Abstract
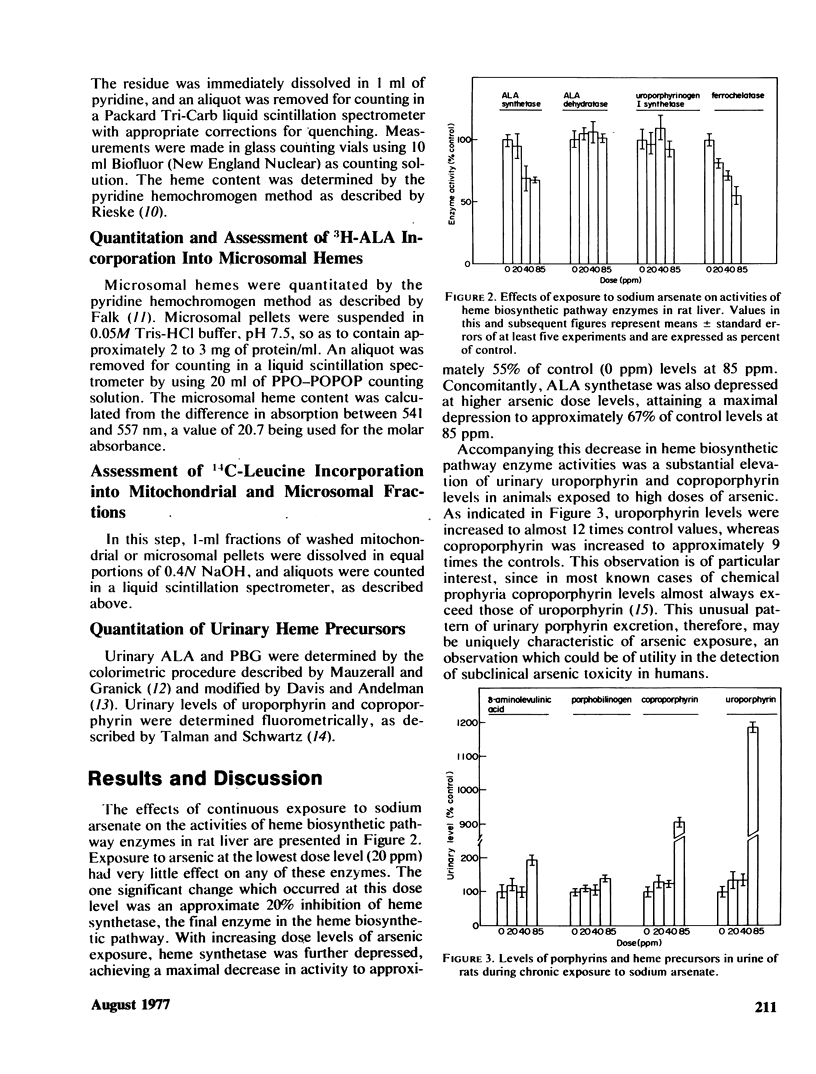
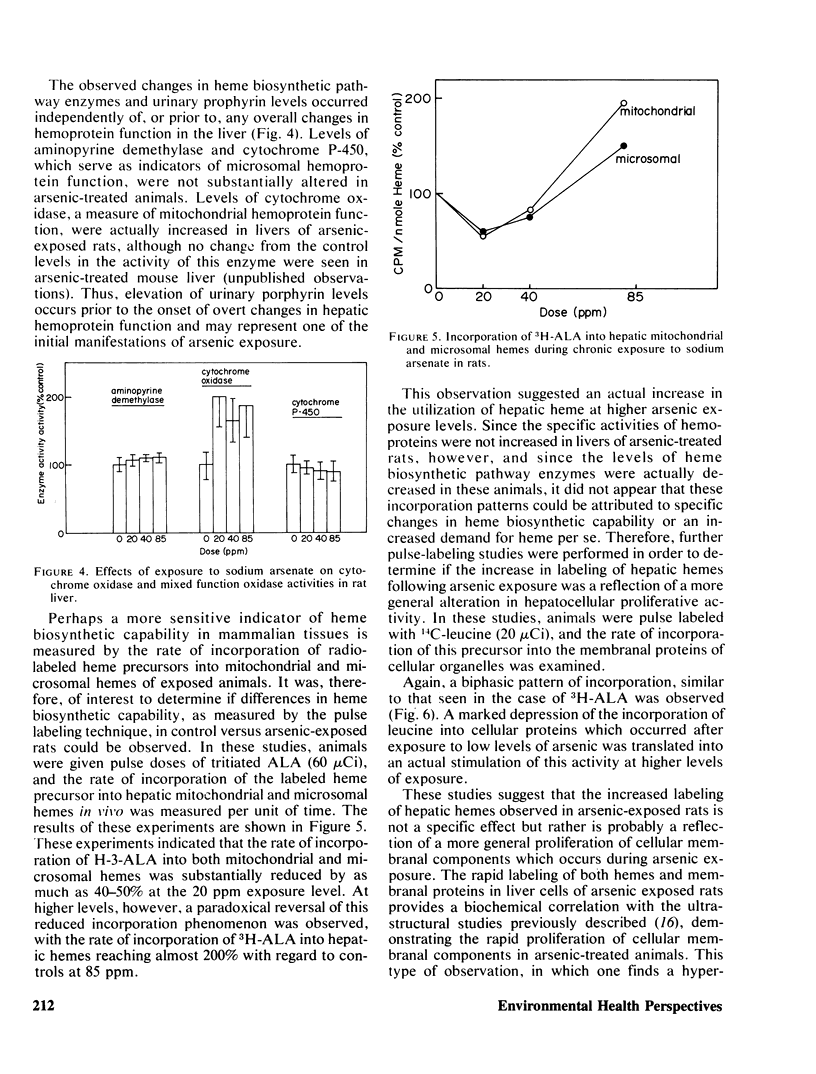
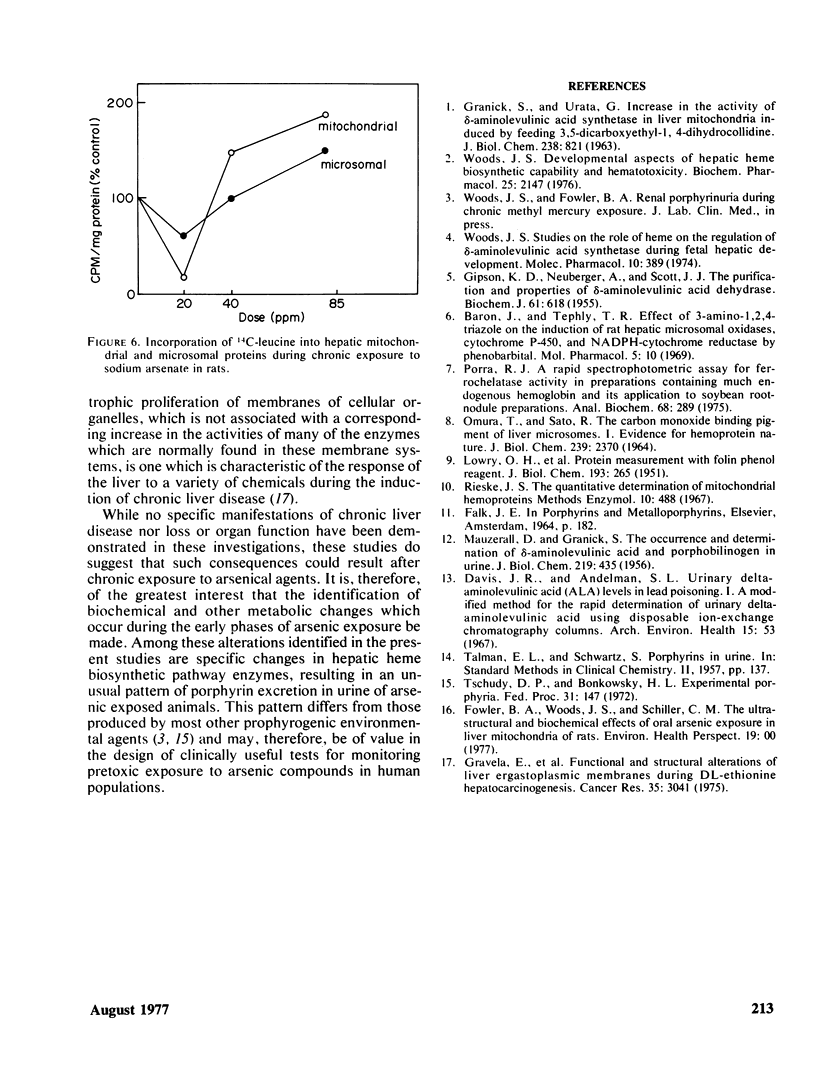
In these studies the effects of ingested arsenic (As+5) on hepatic heme biosynthetic capability and hemoprotein function in adult male rats were investigated. Animals exposed for 6 weeks to 0, 20, 40, or 85 ppm sodium arsenate in the drinking water suffered depression of hepatic δ-aminolevulinic acid (ALA) synthetase and heme synthetase (ferrochelatase) activities, with maximal decreases to 67 and 55% of control levels, respectively, at 85 ppm. Concomitantly, urinary uroporphyrin levels were elevated by as much as 12 times, and coproporphyrin by as much as 9 times, control values. The rate of incorporation of 3H-ALA into mitochondrial and microsomal hemes was depressed by 40–50% at 20 ppm but was increased with regard to controls by as much as 150% at the higher treatment levels. A similar biphasic pattern was observed in regard to 14C-leucine incorporation into cellular membranal proteins. In contrast, the levels of ALA dehydratase, uroporphyrinogen I synthetase, aminopyrine demethylase, and cytochrome P-450 were not significantly changed in As+5-treated rats. These results support the hypothesis that chronic, low level, arsenic exposure results in selective inhibition of mitochondrial-bound heme biosynthetic pathway enzymes (ALA synthetase and heme synthetase) resulting in a substantial increase in urinary porphyrins, uniquely characterized by a greater increase in uroporphyrin than coproporphyrin levels. These changes occur independent of, or prior to, alterations in hepatic hemoprotein-dependent functions and may thus serve in the clinical analysis of pretoxic exposure to arsenic compounds in human populations.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Davis J. R., Andelman S. L. Urinary delta-aminolevulinic acid (ALA) levels in lead poisoning. I. A modified method for the rapid determination of urinary delta-aminolevulinic acid using disposable ion-exchange chromatography columns. Arch Environ Health. 1967 Jul;15(1):53–59. doi: 10.1080/00039896.1967.10664873. [DOI] [PubMed] [Google Scholar]
- GIBSON K. D., NEUBERGER A., SCOTT J. J. The purification and properties of delta-aminolaevulic acid dehydrase. Biochem J. 1955 Dec;61(4):618–629. doi: 10.1042/bj0610618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S., URATA G. Increase in activity of alpha-aminolevulinic acid synthetase in liver mitochondria induced by feeding of 3,5-dicarbethoxy-1,4-dihydrocollidine. J Biol Chem. 1963 Feb;238:821–827. [PubMed] [Google Scholar]
- Gravela E., Feo F., Canuto R. A., Garcea R., Gabriel L. Functional and structural alterations of liver ergastoplasmic membranes during DL-ethionine hepatocarcinogenesis. Cancer Res. 1975 Nov;35(11 Pt 1):3041–3047. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Porra R. J. A rapid spectrophotometric assay for ferrochelatase activity in preparations containing much endogenous hemoglobin and its application to soybean root-nodule preparations. Anal Biochem. 1975 Sep;68(1):289–298. doi: 10.1016/0003-2697(75)90707-1. [DOI] [PubMed] [Google Scholar]
- Tschudy D. P., Bonkowsky H. L. Experimental porphyria. Fed Proc. 1972 Jan-Feb;31(1):147–159. [PubMed] [Google Scholar]
- Woods J. S. Developmental aspects of hepatic heme biosynthetic capability and hematotoxicity. Biochem Pharmacol. 1976 Oct 1;25(19):2147–2152. doi: 10.1016/0006-2952(76)90126-x. [DOI] [PubMed] [Google Scholar]
- Woods J. S. Studies on the role of heme in the regulation of delta-aminolevulinic acid synthetase during fetal hepatic development. Mol Pharmacol. 1974 May;10(3):389–397. [PubMed] [Google Scholar]


