Abstract
Evolutionary processes have not yet developed specific and safe ways to detoxify all chemical species new to our environment. Indeed, some are transformed and/or conjugated by the liver into more toxic species. Environmental factors can modulate hepatic enzyme systems. Particularly responsive are the mixed function oxidases, which initiate the transformation of many xenobiotics to excretable species via reactions which generate electrophilic intermediates such as free radicals, epoxides and aldehydes. Unless these reactive metabolites are rapidly removed by subsequent detoxification reactions or by endogenous defense systems, destructive cytotoxic reactions can be triggered or cell constitutents “attacked” thereby causing either acute injury and/or more latent molecular injury to long chain biopolymers resulting in chromatin damage, or tumors.
In vitro systems using purified, specialized cell fractions may be of considerable value in defining metabolic processes, but the results must be relevant to in vivo conditions. Although human liver is peculiarly resistant to tumorigenesis, liver microsomes (isolated endoplasmic reticulum) are extensively used as biological activators for in vitro mutagenicity test systems. The in vivo defense system of liver cells must be exceptionally efficient! Reactive metabolites generated in liver may be stable enough to migrate and cause injury to other tissues or organ systems. It is important to characterize metabolic pathways of toxic xenobiotics, subsequent molecular sites or modes of injury, and factors which depress or augment cellular defense systems including the biliary system responsible for the excretion of many xenobiotics. Only then can techniques or treatments be developed to screen individuals for risk to specific groups of xenobiotics, to protect those exposed, and to treat those injured.
Full text
PDF


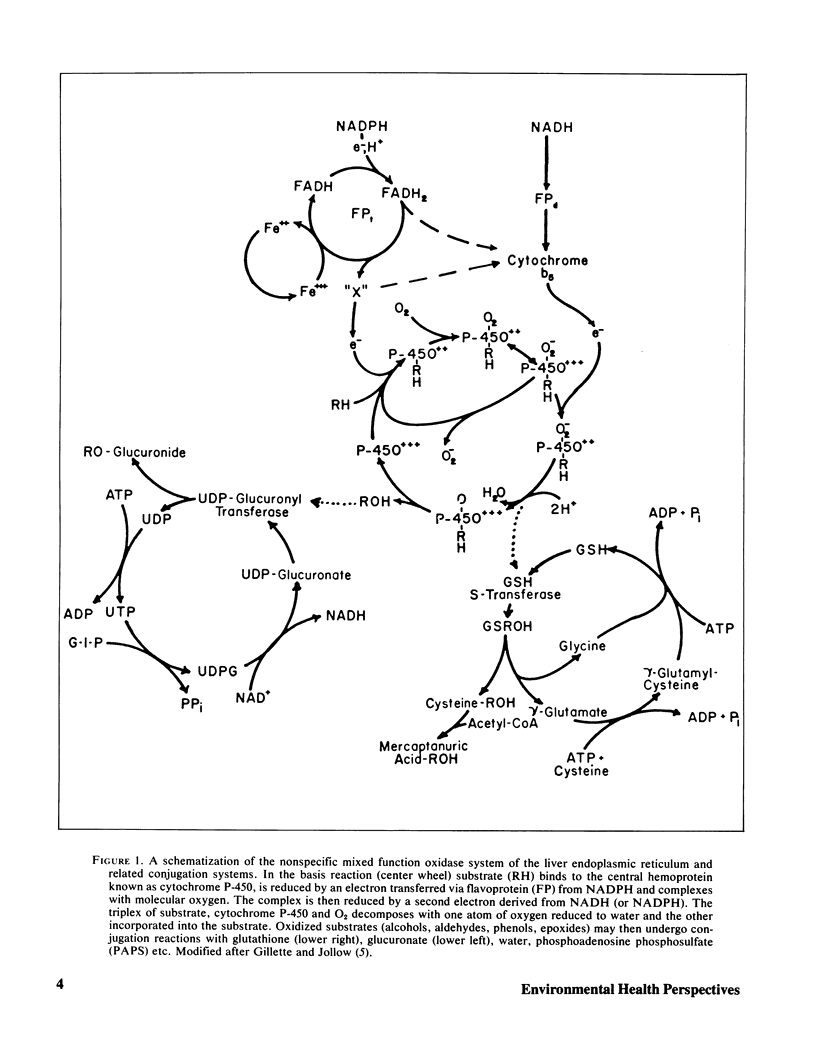


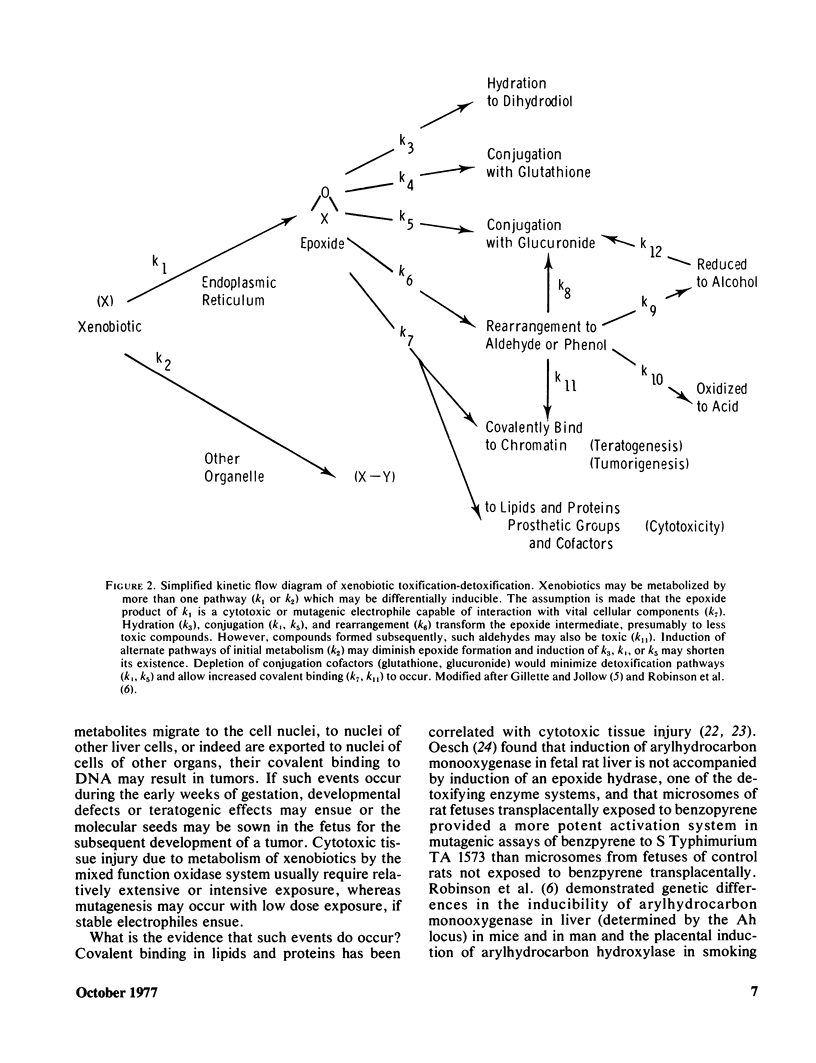




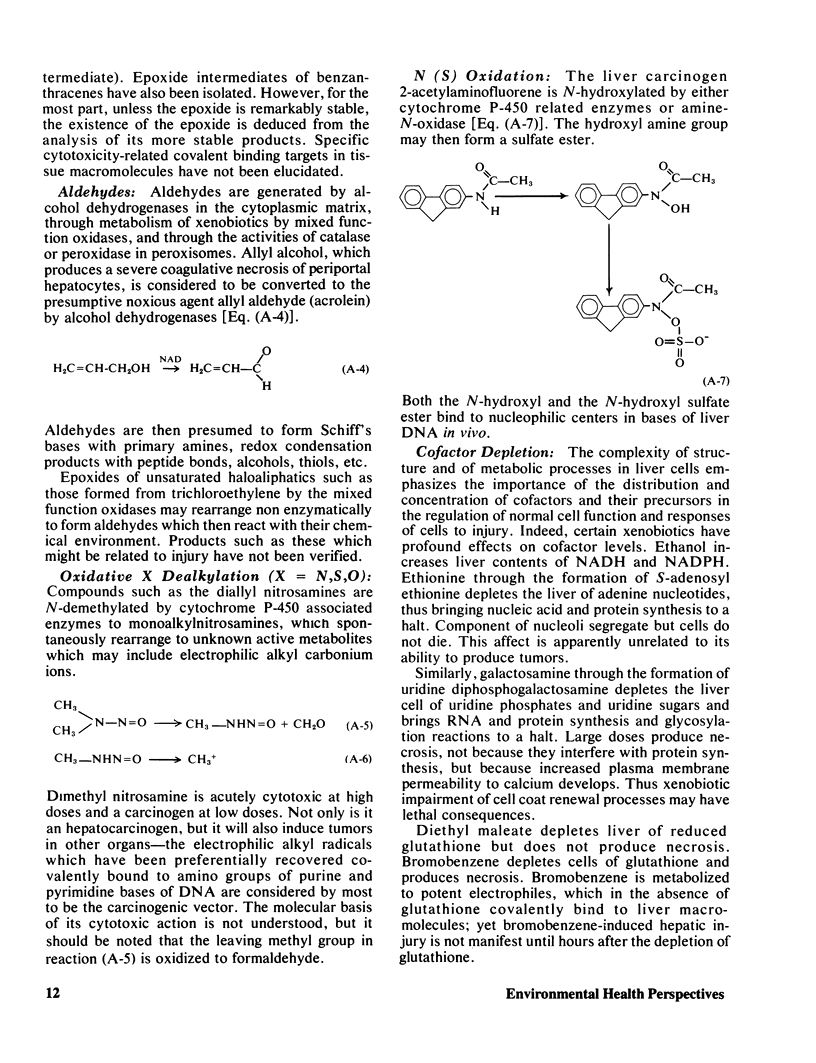

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Duve C., Baudhuin P. Peroxisomes (microbodies and related particles). Physiol Rev. 1966 Apr;46(2):323–357. doi: 10.1152/physrev.1966.46.2.323. [DOI] [PubMed] [Google Scholar]
- Felton J. S., Nebert D. W. Mutagenesis of certain activated carcinogens in vitro associated with genetically mediated increases in monooxygenase activity and cytochrome P 1-450. J Biol Chem. 1975 Sep 10;250(17):6769–6778. [PubMed] [Google Scholar]
- Frantz C. N., Malling H. V. Factors affecting metabolism and mutagenicity of dimethylnitrosamine and diethylnitrosamine. Cancer Res. 1975 Sep;35(9):2307–2314. [PubMed] [Google Scholar]
- Ilett K. F., Reid W. D., Sipes I. G., Krishna G. Chloroform toxicity in mice: correlation of renal and hepatic necrosis with covalent binding of metabolites to tissue macromolecules. Exp Mol Pathol. 1973 Oct;19(2):215–229. doi: 10.1016/0014-4800(73)90080-4. [DOI] [PubMed] [Google Scholar]
- Kappus H., Bold H. M., Buchter A., Bolt W. Rat liver microsomes catalyse covalent binding of 14C-vinyl chloride to macromolecules. Nature. 1975 Sep 11;257(5522):134–135. doi: 10.1038/257134a0. [DOI] [PubMed] [Google Scholar]
- Klaassen C. D. Biliary excretion of xenobiotics. CRC Crit Rev Toxicol. 1975 Oct;4(1):1–30. doi: 10.1080/10408447509163833. [DOI] [PubMed] [Google Scholar]
- Klebanoff S. J. Antimicrobial mechanisms in neutrophilic polymorphonuclear leukocytes. Semin Hematol. 1975 Apr;12(2):117–142. [PubMed] [Google Scholar]
- NOVIKOFF A. B. Cell heterogeneity within the hepatic lobule of the rat: staining reactions. J Histochem Cytochem. 1959 Jul;7(4):240–244. doi: 10.1177/7.4.240. [DOI] [PubMed] [Google Scholar]
- Occupational disease among operating room personnel: a national study. Report of an Ad Hoc Committee on the Effect of Trace Anesthetics on the Health of Operating Room Personnel, American Society of Anesthesiologists. Anesthesiology. 1974 Oct;41(4):321–340. [PubMed] [Google Scholar]
- Reddy J., Chiga M., Bunyaratvej S., Svoboda D. Microbodies in experimentally altered cells. VII. CPID-induced hepatic microbody proliferation in the absence of significant catalase synthesis. J Cell Biol. 1970 Jan;44(1):226–234. doi: 10.1083/jcb.44.1.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid W. D., Krishna G. Centrolobular hepatic necrosis related to covalent binding of metabolites of halogenated aromatic hydrocarbons. Exp Mol Pathol. 1973 Feb;18(1):80–99. doi: 10.1016/0014-4800(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S. Liver endoplasmic reticulum: target site of halocarbon metabolites. Adv Exp Med Biol. 1977;84:117–137. doi: 10.1007/978-1-4684-3279-4_6. [DOI] [PubMed] [Google Scholar]
- Reynolds E. S., Moslen M. T., Szabo S., Jaeger R. J. Vinyl chloride-induced deactivation of cytochrome P-450 and other components of the liver mixed function oxidase system: an in vivo study. Res Commun Chem Pathol Pharmacol. 1975 Dec;12(4):685–694. [PubMed] [Google Scholar]
- Schulte-Hermann R. Induction of liver growth by xenobiotic compounds and other stimuli. CRC Crit Rev Toxicol. 1974 Sep;3(1):97–158. doi: 10.3109/10408447409079856. [DOI] [PubMed] [Google Scholar]
- Sipes I. G., Brown B. R., Jr An animal model of hepatotoxicity associated with halothane anesthesia. Anesthesiology. 1976 Dec;45(6):622–628. doi: 10.1097/00000542-197612000-00005. [DOI] [PubMed] [Google Scholar]
- Thomas L. B., Popper H., Berk P. D., Selikoff I., Falk H. Vinyl-chloride-induced liver disease. From idiopathic portal hypertension (Banti's syndrome) to Angiosarcomas. N Engl J Med. 1975 Jan 2;292(1):17–22. doi: 10.1056/NEJM197501022920104. [DOI] [PubMed] [Google Scholar]


