Abstract
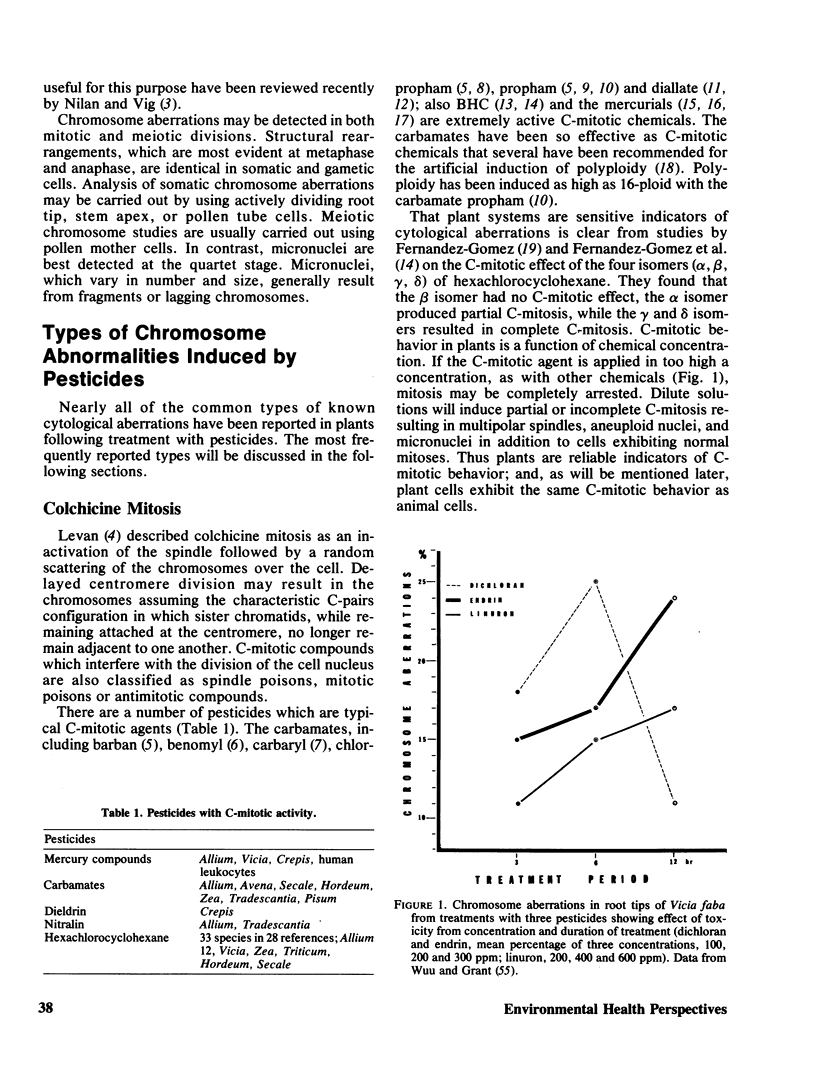
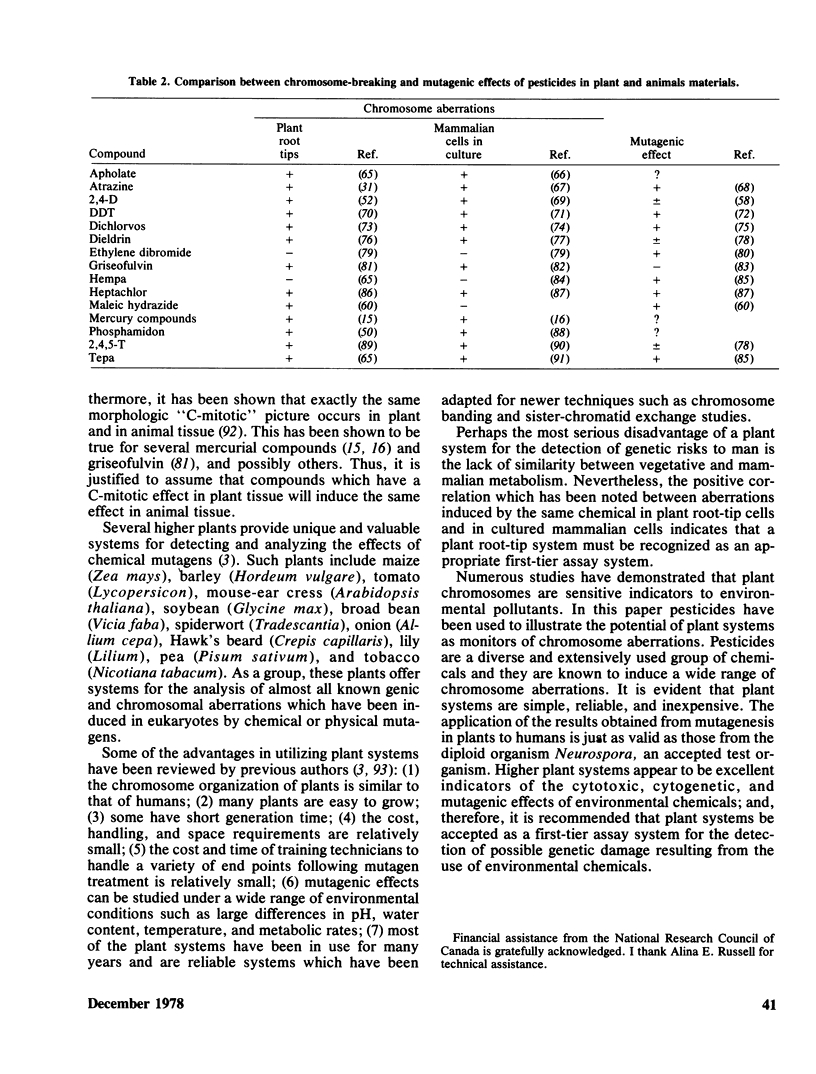
The potential of higher plants as a first-tier assay system for detecting chemical mutagens is evaluated. The use of plant tissue (primarily root tips and pollen mother cells) for studying the induction of chromosomal aberrations is one of the oldest, simplest, most reliable, and inexpensive methods available. Specific types of abnormalities have been induced by different classes of pesticides. Chromosome clumping, contraction, stickiness, paling, fragmentation, dissolution, chromosome and chromatid bridges, C-mitosis, and endoploidy have been reported in the literature. Examples of cytogenetic studies with pesticides demonstrating the usefulness of higher plants as a monitoring system are reviewed. Pesticides which cause chromosome aberrations in plant cells also produce chromosome aberrations in cultured animal cells. Frequently, the aberrations are identical. For example, studies have shown that compounds which have a C-mitotic effect on plant cells have the same effect on animal cells. It is recommended that plant systems be accepted as a first-tier assay system for the detection of possible genetic damage by environmental chemicals.
Full text
PDF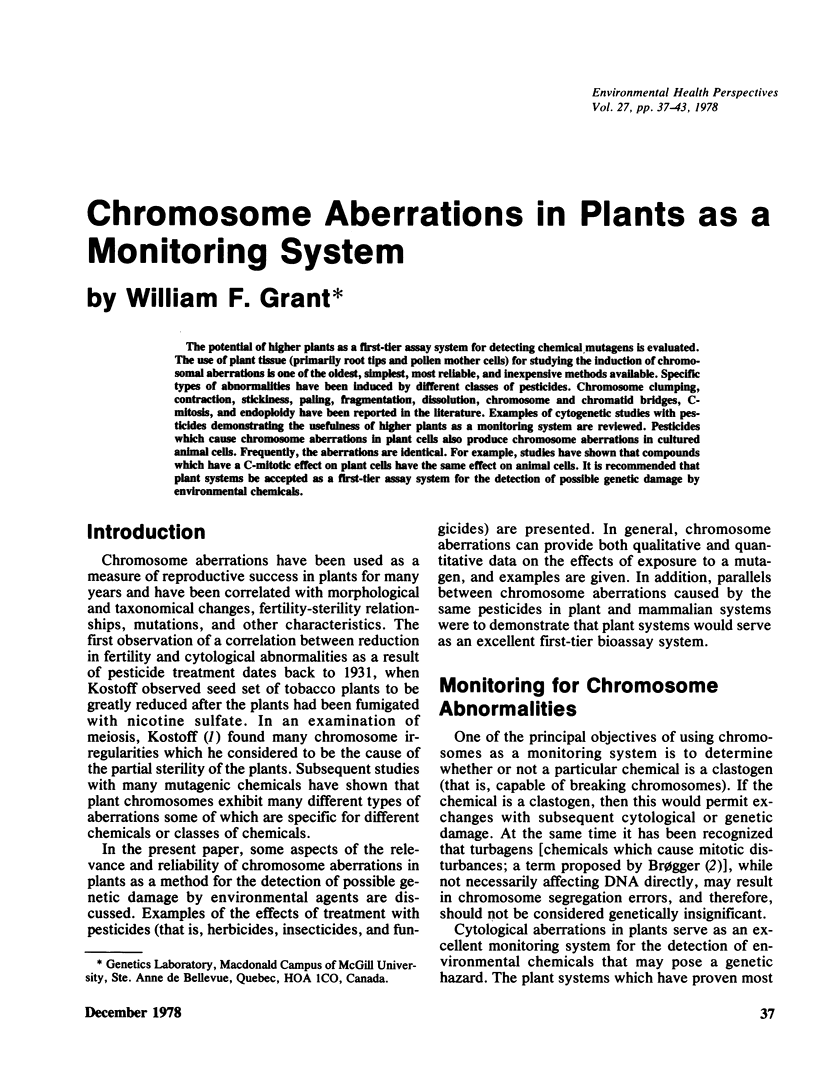




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridges B. A., Mottershead R. P., Green M. H., Gray W. J. Mutagenicity of dichlorvos and methyl methanesulphonate for Escherichia coli WP2 and some derivatives deficient in DNA repair. Mutat Res. 1973 Sep;19(3):295–303. doi: 10.1016/0027-5107(73)90229-7. [DOI] [PubMed] [Google Scholar]
- Chang T. H., Klassen W. Comparative effects of tretamine, tepa, apholate and their structural analogs on human chromsomes in vitro. Chromosoma. 1968;24(3):314–323. doi: 10.1007/BF00336199. [DOI] [PubMed] [Google Scholar]
- Epstein S. S., Shafner H. Chemical mutagens in the human environment. Nature. 1968 Jul 27;219(5152):385–387. doi: 10.1038/219385a0. [DOI] [PubMed] [Google Scholar]
- Fiskesjö G. Some results from allium tests with organic mercury halogenides. Hereditas. 1969;62(3):314–322. doi: 10.1111/j.1601-5223.1969.tb02241.x. [DOI] [PubMed] [Google Scholar]
- Fiskesjö G. The effect of two organic mercury compounds on human leukocytes in vitro. Hereditas. 1970;64(1):142–146. doi: 10.1111/j.1601-5223.1970.tb02283.x. [DOI] [PubMed] [Google Scholar]
- Georgian L. The comparative cytogenetic effects of aldrin and phosphamidon. Mutat Res. 1975 Apr;31(2):103–108. doi: 10.1016/0165-1161(75)90072-2. [DOI] [PubMed] [Google Scholar]
- Georgian L. The comparative cytogenetic effects of aldrin and phosphamidon. Mutat Res. 1975 Apr;31(2):103–108. doi: 10.1016/0165-1161(75)90072-2. [DOI] [PubMed] [Google Scholar]
- HALL T. C. Chemotherapy of cancer. N Engl J Med. 1962 Jan 18;266:129–contd. doi: 10.1056/NEJM196201182660306. [DOI] [PubMed] [Google Scholar]
- Hanna P. J., Dyer K. F. Mutagenicity of organophosphorus compounds in bacteria and Drosophila. Mutat Res. 1975 Jun;28(3):405–420. doi: 10.1016/0027-5107(75)90235-3. [DOI] [PubMed] [Google Scholar]
- Klassen W., Chang T. H., Eide P. E. Effects of apholate on chromosomes of germ cells in the grasshopper testes. Can J Genet Cytol. 1969 Dec;11(4):829–833. doi: 10.1139/g69-098. [DOI] [PubMed] [Google Scholar]
- Klásterská I., Natarajan A. T., Ramel C. An interpretation of the origin of subchromatid aberrations and chromosome stickiness as a category of chromatid aberrations. Hereditas. 1976;83(2):153–162. doi: 10.1111/j.1601-5223.1976.tb01581.x. [DOI] [PubMed] [Google Scholar]
- Majumdar S. K., Hall R. C. Cytogenetic effects of 2,4,5-T on in vivo bone marrow cells of Mongolian gerbils. J Hered. 1973 Jul-Aug;64(4):213–216. doi: 10.1093/oxfordjournals.jhered.a108391. [DOI] [PubMed] [Google Scholar]
- McGill M., Pathak S., Hsu T. C. Effects of ethidium bromide on mitosis and chromosomes: a possible material basis for chromosome stickiness. Chromosoma. 1974;47(2):157–166. doi: 10.1007/BF00331803. [DOI] [PubMed] [Google Scholar]
- Ninan T., Wilson G. B. Chromosome breakage by ethylenimines and related compounds. Genetica. 1969;40(1):103–119. doi: 10.1007/BF01787344. [DOI] [PubMed] [Google Scholar]
- PAGET G. E., WALPOLE A. L. Some cytological effects of griseofulvin. Nature. 1958 Nov 8;182(4645):1320–1321. doi: 10.1038/1821320a0. [DOI] [PubMed] [Google Scholar]
- Palmquist J., LaChance L. E. Comparative mutagenicity of two chemosterilants, tepa and hempa, in sperm of Bracon hebetor. Science. 1966 Nov 18;154(3751):915–917. doi: 10.1126/science.154.3751.915. [DOI] [PubMed] [Google Scholar]
- Perry P., Evans H. J. Cytological detection of mutagen-carcinogen exposure by sister chromatid exchange. Nature. 1975 Nov 13;258(5531):121–125. doi: 10.1038/258121a0. [DOI] [PubMed] [Google Scholar]
- Plewa M. J., Gentile J. M. Mutagenicity of atrazine: a maize-microbe bioassay. Mutat Res. 1976 Aug;38(4):287–292. doi: 10.1016/0165-1161(76)90152-7. [DOI] [PubMed] [Google Scholar]
- Sawamura S. Cytological studies on the effect of herbicides on plant cells in vivo. I. Hormonic herbicides. Cytologia (Tokyo) 1964 Mar;29(1):86–102. doi: 10.1508/cytologia.29.86. [DOI] [PubMed] [Google Scholar]
- Sharma A. K., Gosh S. A comparative study of the effects of certain chemical agents on chromosomes. Acta Biol Acad Sci Hung. 1969;20(1):11–21. [PubMed] [Google Scholar]
- Yoder J., Watson M., Benson W. W. Lymphocyte chromosome analysis of agricultural workers during extensive occupational exposure to pesticides. Mutat Res. 1973 Dec;21(6):335–340. [PubMed] [Google Scholar]



