Abstract
This review represents a summary of the technique of using cultured heart cells as a model system for studying the physiology, pharmacology, biochemistry and toxicology of myocardial cells. The general techniques and types of culture preparations commonly used are given and some of the advantages and disadvantages of working with cultured heart cells are summarized.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carmeliet E. E., Horres C. R., Lieberman M., Vereecke J. S. Developmental aspects of potassium flux and permeability of the embryonic chick heart. J Physiol. 1976 Jan;254(3):673–692. doi: 10.1113/jphysiol.1976.sp011252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley J. H., Campbell G. R. Mitosis of contractile smooth muscle cells in tissue culture. Exp Cell Res. 1974 Mar 15;84(1):105–110. doi: 10.1016/0014-4827(74)90385-1. [DOI] [PubMed] [Google Scholar]
- Dehaan R. L., Fozzard H. A. Membrane response to current pulses in spheroidal aggregates of embryonic heart cells. J Gen Physiol. 1975 Feb;65(2):207–222. doi: 10.1085/jgp.65.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EARLE W. R., SANFORD K. K., EVANS V. J., WALTZ H. K., SHANNON J. E., Jr The influence of inoculum size on proliferation in tissue cultures. J Natl Cancer Inst. 1951 Aug;12(1):133–153. [PubMed] [Google Scholar]
- ENEMAR A., FALCK B. OBSERVATIONS ON THE APPEARANCE OF NOREPINEPHRINE IN THE SYMPATHETIC NERVOUS SYSTEM OF THE CHICK EMBRYO. Dev Biol. 1965 Apr;11:268–283. doi: 10.1016/0012-1606(65)90060-6. [DOI] [PubMed] [Google Scholar]
- FANGE R., PERSSON H., THESLEFF S. Electrophysiologic and pharmacological observations on trypsin-disintegrated embryonic chick hearts cultured in vitro. Acta Physiol Scand. 1956 Dec 31;38(2):173–183. doi: 10.1111/j.1748-1716.1957.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Freer R. J., Pappano A. J., Peach M. J., Bing K. T., McLean M. J., Vogel S., Sperelakis N. Mechanism for the postive inotropic effect of angiotensin II on isolated cardiac muscle. Circ Res. 1976 Aug;39(2):178–183. doi: 10.1161/01.res.39.2.178. [DOI] [PubMed] [Google Scholar]
- Gordon H. P., Brice M. C. Intrinsic factors influencing the maintenance of contractile embryonic heart cells in vitro. II. Biochemical analysis of heart muscle conditioned medium. Exp Cell Res. 1974 Apr;85(2):311–318. doi: 10.1016/0014-4827(74)90132-3. [DOI] [PubMed] [Google Scholar]
- HARARY I., FUJIMOTO A., KURAMITSU H. ENZYME CHANGES IN CULTURED HEART CELLS. Natl Cancer Inst Monogr. 1964 Apr;13:257–275. [PubMed] [Google Scholar]
- Halbert S. P., Bruderer R., Lin T. M. In vitro organization of dissociated rat cardiac cells into beating three-dimensional structures. J Exp Med. 1971 Apr 1;133(4):677–695. doi: 10.1084/jem.133.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermsmeyer K., de Cino P., White R. Spontaneous contractions of dispersed vascular muscle in cell culture. In Vitro. 1976 Sep;12(9):628–634. doi: 10.1007/BF02797461. [DOI] [PubMed] [Google Scholar]
- Hodges G. M., Livingston D. C., Franks L. M. The localization of trypsin in cultured mammalian cells. J Cell Sci. 1973 May;12(3):887–902. doi: 10.1242/jcs.12.3.887. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. Effect of oxygen on growth of cultured myocardial cells. Circ Res. 1971 Feb;28(2):148–157. doi: 10.1161/01.res.28.2.148. [DOI] [PubMed] [Google Scholar]
- Hyde A., Blondel B., Matter A., Cheneval J. P., Filloux B., Girardier L. Homo- and heterocellular junctions in cell cultures: an electrophysiological and morphological study. Prog Brain Res. 1969;31:283–311. doi: 10.1016/S0079-6123(08)63247-1. [DOI] [PubMed] [Google Scholar]
- Jongsma H. J., van Rijn H. E. Electronic spread of current in monolayer cultures of neonatal rat heart cells. J Membr Biol. 1972;9(4):341–360. [PubMed] [Google Scholar]
- Josephson I., Renaud J. F., Vogel S., Mclean M., Sperelakis N. Mechanism of the histamine-induced positive inotropic action in cardiac muscle. Eur J Pharmacol. 1976 Feb;35(2):393–398. doi: 10.1016/0014-2999(76)90243-0. [DOI] [PubMed] [Google Scholar]
- Josephson I., Sperelakis N. Ouabain blockade of inward slow current in cardiac muscle. J Mol Cell Cardiol. 1977 May;9(5):409–418. doi: 10.1016/s0022-2828(77)80007-2. [DOI] [PubMed] [Google Scholar]
- Karsten U., Kössler A., Janiszewski E., Wollenberger A. Influence of variations in pericellular oxygen tension on individual cell growth, muscle-characteristic proteins, and lactate dehydrogenase isoenzyme heart cells. In Vitro. 1973 Nov-Dec;9(3):139–146. doi: 10.1007/BF02618430. [DOI] [PubMed] [Google Scholar]
- Kasten F. H. Rat myocardial cells in vitro: mitosis and differentiated properties. In Vitro. 1972 Nov-Dec;8(3):128–150. doi: 10.1007/BF02619489. [DOI] [PubMed] [Google Scholar]
- Kelly A. M., Chacko S. Myofibril organisation and mitosis in cultured cardiac muscle cells. Dev Biol. 1976 Feb;48(2):421–430. doi: 10.1016/0012-1606(76)90103-2. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Brandt B. L. Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res. 1976 Mar 15;98(2):349–366. doi: 10.1016/0014-4827(76)90446-8. [DOI] [PubMed] [Google Scholar]
- Langer G. A., Sato E., Seraydarian M. Calcium exchange in a single layer of rat cardiac cells studied by direct counting of cellular activity of labeled calcium. Circ Res. 1969 May;24(5):589–597. doi: 10.1161/01.res.24.5.589. [DOI] [PubMed] [Google Scholar]
- Le Douarin G., Obrecht G., Coraboeuf E. Déterminations régionales dans l'aire cardiaque présomptive mises en évidence chez l'embryon de poulet par la méthode microélectrophysiologique. J Embryol Exp Morphol. 1966 Apr;15(2):153–167. [PubMed] [Google Scholar]
- Lehmkuhl D., Sperelakis N. Electrotonic spread of current in cultured chick heart cells. J Cell Physiol. 1965 Aug;66(1):119–133. doi: 10.1002/jcp.1030660113. [DOI] [PubMed] [Google Scholar]
- Lieberman M. Electrophysiological studies of a synthetic strand of cardiac muscle. Physiologist. 1973 Nov;16(4):551–563. [PubMed] [Google Scholar]
- Lieberman M., Roggeveen A. E., Purdy J. E., Johnson E. A. Synthetic strands of cardiac muscle: growth and physiological implication. Science. 1972 Feb 25;175(4024):909–911. doi: 10.1126/science.175.4024.909. [DOI] [PubMed] [Google Scholar]
- METTLER F. A., GRUNDFEST H., CRAIN S. M., MURRAY M. R. Spontaneous electrical activity from tissue cultures. Trans Am Neurol Assoc. 1952;56(77TH):52–53. [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
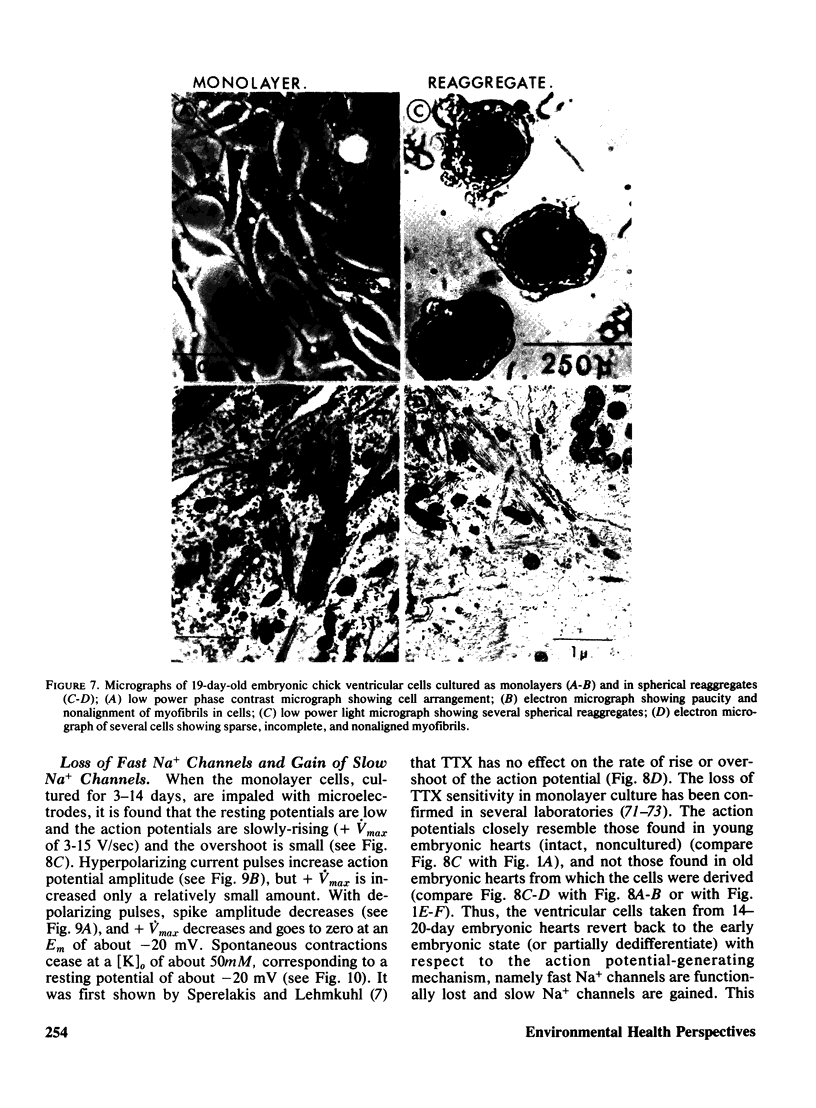
- Manasek F. J., Monroe R. G. Early cardiac morphogenesis is independent of function. Dev Biol. 1972 Apr;27(4):584–588. doi: 10.1016/0012-1606(72)90196-0. [DOI] [PubMed] [Google Scholar]
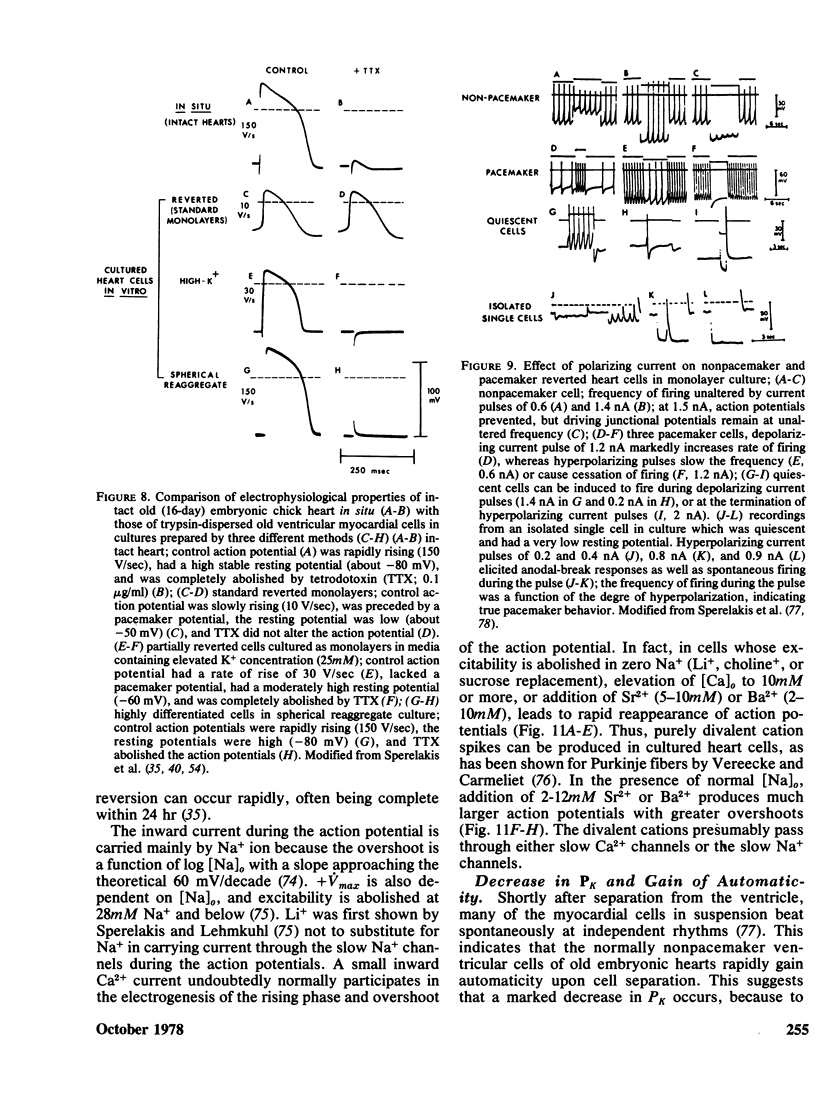
- Mark G. E., Strasser F. F. Pacemaker activity and mitosis in cultures of newborn rat heart ventricle cells. Exp Cell Res. 1966 Nov-Dec;44(2):217–233. doi: 10.1016/0014-4827(66)90427-7. [DOI] [PubMed] [Google Scholar]
- McDonald T. F., Sachs H. G., DeHaan R. L. Development of sensitivity to tetrodotoxin in beating chick embryo hearts, single cells, and aggregates. Science. 1972 Jun 16;176(4040):1248–1250. doi: 10.1126/science.176.4040.1248. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Lapsley R. A., Shigenobu K., Murad F., Sperelakis N. High cyclic AMP levels in young chick embryonic hearts. Dev Biol. 1975 Jan;42(1):196–201. doi: 10.1016/0012-1606(75)90324-3. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Renaud J., Sperelakis N., Niu M. C. Messenger RNA induction of fast sodium ion channels in cultured cardiac myoblasts. Science. 1976 Jan 23;191(4224):297–299. doi: 10.1126/science.1246615. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Sperelakis N. Electrophysiological recordings from spontaneously contracting reaggregates of cultured vascular smooth muscle cells from chick enbryos. Exp Cell Res. 1977 Feb;104(2):309–318. doi: 10.1016/0014-4827(77)90096-9. [DOI] [PubMed] [Google Scholar]
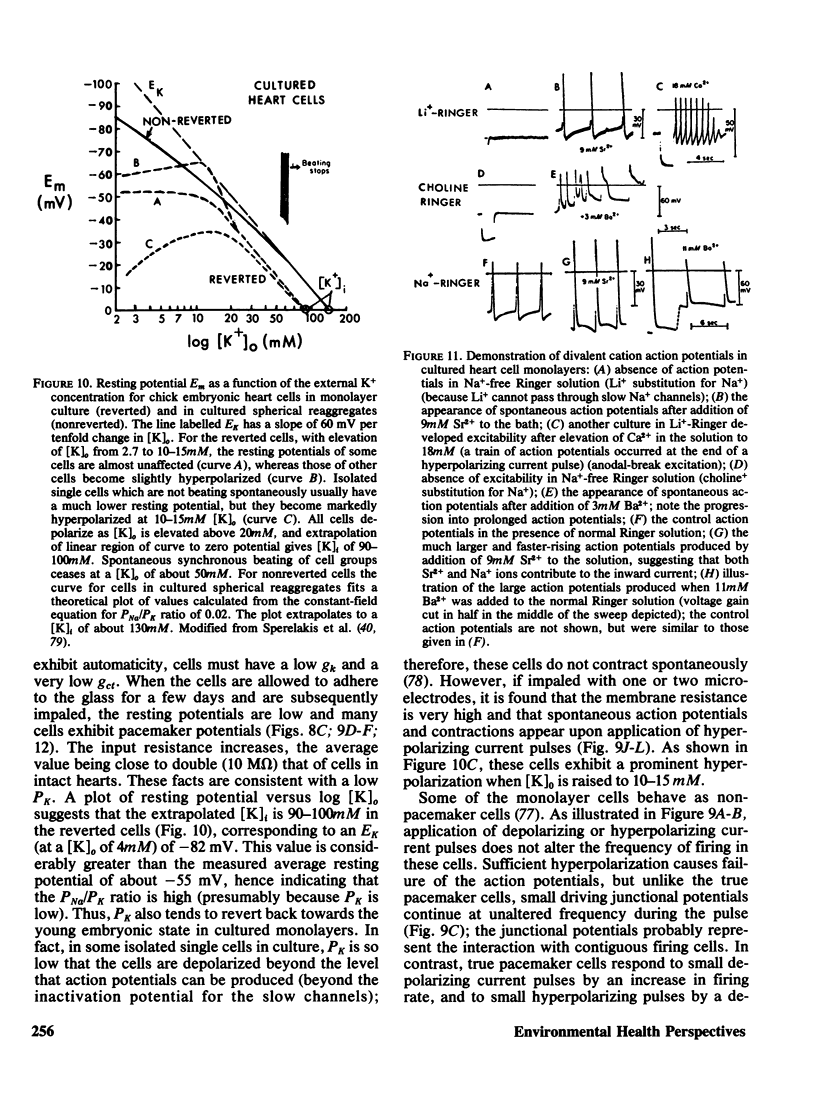
- McLean M. J., Sperelakis N. Rapid loss of sensitivity to tetrodotoxin by chick ventricular myocardial cells after separation from the heart. Exp Cell Res. 1974 Jun;86(2):351–364. doi: 10.1016/0014-4827(74)90723-x. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Sperelakis N. Retention of fully differentiated electrophysiological properties of chick embryonic heart cells in culture. Dev Biol. 1976 May;50(1):134–141. doi: 10.1016/0012-1606(76)90073-7. [DOI] [PubMed] [Google Scholar]
- PATTEN B. M. The development of the sinoventricular conduction system. Med Bull (Ann Arbor) 1956 Jan;22(1):1–21. [PubMed] [Google Scholar]
- Pappano A. J., Sperelakis N. Low K+ conductance and low resting potentials of isolated single cultured heart cells. Am J Physiol. 1969 Oct;217(4):1076–1082. doi: 10.1152/ajplegacy.1969.217.4.1076. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Sperelakis N. Spike electrogenesis in cultured heart cells. Am J Physiol. 1969 Aug;217(2):615–624. doi: 10.1152/ajplegacy.1969.217.2.615. [DOI] [PubMed] [Google Scholar]
- Pappano A. J., Sperelakis N. Spontaneous contractions of cultured heart cells in high K+ media. Exp Cell Res. 1969 Jan;54(1):58–68. doi: 10.1016/0014-4827(69)90293-6. [DOI] [PubMed] [Google Scholar]
- Phillips H. J. Some metabolic changes resulting from treating kidney tissue with trypsin. Can J Biochem. 1967 Oct;45(10):1495–1504. doi: 10.1139/o67-180. [DOI] [PubMed] [Google Scholar]
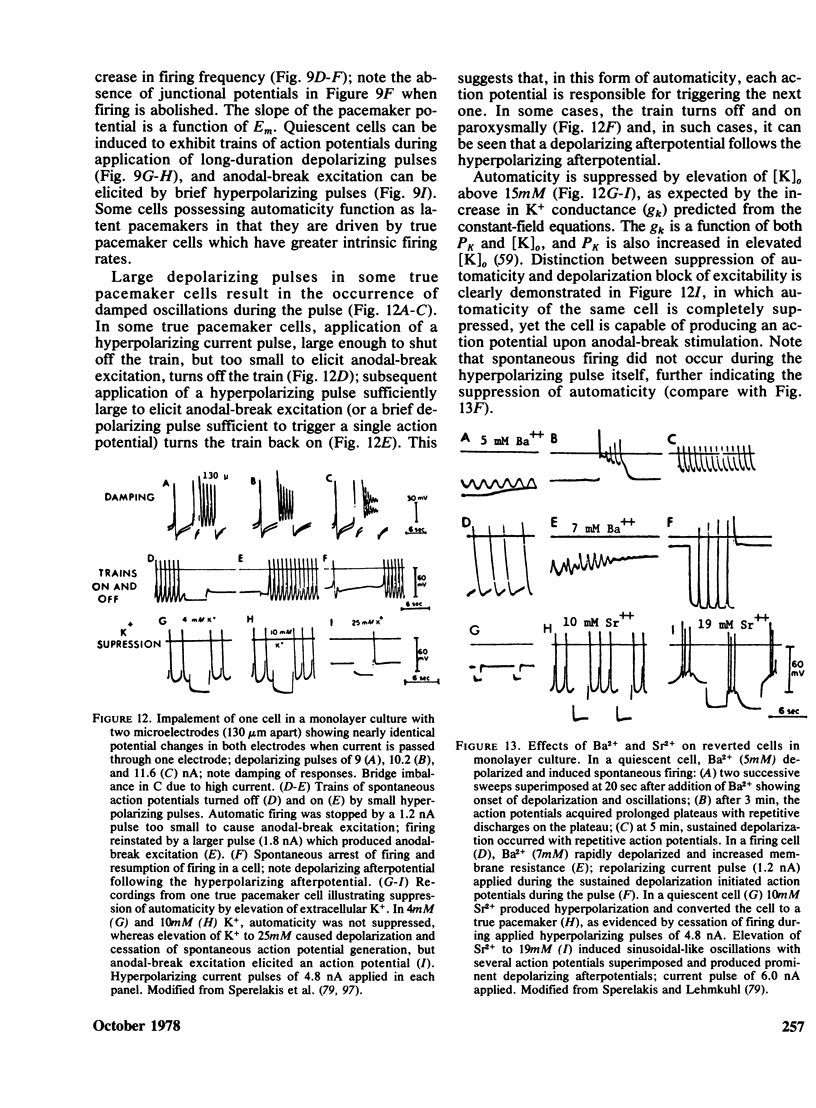
- Renaud J. F., Sperelakis N. Electrophysiological properties of chick embryonic hearts grafted and organ cultured in vitro. J Mol Cell Cardiol. 1976 Nov;8(11):889–900. doi: 10.1016/0022-2828(76)90071-7. [DOI] [PubMed] [Google Scholar]
- Renaud J. F., Sperelakis N., Le Douarin G. Increase of cyclic AMP levels induced by isoproterenol in cultured and non-cultured chick embryonic hearts. J Mol Cell Cardiol. 1978 Mar;10(3):281–286. doi: 10.1016/0022-2828(78)90350-4. [DOI] [PubMed] [Google Scholar]
- Roseen J. S., Fuhrman F. A. Comparison of the effects of atelopidtoxin with those of tetrodotoxin, saxitoxin and batrachotoxin on beating of cultured chick heart cells. Toxicon. 1971 Oct;9(4):411–415. doi: 10.1016/0041-0101(71)90140-1. [DOI] [PubMed] [Google Scholar]
- Rosenthal M. D., Warshaw J. B. Interaction of fatty acid and glucose oxidation by cultured heart cells. J Cell Biol. 1973 Aug;58(2):332–339. doi: 10.1083/jcb.58.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPERELAKIS N., LEHMKUHL D. EFFECT OF CURRENT ON TRANSMEMBRANE POTENTIALS IN CULTURED CHICK HEART CELLS. J Gen Physiol. 1964 May;47:895–927. doi: 10.1085/jgp.47.5.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs H. G., McDonald T. F., DeHaan R. L. Tetrodotoxin sensitivity of cultured embryonic heart cells depends on cell interactions. J Cell Biol. 1973 Jan;56(1):255–258. [PMC free article] [PubMed] [Google Scholar]
- Schneider J. A., Sperelakis N. Slow Ca2+ and Na+ responses induced by isoproterenol and methylxanthines in isolated perfused guinea pig hearts exposed to elevated K+. J Mol Cell Cardiol. 1975 Apr;7(4):249–273. doi: 10.1016/0022-2828(75)90084-x. [DOI] [PubMed] [Google Scholar]
- Schneider J. A., Sperelakis N. The demonstration of energy dependence of the isoproterenol-induced transcellular Ca2+ current in isolated perfused guinea pig hearts--an explanation for mechanical failure of ischemic myocardium. J Surg Res. 1974 Apr;16(4):389–403. doi: 10.1016/0022-4804(74)90060-2. [DOI] [PubMed] [Google Scholar]
- Seraydarian M. W., Sato E., Harary I. The effect of inhibitors on the calcium exchange of heart cells in tissue culture. J Mol Cell Cardiol. 1970 Dec;1(4):436–444. doi: 10.1016/0022-2828(70)90040-4. [DOI] [PubMed] [Google Scholar]
- Shigenobu K., Schneider J. A., Sperelakis N. Verapamil blockade of slow Na+ and Ca++ responses in myocardial cells. J Pharmacol Exp Ther. 1974 Aug;190(2):280–288. [PubMed] [Google Scholar]
- Shigenobu K., Sperelakis N. Calcium current channels induced by catecholamines in chick embryonic hearts whose fast sodium channels are blocked by tetrodotoxin or elevated potassium. Circ Res. 1972 Dec;31(6):932–952. doi: 10.1161/01.res.31.6.932. [DOI] [PubMed] [Google Scholar]
- Shigenobu K., Sperelakis N. Development of sensitivity to tetrodotoxin of chick embryonic hearts with age. J Mol Cell Cardiol. 1971 Dec;3(3):271–286. doi: 10.1016/0022-2828(71)90046-0. [DOI] [PubMed] [Google Scholar]
- Shigenobu K., Sperelakis N. Failure of development of fast Na+ channels during organ culture of young embryonic chick hearts. Dev Biol. 1974 Aug;39(2):326–330. doi: 10.1016/0012-1606(74)90245-0. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Moscona A. A., Fischman D. A. Scanning electron microscopy of cell aggregation: cardiac and mixed retina-cardiac cell suspensions. Dev Biol. 1974 Feb;36(2):428–446. doi: 10.1016/0012-1606(74)90063-3. [DOI] [PubMed] [Google Scholar]
- Sperelakis N. (Na + , K + )-ATPase activity of embryonic chick heart and skeletal muscles as a function of age. Biochim Biophys Acta. 1972 Apr 14;266(1):230–237. doi: 10.1016/0005-2736(72)90137-x. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Forbes M. S., Shigenobu K., Coburn S. Organ-cultured chick embryonic hearts of various ages. II. Ultrastructure. J Mol Cell Cardiol. 1974 Oct;6(5):473–483. doi: 10.1016/0022-2828(74)90028-5. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Lee E. C. Characterization of (Na + ,K + )-ATPase isolated from embryonic chick hearts and cultured chick heart cells. Biochim Biophys Acta. 1971 Jun 1;233(3):562–579. doi: 10.1016/0005-2736(71)90155-6. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Lehmkuhl D. Ba 2+ AND Sr 2+ reversal of the inhibition produced by ouabain and local anesthetics on membrane potentials of cultured heart cells. Exp Cell Res. 1968 Feb;49(2):396–410. doi: 10.1016/0014-4827(68)90189-4. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Lehmkuhl D. Insensitivity of cultured chick heart cells to autonomic agents and tetrodotoxin. Am J Physiol. 1965 Oct;209(4):693–698. doi: 10.1152/ajplegacy.1965.209.4.693. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Lehmkuhl D. Ionic interconversion of pacemaker and nonpacemaker cultured chick heart cells. J Gen Physiol. 1966 May;49(5):867–895. doi: 10.1085/jgp.49.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., McLean M. J. Electrical properties of cultured heart cells. Recent Adv Stud Cardiac Struct Metab. 1976 May 26;12:645–666. [PubMed] [Google Scholar]
- Sperelakis N., Schneider J. A. A metabolic control mechanism for calcium ion influx that may protect the ventricular myocardial cell. Am J Cardiol. 1976 Jun;37(7):1079–1085. doi: 10.1016/0002-9149(76)90428-8. [DOI] [PubMed] [Google Scholar]
- Sperelakis N., Schneider M. F., Harris E. J. Decreased K+ conductance produced by Ba++ in frog sartorius fibers. J Gen Physiol. 1967 Jul;50(6):1565–1583. doi: 10.1085/jgp.50.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Shigenobu K. Changes in membrane properties of chick embryonic hearts during development. J Gen Physiol. 1972 Oct;60(4):430–453. doi: 10.1085/jgp.60.4.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Shigenobu K. Organ-cultured chick embryonic hearts of various ages. I. Electrophysiology. J Mol Cell Cardiol. 1974 Oct;6(5):449–471. doi: 10.1016/0022-2828(74)90027-3. [DOI] [PubMed] [Google Scholar]
- Steinberg M. S., Armstrong P. B., Granger R. E. On the recovery of adhesiveness by trypsin-dissociated cells. J Membr Biol. 1973;13(2):97–128. doi: 10.1007/BF01868223. [DOI] [PubMed] [Google Scholar]
- TROWELL O. A. The culture of mature organs in a synthetic medium. Exp Cell Res. 1959 Jan;16(1):118–147. doi: 10.1016/0014-4827(59)90201-0. [DOI] [PubMed] [Google Scholar]
- Vogel S., Sperelakis N., Josephson I., Brooker G. Fluoride stimulation of slow Ca2+ current in cardiac muscle. J Mol Cell Cardiol. 1977 Jun;9(6):461–475. doi: 10.1016/s0022-2828(77)80026-6. [DOI] [PubMed] [Google Scholar]
- Warshaw J. B., Rosenthal M. D. Changes in glucose oxidation during growth of embryonic heart cells in culture. J Cell Biol. 1972 Feb;52(2):283–291. doi: 10.1083/jcb.52.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildenthal K. Long-term maintenance of spontaneously beating mouse hearts in organ culture. J Appl Physiol. 1971 Jan;30(1):153–157. doi: 10.1152/jappl.1971.30.1.153. [DOI] [PubMed] [Google Scholar]




