Abstract
The central role of the hypothalamus in the origination and/or processing of feeding-related stimuli may be modulated by the activity of other functional areas of the brain including the insular cortex (involved in enteroceptive monitoring) and the prefrontal cortex (involved in the inhibition of inappropriate response tendencies). Regional cerebral blood flow (rCBF), a marker of neuronal activity, was measured in 11 healthy, normal-weight men by using positron emission tomography in a state of hunger (after 36-h fast) and a state of satiation (after a liquid meal). Hunger was associated with significantly increased rCBF in the vicinity of the hypothalamus and insular cortex and in additional paralimbic and limbic areas (orbitofrontal cortex, anterior cingulate cortex, and parahippocampal and hippocampal formation), thalamus, caudate, precuneus, putamen, and cerebellum. Satiation was associated with increased rCBF in the vicinity of the ventromedial prefrontal cortex, dorsolateral prefrontal cortex, and inferior parietal lobule. Changes in plasma insulin concentrations in response to the meal were negatively correlated with changes in rCBF in the insular and orbitofrontal cortex. Changes in plasma free fatty acid concentrations in response to the meal were negatively correlated with changes in rCBF in the anterior cingulate and positively correlated with changes in rCBF in the dorsolateral prefrontal cortex. In conclusion, these findings raise the possibility that several regions of the brain participate in the regulation of hunger and satiation and that insulin and free fatty acids may be metabolic modulators of postprandial brain neuronal events. Although exploratory, the present study provides a foundation for investigating the human brain regions and cognitive operations that respond to nutritional stimuli.
The brain plays a critical role in maintaining whole-body energy balance by regulating energy intake and energy expenditure (1). In laboratory animals, the hypothalamus is a major center for the regulation of food intake (2, 3). The original dual center hypothesis, which identified the ventromedial hypothalamus as the satiety center and the lateral hypothalamus as the hunger center (4, 5), has been revised in recent years to account for the autonomic and endocrine responses elicited by hypothalamic lesions (6, 7). The arcuate nucleus, which has neuropeptide Y-producing neurons that project to the ventromedial hypothalamus and the nucleus of the solitary tract, and the paraventricular nucleus, which has corticotropin releasing hormone-producing neurons that project to the arcuate nucleus and locus ceruleus, are other important centers that regulate food intake (8). The production of neurohormones that regulate feeding may be modulated by insulin (9), leptin (10–12), and other metabolic/hormonal signals, allowing the brain to monitor nutritional status and peripheral energy stores. Other areas of the brain known to play a role in the regulation of food intake in animals include the thalamus, a relay center for taste perception and learning, and the limbic areas, related to behavioral regulation and arousal during a meal (13).
Very little is known about the regions of the human brain involved in the control of food intake, and most information has been inferred from pathological conditions (14). Stimulation of gustatory and olfactory receptors is associated with activation of the insular cortex, the orbitofrontal cortex (taste), and the piriform cortex (olfaction) (15–18). Because the anterior and middle aspect of the insular cortex appears to participate in the evaluation of potentially distressing internal stimuli (19, 20), we postulated that this region may be preferentially involved in the response to hunger or satiation. Because regions of the dorsolateral and medial prefrontal cortex have been shown to participate in the inhibition of inappropriate behavioral response tendencies (19, 21, 22), we postulated that these regions of the brain may participate in the inhibition of excessive food consumption. In this study, positron emission tomography (PET) measurements of regional cerebral blood flow (rCBF), a marker of neuronal activity, were used to investigate the regions of the human brain that are preferentially affected during hunger and satiation. We tested the hypotheses that the hypothalamus, insular cortex, and prefrontal cortex are preferentially involved, and we explored the possibility that additional brain regions play a role in these appetitive states.
MATERIALS AND METHODS
Subjects.
Eleven right-handed male Caucasian volunteers (35 ± 8 years, 73 ± 9 kg, 19 ± 6% body fat; mean ± SD) were recruited from the greater Phoenix area by newspaper advertisement. Subjects were in good health as determined by medical history, physical examination, and laboratory screening tests. Exclusion criteria included family history of obesity/diabetes, history of substance abuse or addiction, endocrine disorders including abnormal thyroid function and non-insulin dependent diabetes mellitus, pulmonary disorders, cardiovascular disease, hypertension, gastrointestinal disease and swallowing disorders, hepatic or renal disease, and central nervous system disorders. Behavioral or psychiatric conditions incompatible with safe and successful participation in the study (claustrophobia, major depression, presence of psychotic symptoms, bulimia nervosa) were screened for with the Structured Clinical Interview for DSM-III-R (23). Absence of dietary restraint was assessed by using a three-factor eating questionnaire (24). Subjects took no medications for at least 1 month before admission. All subjects were admitted for approximately 1 week to the Clinical Diabetes and Nutrition Section of the National Institutes of Health in Phoenix. Subjects were restricted to the metabolic ward and were limited to sedentary activity for the duration of the study. The protocol was approved by the Institutional Review Boards of the National Institute of Diabetes and Digestive and Kidney Diseases and the Good Samaritan Regional Medical Center, and informed, written consent was obtained from all subjects before participation.
Fasting Condition.
On admission, all subjects were placed on a weight-maintaining diet (50% carbohydrate, 30% fat, 20% protein). Body composition was assessed by dual energy x-ray absorptiometry (DPX-l, Lunar, Madison, WI). On the second morning, after a 12-h overnight fast, resting energy expenditure was measured for 45 min by using a ventilated hood system (DeltaTrac, SensorMedics, Yorba Linda, CA). Before brain imaging procedures, subjects fasted for 36 h. Water and noncaloric, noncaffeinated beverages were provided ad libitum during the fast.
Imaging Procedures.
PET and MRI procedures were conducted at Good Samaritan Regional Medical Center (Phoenix, AZ). MRI was performed by using a 1.5 T Signa system (General Electric). A T1-weighted, three-dimensional, volume spoiled gradient acquisition (SPGR, TE = 5 msec, TR = 33 msec, angle = 30°, NEX = 1, FOV = 24 cm, imaging matrix = 256 × 192) was used to acquire 124 contiguous, 1.5-mm thick, horizontal slices of the brain to rule out gross anatomical abnormalities and facilitate comparisons between brain structure and function. For the PET procedure, a transmission scan using a 68Germanium/68Gallium ring source was performed to correct subsequent emission images for radiation attenuation. During each scan, subjects rested quietly in the supine position without movement and were asked to keep their eyes closed and pointing forward. PET images of regional brain activity (counts per pixel per min) were obtained from each subject by using an ECAT 951/31 scanner (Siemens, Iselin, NJ). For each scan, a 50-mCi (1 Ci = 37 GBq) intravenous bolus of 15O-labeled water was injected. Two 1-minute scans were obtained at baseline and two after feeding, with intervals of 10–15 min between scans. PET images were reconstructed with an in-place resolution of 10 mm full width at half-maximum (FWHM) and a slice thickness of 5 mm FWHM. Blood samples were collected immediately after each scan for measurement of plasma glucose, free fatty acids, insulin, and leptin concentrations.
Feeding Procedure.
A liquid formula meal [Ensure-Plus 1.5 kcal/ml (1 Ca = 4.18 J), Ross-Abbott Laboratories, Columbus, OH] was administered orally to induce satiation. With the patient supine on the PET table, a plastic extension tube was inserted into the mouth to the middle of the tongue. To eliminate possible confounding factors such as tactile stimulation of the tongue and motor neuron activity, swallowing was consistently induced by administering 2 ml of water before each of the four PET scans. Between the PET scans in the baseline and satiated states, a peristaltic pump (IMED 980, Imed, San Diego) was set to deliver, over a period of 25 min, a liquid meal providing 50% of the previously measured daily resting energy expenditure. Subjective ratings of hunger and satiation were recorded after each PET scan (25). To familiarize each subject with the experimental setting and minimize the risk of learning-related artifacts, the feeding procedure was practiced on the research ward before PET scanning.
Image Processing and Statistical Analysis.
Automated algorithms were used to align each subject’s sequential PET images (26), transform PET images into spatial coordinates of a standard brain atlas (27), investigate increases in rCBF independent of variations in whole-brain measurements using analysis of covariance (28), and generate normalized t-value (i.e., z-score) maps of increases in rCBF during hunger (average of the two images in the hunger condition minus average of the two images in the satiation condition) and satiation (average of the two images in the satiation condition minus average of the two images in the hunger condition). For data analysis, a Gaussian filter yielded an in-plane resolution of about 20 mm FWHM and a slice thickness of 10 mm FWHM. To reduce type I errors, a critical z score ≥ 2.58 (P < 0.005 uncorrected for multiple comparisons) was used to characterize significant changes in regional brain activity. Automated algorithms were used to transform each subject’s brain MRI into standard atlas coordinates (29), compute an average of the 11 subjects’ MRIs, and superimpose each z-score map onto the averaged MRI to allow visual inspection of the composite images. Pearson’s product moment correlations were used to test the relationship between state-dependent changes in rCBF in the areas of interest and state-dependent changes in subjective hunger/satiation ratings and plasma levels of the hormones and metabolites.
RESULTS
After a 36-h fast, subjects reported high ratings of hunger (74 ± 25 mm on a 0- to 100-mm visual analog scale) and low ratings of satiation (23 ± 24 mm). In contrast, after the administration of a liquid meal delivering 50% of the daily resting energy requirement, subjects reported significantly lower ratings of hunger (31 ± 21 mm) and higher ratings of satiation (70 ± 20 mm) (P < 0.005 for both). Whereas plasma leptin concentrations did not change after the meal, the expected increases in plasma insulin and glucose concentrations and decreases in plasma free fatty acid concentrations were observed (Table 1).
Table 1.
Hormones and metabolites before and after a liquid meal in 11 right-handed, normal weight male individuals
| Time | Glucose, mg/dl | Free fatty acids, mmol/liter | Insulin, microunit/ml | Leptin, mg/ml |
|---|---|---|---|---|
| Before meal | 76 ± 11 | 0.64 ± 0.36 | 4 ± 2 | 0.47 ± 0.24 |
| After meal | 124 ± 15* | 0.48 ± 0.06 | 53 ± 31* | 0.46 ± 0.25 |
Values given are mean ± SD.
P < 0.05 vs before meal values.
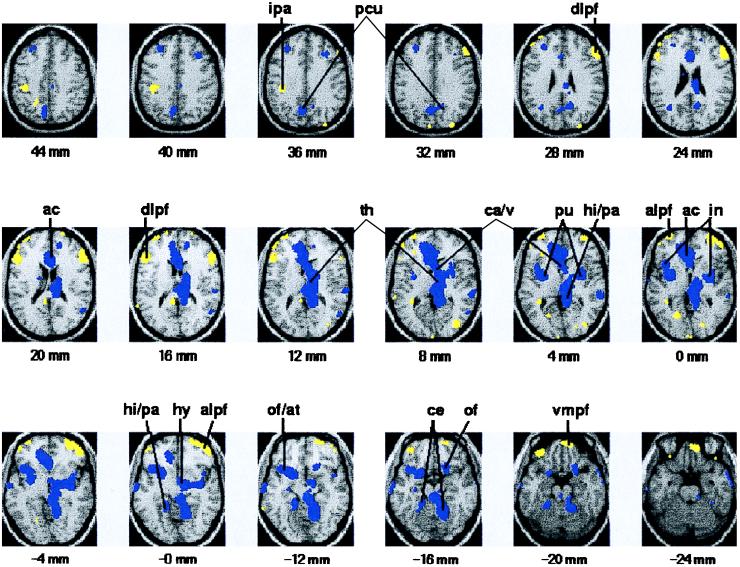
In comparison with satiation, hunger was associated with increased rCBF in the vicinity of the hypothalamus and insular cortex and in additional paralimbic and limbic areas (anterior cingulate cortex, parahippocampal and hippocampal formation, and a region that includes the anterior temporal and posterior orbitofrontal cortex), thalamus, caudate, precuneus, putamen, and cerebellum (Table 2; Fig. 1).
Table 2.
Significantly greater increases in regional brain activity after a ∼36-h fast (hunger) as compared to those after a liquid meal (satiation)
| Region | Brodmann’s Area | Atlas Coordinates
|
z score* | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Hypothalamus | 2 | −6 | −8 | 2.71 | |
| Insular cortex/claustrum | −42 | 16 | 0 | 3.14 | |
| 34 | −8 | 0 | 2.79 | ||
| Thalamus | −4 | −24 | 8 | 2.59 | |
| 12 | −28 | 12 | 3.63 | ||
| Anterior cingulate cortex | 24, 32 | −12 | 36 | 0 | 3.73 |
| 2 | 22 | 20 | 3.16 | ||
| Posterior orbitofrontal/ anterior temporal cortex | 11, 25, 47, 38 | −42 | 14 | −12 | 2.91 |
| 20 | 18 | −16 | 2.61 | ||
| Hippocampus/parahippocampal gyrus | 27, 30, 35, 36 | −20 | −38 | −8 | 2.80 |
| 10 | −36 | 4 | 2.68 | ||
| Precuneus | 7 | −10 | −64 | 36 | 3.00 |
| 12 | −58 | 32 | 2.65 | ||
| Caudate/ventricle | −4 | 24 | 4 | 2.98 | |
| 2 | 10 | 8 | 3.09 | ||
| Putamen | −28 | 0 | 4 | 2.59 | |
| 30 | −2 | 4 | 2.60 | ||
| Cerebellum | −22 | −38 | −16 | 2.81 | |
| 14 | −58 | −16 | 3.54 | ||
Atlas coordinates are from the brain atlas of Tailarach and Tournoux (27), such that x is the distance in mm to the right (+) or left (−) of midline, y is the distance in mm anterior (+) or posterior (−) to the anterior commissure, and z is the distance in mm superior (+) or inferior (−) to a horizontal plane through the anterior and posterior commissures. Hypothalamus and insular cortex/claustrum were originally postulated to participate in aspects of hunger.
P < .005, without correction for multiple comparisons.
Figure 1.
Images of brain activation in response to hunger and satiation. Brain regions with significant increase in rCBF in response to hunger are shown in blue; brain regions with significant increase in rCBF in response to satiation are shown in yellow. Images were generated by using PET and MRI data from 11 healthy, lean individuals. Color-coded images are superimposed onto an average of the subjects’ brain MRIs (grayscale image). Horizontal brain sections correspond to the coordinates of the Talairach and Tournoux brain atlas (27). The number under each section reflects the distance in mm superior (+) or inferior (−) to a horizontal plane between the anterior and posterior commissures. The right hemisphere in each section is on the reader’s right. Compared with satiation, hunger was associated with significantly increased rCBF (P < 0.005 uncorrected for multiple comparisons) in the hypothalamus (hy), thalamus (th), anterior cingulate (ac), insula/claustrum (in), orbitofrontal and temporal cortex (of/at), hippocampus/parahippocampul gyrus (hi/pa), precuneus (pcu), caudate/ventricle (ca/v), putamen (pu), and cerebellum (ce). Compared with hunger, satiation was associated with increased rCBF (P < 0.005 uncorrected for multiple comparisons) in the ventromedial prefrontal cortex (vmpf), dorsolateral prefrontal cortex (dlpf), ventrolateral prefrontal cortex (alpf), and inferior parietal lobule (ipa). The location and magnitude of maximal difference are shown in Tables 2 and 3.
In comparison with hunger, satiation was associated with increased rCBF bilaterally in the vicinity of the ventromedial prefrontal cortex, dorsolateral prefrontal cortex, and the inferior parietal lobule (Table 3; Fig. 1).
Table 3.
Significantly greater increases in regional brain activity after a liquid meal (satiation) as compared to those after a ∼36-h fast (hunger)
| Region | Brodmann’s Area | Atlas Coordinates
|
z score* | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Dorsolateral prefrontal cortex | 9, 10, 46 | −36 | 60 | 16 | 2.99 |
| 54 | 32 | 28 | 3.36 | ||
| Ventromedial prefrontal cortex | 11 | 10 | 60 | −20 | 3.39 |
| Inferior parietal lobule | 40 | −40 | −32 | 36 | 2.63 |
Coordinates from the brain atlas of Tailarach and Tournoux (27), such that x is the distance in mm to the right (+) or left (−) of midline, y is the distance in mm anterior (+) or posterior (−) to the anterior commissure, and z is the distance in mm superior (+) or inferior (−) to a horizontal plane through the anterior and posterior commissures. Dorsolateral prefrontal cortex and ventromedial prefrontal cortex were originally postulated to participate in aspects of satiation.
P < .005, without correction for multiple comparisons.
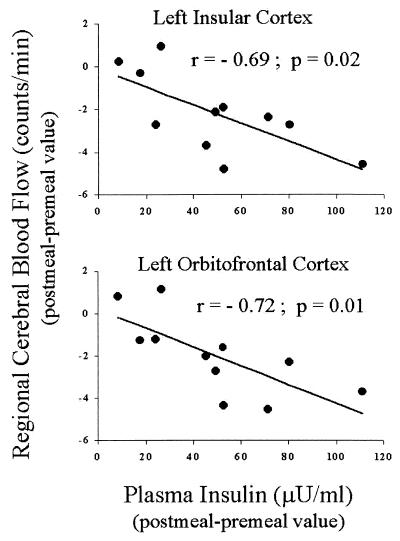
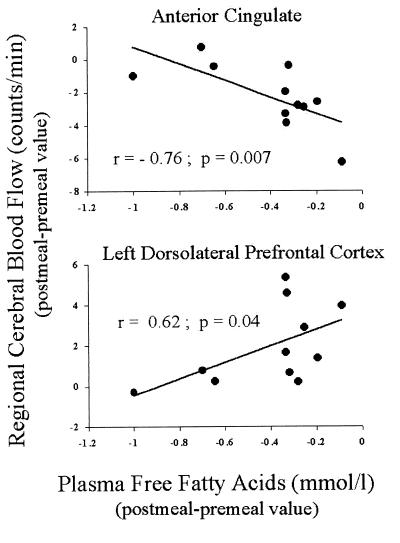
Postmeal insulin changes were negatively correlated with postmeal rCBF changes in the vicinity of the insular cortex (left hemisphere: r = −0.69, P = 0.02, Fig. 2; right hemisphere: r = −0.57, P = 0.06). A similar negative correlation was observed for the orbitofrontal cortex (left hemisphere: r = −0.72, P = 0.01, Fig. 2; right hemisphere: r = −0.59, P = 0.05). As shown in Fig. 3, postmeal free fatty acids changes were negatively correlated with postmeal rCBF changes in the vicinity of anterior cingulate (r = −0.76, P = 0.007), and positively correlated with postmeal rCBF changes in the dorsolateral prefrontal cortex (left hemisphere: r = 0.62, P = 0.04; right hemisphere: r = 0.28, P = 0.40).
Figure 2.
Correlations between changes in plasma insulin concentrations elicited by satiation and changes in normalized PET counts in the left insular cortex centered around Talairach atlas coordinates (x = −40, y = 12, z = 5; Upper) and left orbitofrontal cortex centered around Talairach atlas coordinates (x = −28, y = 16, z = −12; Lower).
Figure 3.
Correlations between changes in plasma free fatty acids concentrations elicited by satiation and changes in normalized PET counts in the anterior cingulate centered around Talairach atlas coordinates (x = 0, y = 25, z = 18; Upper) and left dorsolateral prefrontal cortex centered around Talairach atlas coordinates (x = −52, y = 32, z = 22; Lower).
DISCUSSION
This exploratory study provides information about regions of the human brain that are preferentially affected during hunger and satiation. Hunger was associated with increased neuronal activity in the vicinity of the hypothalamus and thalamus, areas previously described as important in the regulation of feeding behavior in animals. Hunger also was associated with increased activation in the vicinity of the limbic and paralimbic areas, regions involved in affect and motivation. In contrast, satiation was associated with increased activity in prefrontal cortical areas implicated in aspects of response inhibition. In addition, our findings indicate involvement of regions of the brain not previously associated with the regulation of food intake such as the putamen and cerebellum. The correlations observed between postmeal changes in neuronal activity and plasma concentrations of insulin and free fatty acids suggest metabolic modulation of postprandial brain neuronal events.
Neuroanatomical Correlates of Hunger.
Our data indicate that hunger elicits a response in the human brain that is more complex than anticipated in our hypothesis based on animal neurophysiology. In primates, the lateral hypothalamus projects to an area of the orbitofrontal cortex corresponding to Brodmann’s area 11 in humans (2). The orbitofrontal cortex, in turn, integrates the sensory and visceral afferents and feeds back onto the hypothalamus and limbic areas, thus motivating the individual to behave in a manner intended to alleviate hunger (2). Our PET functional data and the correlation between rCBF and plasma insulin concentrations seem to support such a role for the orbitofrontal cortex in humans. The human cortical representation of hunger may, however, be more complex, because we observed concomitant activation in the vicinity of the orbitofrontal, temporal, and parietal cortex. Involvement of the temporal cortex in hunger has been previously suggested by a clinical study (30).
The orbitofrontal cortex projects to limbic and paralimbic areas in rodents and primates (2). Our findings of increased activity in the hippocampus and parahippocampal gyrus are consistent with this neuronal pathway and with the role of these areas in the generation of affective responses to internal stimuli (21). In addition, the unpleasant nature of some of the sensations that often accompany hunger (discomfort, pain, anxiety) may explain the increased activity observed in the anterior cingulate, a region of the human cortex that is selectively activated by noxious stimuli (31). Free fatty acids may have a modulatory effect on this response.
The insula represents an important relay of the neuronal circuitry connecting the hypothalamus, orbitofrontal cortex, and limbic system (2). The insular cortex is known to contribute to the autonomic response to emotional states, and insular stimulation elicits gastrointestinal responses (22). The insular cortex is also a visceral sensory area (20), and recent evidence suggests that it monitors distressing and potentially dangerous internal sensations (19). We, therefore, postulate that this region is selectively affected by the metabolic, hormonal, or neuronal events that signal the threatening state of hunger and that insulin may have a modulatory effect on this response.
Neuroanatomical Correlates of Satiation.
Satiation represents a series of physiologic events that result in the voluntary termination of a feeding episode. Satiation is, therefore, a relatively acute event compared with satiety, which covers the intermeal period (25). Our data indicate that satiation is associated with increased neuronal activity in the prefrontal cortex and inferior parietal lobule. The prefrontal cortex is vital in primates and humans for the storage of temporary information known as working memory (32, 33) and has been implicated in the control of such complex functions as spatial, object and verbal working memory, self-ordered tasks, and analytic reasoning (33). The link between these higher brain activities and the internal and external stimuli that terminate a meal is unknown. Based on the evidence that regions of the prefrontal cortex participate in the inhibition of inappropriate behavioral responses (19), we postulate that, as in animals, these regions exert inhibitory effects on the hypothalamic regions that respond to hunger in humans, thus promoting the termination of a meal.
Correlations Between Brain Activity and Insulin and Free Fatty Acids.
Knowing the hormonal and nutritional modulation of hunger and satiation is crucial for understanding the pathophysiology of food intake regulation in humans. Although insulin does not regulate glucose uptake in the brain, it efficiently crosses the blood–brain barrier (34) and binds to widely expressed receptors in the central nervous system (35). It has been proposed that insulin may act as an anorectic signal in the brain, and the correlation we found between changes in plasma insulin concentrations and rCBF in the vicinity of the insular and orbitofrontal cortex is consistent with this hypothesis. Our results are also consistent with two prospective studies showing that reduced insulin secretion in response to glucose is associated with an increased risk for weight gain (36) and that insulin sensitivity (which is associated with lower insulin concentrations) predicts future weight gain (37). The role of free fatty acids in the regulation of food intake is not well defined. However, free fatty acids can cross the blood–brain barrier (38). The correlations observed in our study between changes in free fatty acid concentrations and rCBF in the vicinity of the anterior cingulate and dorsolateral prefrontal cortex suggest a possible anorectic role of these lipids in humans. A correlation between plasma concentrations of insulin or free fatty acids and brain activity does not prove a causal relationship, but this can be tested experimentally. Also, we cannot rule out the possibility that brain activity directly affects peripheral metabolites and hormone secretion.
Potential Study Limitations.
PET results must be interpreted in light of the major limitations of this imaging technique (39). Spatial resolution, contrast resolution of individual subtraction images, and accuracy of the image deformation algorithm make it difficult to specify in greater detail the structures that are responsible for the observed increases in regional brain activity (i.e., to identify hypothalamic and thalamic nuclei). Because increases in regional brain activity appear to reflect the activity of terminal neuronal fields (40) (including those from local interneurons and afferent projections arising in other sites), it is not possible to specify the neuronal projections that account for the observed increases in regional activity. To reduce the possibility of chance findings caused by multiple comparisons, we tested specific hypotheses about alterations in hypothalamic, insular, and prefrontal cortex rCBF and we retained changes in other areas when bilateral. The use of a critical z score of 2.58 raises the possibility that some of the observed changes in regional brain activity could be attributable to the large number of regions compared in our statistical maps. On the basis of theoretical considerations alone, this criterion might seem too liberal, yielding an unacceptably high likelihood of statistical type I errors. However, it was chosen on the basis of a study in which we empirically evaluated the number of true signals and false signals associated with different critical values. By using the same radiotracer methods, imaging system, and brain mapping algorithms used in this study, we previously analyzed PET images acquired in a separate group of normal volunteers during a well characterized motor task and two baseline tasks to simulate true signals and false signals. We found that a critical z score of 2.58 was associated with 0–1 false signal in the entire statistical map (far fewer than one would expect considering the large number of resolution elements in the data set), that it improved the sensitivity for detecting true signals, and that it provided the best tradeoff between type I and type II errors (unpublished data). Because brain activity changes in the vicinity of the insular cortex, orbitofrontal cortex, hippocampal formation, cerebellum, and dorsolateral prefrontal cortex were bilateral, we believe these changes are unlikely to reflect statistical type I errors. Still, our results were not statistically correct for the potential number of independent comparisons and, thus, should be considered preliminary. Additional studies are needed to replicate the observed increases in regional brain activity associated with hunger and satiation.
Limitations in the study design also must be acknowledged. Because of the exploratory nature of the study, we elected to induce rather extreme (and somewhat unphysiological) states of hunger and satiation. This was done to produce behavioral states of sufficient intensity to maximize our chances to detect brain regions selectively affected during these conditions. Subjects were studied in a resting condition, rather than during a systematically manipulated mental activity (i.e., visuospatial task). Although we cannot exclude the possibility that the subjects engaged in more spontaneous visual imagery in one condition vs. the other, it is our experience that the subject’s behavioral state is not significantly different during sequential 1-minute scans. Finally, while acknowledging the potentially confounding effects of scan order, we note that: (i) we could not counterbalance the conditions in a within-session study; (ii) between-session studies have lower statistical power. We also examined the effects of scan order by comparing PET rCBF images acquired in the same resting state (with an interscan duration of 40–60 min) in eight previously studied normal volunteers. Although the second scan was associated with increased rCBF in a region of the left dorsolateral prefrontal cortex (x = −54, y = 40, z = 12), it was not associated with rCBF increases or decreases in any of the regions reported in the present study. Although the observed changes in regional rCBF do not appear to be entirely attributable to the effects of scan order, we cannot exclude the possibility that they were at least partly attributable to the interaction between scan order and the fasting state.
Conclusions.
The present study functionally describes the regions of the human brain involved in hunger and satiation. These findings raise the possibility that several regions of the brain participate in the regulation of hunger and satiation and that insulin and free fatty acids may be metabolic modulators of postprandial brain neuronal events. Although exploratory, the present study provides a foundation for investigating the human brain regions and cognitive operations that respond to nutritional stimuli. PET functional brain imaging may be a suitable technique to investigate whether hyperphagia (obesity) and anorexia are associated with selective differences in regional brain activity.
Acknowledgments
We thank Lang Sheng Yun, Sandy Goodwin, Leslie Mullen, Tricia Giurlani, David Stith, and Frank Gucciardo for technical assistance, Jennifer Frost and Amelia Dorris for editorial preparation of figures and tables, Manish Amin and Vaishali Patel for their logistic help, and the nursing, dietary, and technical staffs of the Clinical Research Center.
ABBREVIATIONS
- PET
positron emission tomography
- rCBF
regional cerebral blood flow
- FWHM
full width half maximum
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Levin B E, Routh V H. Am J Physiol. 1996;271:R491–R500. doi: 10.1152/ajpregu.1996.271.3.R491. [DOI] [PubMed] [Google Scholar]
- 2.Oomura Y. In: Handbook of the Hypothalamus: Physiology of the Hypothalamus. Morgane P J, Panksepp J, editors. New York: Dekker; 1981. pp. 557–620. [Google Scholar]
- 3.Kuenzel W J. J Nutr. 1994;124:1355S–1370S. [PubMed] [Google Scholar]
- 4.Stellar E. Psychol Rev. 1954;61:5–22. doi: 10.1037/h0060347. [DOI] [PubMed] [Google Scholar]
- 5.Anand B, Brobeck J R. Yale J Biol Med. 1951;24:123–146. [PMC free article] [PubMed] [Google Scholar]
- 6.Rohner-Jeanrenaud F. Int J Obes. 1995;19:517–534. [PubMed] [Google Scholar]
- 7.Flier J S, Maratos-Flier E. Cell. 1998;92:437–440. doi: 10.1016/s0092-8674(00)80937-x. [DOI] [PubMed] [Google Scholar]
- 8.Kaiyala, K. J., Woods, S. C. & Schwartz, M. W. (1995) Am. J. Clin. Nutr.62 Suppl., 1123S–1134S. [DOI] [PubMed]
- 9.Porte D, Jr, Seeley R J, Woods S C, Baskin D G, Figlewicz D P, Schwartz M W. Diabetologia. 1998;41:863–881. doi: 10.1007/s001250051002. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz M W, Baskin D G, Bukowski T R, Kuijper J L, Foster D, Lasser G, Prunkard D E, Porte D, Jr, Woods S C, Seeley R J, et al. Diabetes. 1996;45:531–535. doi: 10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 11.Heiman M L, Ahima R S, Craft L S, Schoner B, Stephens T W, Flier J S. Endocrinology. 1997;138:3859–3863. doi: 10.1210/endo.138.9.5366. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 13.Doyle P, Rohner-Jeanrenaud F, Jeanrenaud B. Am J Physiol. 1993;264:E29–E36. doi: 10.1152/ajpendo.1993.264.1.E29. [DOI] [PubMed] [Google Scholar]
- 14.Bray G A, Gallagher T F., Jr Medicine. 1975;54:301–330. doi: 10.1097/00005792-197507000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Scott T R, Yan J, Rolls E T. Neurobiology. 1995;3:281–292. [PubMed] [Google Scholar]
- 16.Kinomura S, Kawashima R, Yamada K, Ono S, Itoh M, Yoshioka S, Yamaguchi T, Matsui H, Miyazawa H, Itoh H, et al. Brain Res. 1994;659:263–266. doi: 10.1016/0006-8993(94)90890-7. [DOI] [PubMed] [Google Scholar]
- 17.Zatorre R J, Jones-Gotman M, Evans A C, Meyer E. Nature (London) 1992;360:339–340. doi: 10.1038/360339a0. [DOI] [PubMed] [Google Scholar]
- 18.Zald D H, Lee J T, Fluegel K W, Pardo J V. Brain. 1998;121:1143–1154. doi: 10.1093/brain/121.6.1143. [DOI] [PubMed] [Google Scholar]
- 19.Reiman, E. M. (1997) J. Clin. Psychiatry58 Suppl., 4–12. [PubMed]
- 20.Augustine J R. Brain Res Rev. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- 21.Le Doux J E. In: Handbook of Physiology. Plum F, Mountcastle V B, editors. Washington, DC: Am. Physiol. Soc.; 1987. pp. 419–459. [Google Scholar]
- 22.Mesulam M M. Principles of Behavioral Neurology. Philadelphia: Davis; 1985. [Google Scholar]
- 23.Spitzer R L, Williams J B W, Gibbon M, First M B. Structured Clinical Review for DSM-III-R Non-Patient Edition. Washington, DC: Am. Psychiatric Press; 1990. [Google Scholar]
- 24.Stunkard A J, Messick S. J Psychosomatic Res. 1985;29:71–83. doi: 10.1016/0022-3999(85)90010-8. [DOI] [PubMed] [Google Scholar]
- 25.Lawton C L, Burley V J, Wales J K, Blundell J E. Int J Obesity. 1993;17:409–416. [PubMed] [Google Scholar]
- 26.Woods R P, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 27.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 28.Friston K J, Frith C D, Liddle P F, Frackowiak R S J. J Cerebr Blood Flow Metab. 1991;11:690–699. doi: 10.1038/jcbfm.1991.122. [DOI] [PubMed] [Google Scholar]
- 29.Collins D L, Neelin P, Peters T M, Evans A C. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- 30.Fisher C M. Neurology. 1994;44:1577–1579. doi: 10.1212/wnl.44.9.1577. [DOI] [PubMed] [Google Scholar]
- 31.Craig A D, Reiman E M, Evans A, Bushnell M C. Nature (London) 1996;384:258–260. doi: 10.1038/384258a0. [DOI] [PubMed] [Google Scholar]
- 32.Cohen J D, Perlstein W M, Braver T S, Nystrom L E, Noll D C, Jonides J, Smith E E. Nature (London) 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 33.Courtney S M, Ungerleider L G, Keil K, Haxby J V. Nature (London) 1997;386:608–611. doi: 10.1038/386608a0. [DOI] [PubMed] [Google Scholar]
- 34.Baura G D, Foster D M, Porte D, Jr, Kahn S E, Bergman R N, Cobelli C, Schwartz M W. J Clin Invest. 1993;92:1824–1830. doi: 10.1172/JCI116773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz M W, Figlewicz D P, Baskin D G, Woods S C, Porte D., Jr Endocr Rev. 1992;13:387–414. doi: 10.1210/edrv-13-3-387. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz M W, Boyko E J, Kahn S E, Ravussin E, Bogardus C. J Clin Endocrinol Metab. 1995;80:1571–1576. doi: 10.1210/jcem.80.5.7745002. [DOI] [PubMed] [Google Scholar]
- 37.Swinburn B A, Nyomba B L, Saad M F, Zurlo F, Raz I, Knowler W C, Lillioja S, Bogardus C, Ravussin E. J Clin Invest. 1991;88:168–173. doi: 10.1172/JCI115274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spector R. J Neurochem. 1988;50:639–643. doi: 10.1111/j.1471-4159.1988.tb02958.x. [DOI] [PubMed] [Google Scholar]
- 39.Reiman E M, Lane R D, Ahern G L, Schwartz G E, Davidson R J, Friston K J, Yun L S, Chen K. Am J Psychiatry. 1997;154:918–925. doi: 10.1176/ajp.154.7.918. [DOI] [PubMed] [Google Scholar]
- 40.Schwartz W J, Smith C B, Davidsen L, Savaki H, Sokoloff L, Mata M, Fink D J, Gainer H. Science. 1979;205:723–725. doi: 10.1126/science.462184. [DOI] [PubMed] [Google Scholar]