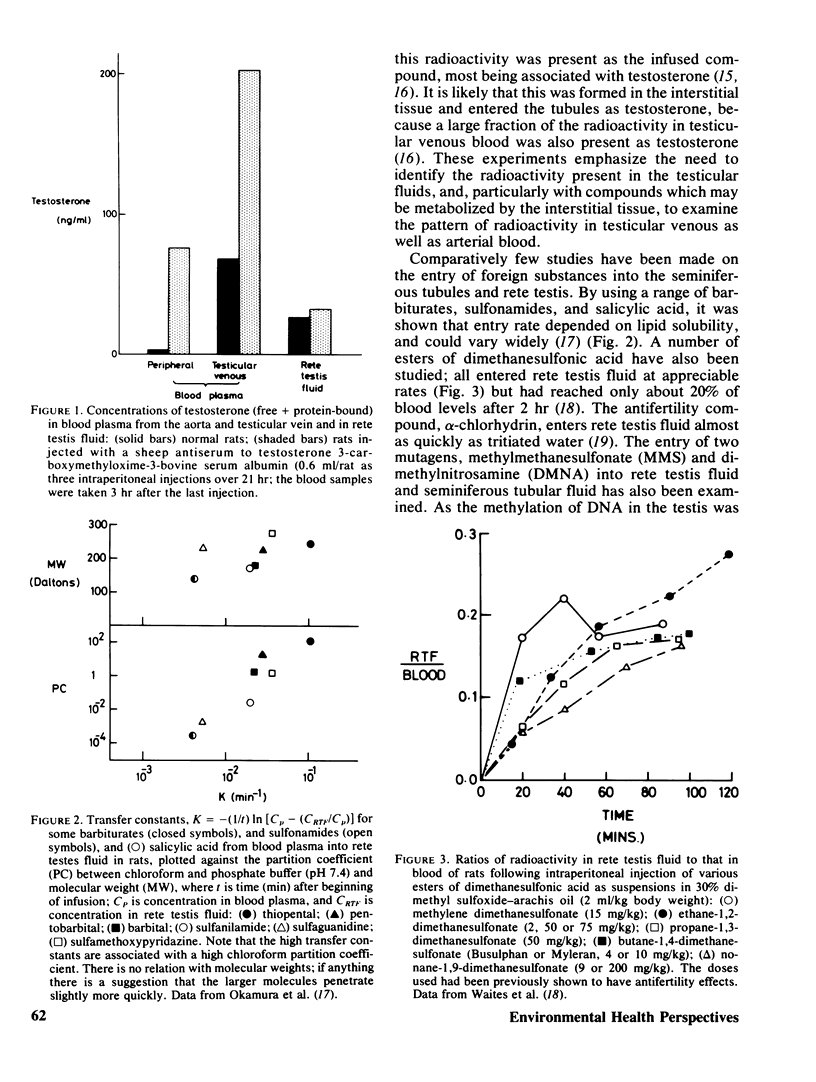
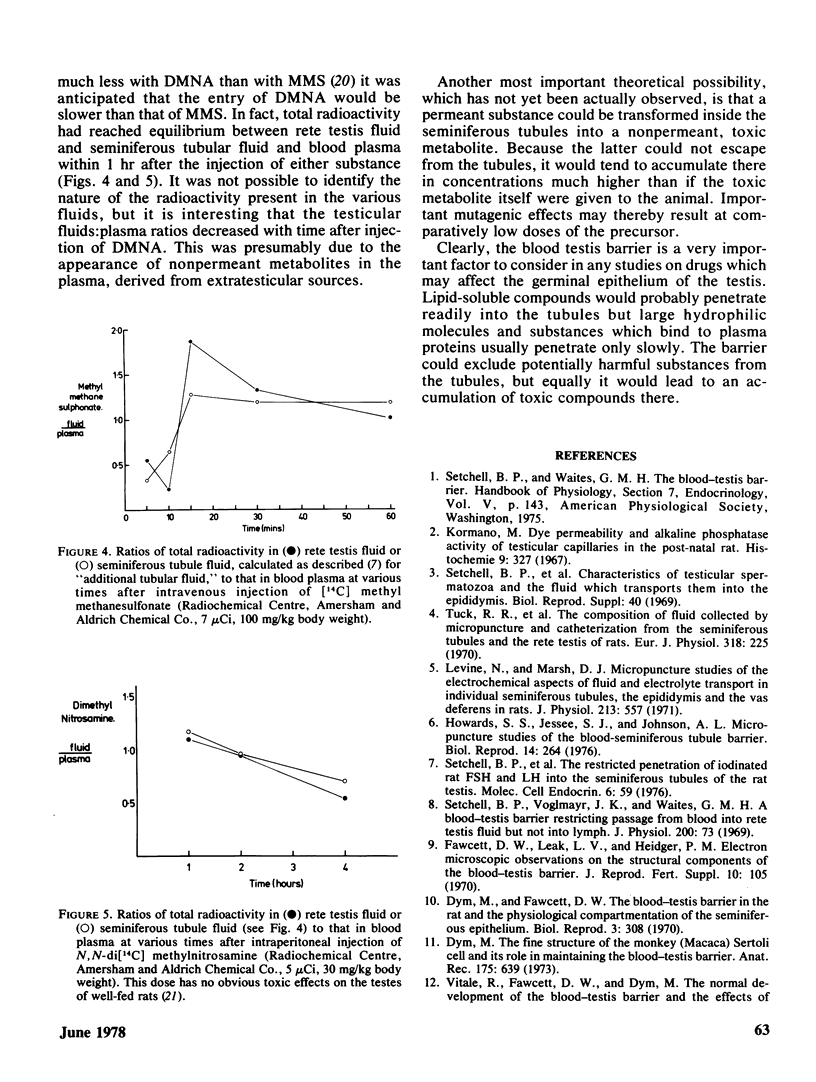
The functional and morphological evidence for the blood–testis barrier is discussed, together with evidence for the various processes (simple diffusion and facilitated diffusion) by which various substances enter the seminiferous tubule. Data are presented to show that methylmethane-sulfonate (MMS) and dimethylnitrosamine (DMNA) both enter the seminiferous tubules rapidly, although from the published rates of methylation of testicular DNA, by these two compounds, it might be expected that the entry of DMNA would be slower than that of MMS. It appears, however, that DMNA in blood is gradually converted to some nonpermeant compound. The possibility, as yet unsubstantiated, is discussed that a nontoxic permeant precursor may be converted into a nonpermeant toxic substance inside the tubules, thereby effectively concentrating the toxic compound inside the tubules.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper T. G., Waites G. M. Steroid entry into rete testis fluid and the blood-testis barrier. J Endocrinol. 1975 May;65(2):195–205. doi: 10.1677/joe.0.0650195. [DOI] [PubMed] [Google Scholar]
- Dym M., Fawcett D. W. The blood-testis barrier in the rat and the physiological compartmentation of the seminiferous epithelium. Biol Reprod. 1970 Dec;3(3):308–326. doi: 10.1093/biolreprod/3.3.308. [DOI] [PubMed] [Google Scholar]
- Dym M. The fine structure of the monkey (Macaca) Sertoli cell and its role in maintaining the blood-testis barrier. Anat Rec. 1973 Apr;175(4):639–656. doi: 10.1002/ar.1091750402. [DOI] [PubMed] [Google Scholar]
- Fawcett D. W., Leak L. V., Heidger P. M., Jr Electron microscopic observations on the structural components of the blood-testis barrier. J Reprod Fertil Suppl. 1970;10:105–122. [PubMed] [Google Scholar]
- Gilula N. B., Fawcett D. W., Aoki A. The Sertoli cell occluding junctions and gap junctions in mature and developing mammalian testis. Dev Biol. 1976 May;50(1):142–168. doi: 10.1016/0012-1606(76)90074-9. [DOI] [PubMed] [Google Scholar]
- Hard G. C., Butler W. H. Toxicity of dimethylnitrosamine for the rat testis. J Pathol. 1970 Dec;102(4):201–207. doi: 10.1002/path.1711020403. [DOI] [PubMed] [Google Scholar]
- Howards S. S., Jessee S. J., Johnson A. L. Micropuncture studies of the blood-seminiferous tubule barrier. Biol Reprod. 1976 Apr;14(3):264–269. doi: 10.1095/biolreprod14.3.264. [DOI] [PubMed] [Google Scholar]
- Kormano M. Dye permeability and alkaline phosphatase activity of testicular capillaries in the postnatal rat. Histochemie. 1967;9(4):327–338. doi: 10.1007/BF00305816. [DOI] [PubMed] [Google Scholar]
- Levine N., Marsh D. J. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis and the vas deferens in rats. J Physiol. 1971 Mar;213(3):557–570. doi: 10.1113/jphysiol.1971.sp009400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setchell B. P., Voglmayr J. K., Waites G. M. A blood-testis barrier restricting passage from blood into rete testis fluid but not into lymph. J Physiol. 1969 Jan;200(1):73–85. doi: 10.1113/jphysiol.1969.sp008682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann P. F., Magee P. N. Nitrosamine-induced carcinogenesis. The alklylation of nucleic acids of the rat by N-methyl-N-nitrosourea, dimethylnitrosamine, dimethyl sulphate and methyl methanesulphonate. Biochem J. 1968 Nov;110(1):39–47. doi: 10.1042/bj1100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites G. M., Jones A. R., Main S. J., Cooper T. G. The entry of antifertility and other compounds into the testis. Adv Biosci. 1973;10:101–116. [PubMed] [Google Scholar]


