Abstract
Electron transfer flavoprotein-ubiquinone oxidoreductase (ETF-QO) is a 4Fe4S flavoprotein located in the inner mitochondrial membrane. It catalyzes ubiquinone (UQ) reduction by ETF, linking oxidation of fatty acids and some amino acids to the mitochondrial respiratory chain. Deficiencies in ETF or ETF-QO result in multiple acyl-CoA dehydrogenase deficiency, a human metabolic disease. Crystal structures of ETF-QO with and without bound UQ were determined, and they are essentially identical. The molecule forms a single structural domain. Three functional regions bind FAD, the 4Fe4S cluster, and UQ and are closely packed and share structural elements, resulting in no discrete structural domains. The UQ-binding pocket consists mainly of hydrophobic residues, and UQ binding differs from that of other UQ-binding proteins. ETF-QO is a monotopic integral membrane protein. The putative membrane-binding surface contains an α-helix and a β-hairpin, forming a hydrophobic plateau. The UQ—flavin distance (8.5 Å) is shorter than the UQ—cluster distance (18.8 Å), and the very similar redox potentials of FAD and the cluster strongly suggest that the flavin, not the cluster, transfers electrons to UQ. Two possible electron transfer paths can be envisioned. First, electrons from the ETF flavin semiquinone may enter the ETF-QO flavin one by one, followed by rapid equilibration with the cluster. Alternatively, electrons may enter via the cluster, followed by equilibration between centers. In both cases, when ETF-QO is reduced to a two-electron reduced state (one electron at each redox center), the enzyme is primed to reduce UQ to ubiquinol via FAD.
Keywords: fatty acid oxidation, iron-sulfur flavoprotein, mitochondrial respiratory chain, membrane protein, acyl-CoA dehydrogenases
Electron transfer flavoprotein-ubiquinone oxidoreductase (ETF-QO) is an intrinsic membrane protein located in the inner mitochondrial membrane. It contains single equivalents of FAD and a [4Fe4S]2+,1+ cluster (1). The protein is the single input site to the main respiratory chain for electrons from nine flavoprotein acyl-CoA dehydrogenases and two N-methyl dehydrogenases (2, 3). The electron acceptor for the dehydrogenases is the ETF, which is the reductant of ETF-QO. ETF-QO is oxidized by the diffusible ubiquinone (UQ) pool that also is accessed by NADH-UQ oxidoreductase (Complex I), succinate-UQ oxidoreductase (Complex II), the flavin-linked glycerol-3-phosphate dehydrogenase, and dihydroorotate dehydrogenase, another flavin-linked UQ oxidoreductase (4). The ubiquinol product of these oxidoreductases transfers electrons to the bc1 complex (Complex III). Thus, ETF and ETF-QO link the oxidation of fatty acids and some amino acids to the mitochondrial respiratory system, and the overall electron flow can be summarized as follows: Acyl-CoA → Acyl-CoA dehydrogenases → ETF → ETF-QO → UQ → Complex III. Inherited deficiencies of ETF-QO or ETF cause a metabolic disease, multiple acyl-CoA dehydrogenase deficiency, also known as glutaric acidemia type II (5). This metabolic disease is characterized in its most severe form by delayed neuronal migration, an energy-intensive process, and polycystic kidneys (6).
Reductive titration of ETF-QO by octanoyl-CoA in the presence of catalytic medium-chain acyl-CoA dehydrogenase and ETF proceeds to the two-electron reduced state, [4Fe4S]1+, and an anionic flavin semiquinone. Electron transfer in this pathway is firmly established only for the initial transfer from the primary dehydrogenases to ETF: the reactions proceed as two one-electron transfer steps from the dehydrogenase dihydroquinone to two equivalents of ETF (7, 8). However, the electron transfer pathway is less clear at this point. ETF-QO catalyzes the disproportionation of ETF semiquinone generated by the primary dehydrogenases at a rate that is catalytically competent to participate in the overall transfer of electrons from an acyl-CoA substrate to UQ (9). This overall reaction in vitro was established only in a soluble uncompartmentalized system, with a short-chain, water-soluble UQ homolog (9).
ETF-QO, along with the other mitochondrial UQ oxidoreductases, plays a central role in the bioenergetics of aerobic organisms and some anaerobic organisms. Three-dimensional structures have been determined for several UQ oxidoreducatases, including succinate-UQ oxidoreductase (10–12), the related quinol-fumarate oxidoreductase (13, 14), dihydroorotate dehydrogenase (15), and the bc1 complex (16–18). These structures have contributed to an understanding of the distance-dependence of electron transfer (19) and some generalizations regarding the UQ-binding motifs (20). However, no detailed structural information has been available for ETF-QO.
We undertook a structural investigation of porcine ETF-QO by using x-ray crystallography to obtain insight into the inter- and intramolecular electron transfers of the protein, the 2e−/2H+ reduction of UQ, and the possible mode of binding of ETF-QO to the membrane.
Results and Discussion
The Overall Structure.
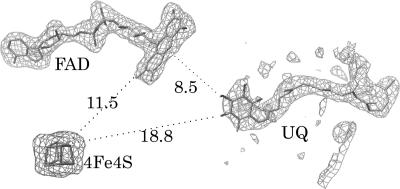
In the final structure of UQ containing ETF-QO, the entire polypeptide chain was visible except the first three residues in one of the two molecules in the asymmetric unit and the first six residues in the other molecule. The residue numbering system used hereafter corresponds to the mature protein sequence and can be related to the complete human sequence by addition of 33 residues, the human mitochondrial signal peptide (21). Each molecule in the asymmetric unit contains one FAD, one 4Fe4S cluster, and one UQ molecule. However, only 5 of the presumed 10 isoprene units could be seen in both of the UQ molecules. The final Rwork and Rfree of the structure were 21.9% and 25.2%, respectively, for all of the data between 30.0-Å and 2.5-Å resolutions. The ETF-QO structure without bound UQ was determined to 2.7 Å, and the Rwork and Rfree of the final model were 22.8% and 25.5%, respectively. The folding of porcine ETF-QO is essentially the same, with or without UQ, with an rms deviation of 0.26 Å between the two structures (a detailed comparison is given in Supporting Results, which is published as supporting information on the PNAS web site). A ribbon diagram of the UQ-bound structure is shown in Fig. 1, and a sequence alignment of ETF-QO from several species along with the secondary structural elements is shown in Fig. 5, which is published as supporting information on the PNAS web site. The structure contains 11 α-helices and 19 β-strands, and the molecule forms a single structural domain having three functional domains: a FAD domain, a 4Fe4S cluster domain, and a UQ-binding domain. The three domains are closely packed and share structural elements, and there are no isolated, discrete domains that would be capable of local segmental motion as seen in the Fe-S protein of the bc1 complex (17). The 4Fe4S cluster domain in ETF-QO consists of N-terminal residues C4–Y16 and C-terminal residues D484–M584. The FAD domain comprises residues P17–N106, V141–P235, and S340–V418 and is primarily an α/β structure. There are two β-sheets in the FAD domain. β-Sheet 1 is a mixed parallel/antiparallel sheet made of strands 1, 6, 7, and 8 and is located at the surface of the molecule capping the 4Fe4S domain. β-Sheet 2 is composed of strands 1, 2, 5, 8, 14, and 15, sandwiched between β-sheet 1 and helices 1, 3, 6, and 7. The UQ domain comprises residues T107–V140, Q236–Q339, and S417–F483. It is dominated by β-sheet 3, which is a twisted, mixed parallel/antiparallel sheet comprising strands 3 (3a), 4, 9, 10, 11, 12, and 13. A structural similarity search using DALI (22) indicates that the fold of the core of the 4Fe4S domain is most similar to that of Clostridium acidurici ferredoxin (23). The Cα rms difference for the 53 equivalent residues for the two molecules is 2.5 Å. The overall fold of the combined FAD and UQ domains is similar to the p-hydroxylbenzoate hydroxylase fold (24). The Cα rms differences for the FAD and UQ/substrate-binding domains of ETF-QO and the hydroxylase are 1.9 Å (145 equivalent residues) and 2.2 Å (75 residues), respectively. The fold of the FAD domain of ETF-QO also is similar to that of flavocytochrome c3-fumarate reductase (rms deviation <2.0 Å for 127 Cα atoms) (25–27) and quinol-fumarate reductase flavin subunit (1.9 Å for 145 residues) (28). These domain- or subdomain-level structural similarities imply divergent evolution and gene fusions among these functionally related proteins. The FAD and 4Fe4S are buried completely in the ETF-QO structure, as is the benzoquinone ring of UQ. The Fo − Fc omit-map and the relative positions of the three redox centers, FAD, 4Fe4S, and UQ, are shown in Fig. 2.
Fig. 1.
Ribbon diagram of ETF-QO. The structure comprises three domains: FAD domain (blue), 4Fe4S cluster domain (red), and UQ-binding domain (green). Three redox centers are shown in sticks: FAD (golden yellow), 4Fe4S (magenta), and UQ (dark red). α-Helices and β-strands are numbered sequentially from the N terminus to the C terminus. The putative membrane-associated surface regions are shown in cyan. Mitochondrial membrane is depicted as blue shaded area.
Fig. 2.
Electron densities in Fo − Fc omit maps for FAD (3.0σ), 4Fe4S (4.0σ), and UQ (2.5σ). The relative positions and distances (in angstroms) among the three redox centers are shown.
The FAD Environment.
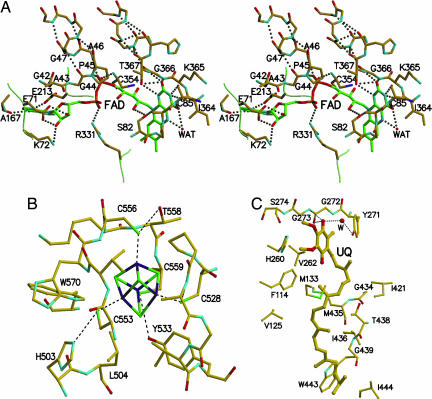
FAD has an extended conformation and is buried completely in the protein (Fig. 1). It is positioned at the carboxyl side of the parallel β-sheet 2 and the C termini of α1 and α6 helices (Fig. 3A). The C7 and C8 methyl groups of the isoalloxazine ring make van der Waal's contacts with the main-chain N atom of R331 in the plane of β-sheet 3. As in other flavoproteins, the pyrimidine side of the isoalloxazine ring is hydrogen-bonded to the polypeptide; O2 forms hydrogen bonds with the main-chain nitrogens of G366 and T367 and the hydroxyl of T367, continuing the hydrogen-bonding pattern of the α6-helix (Fig. 3A). The interaction of the isoalloxazine ring and the α6-helix is strengthened further by hydrogen bonds between the hydroxyl of T367 and the O2 and N1 atoms of the isoalloxazine ring. Other hydrogen-bonding interactions occur between the O4 and C85 N atoms, N3 and C85 carbonyl oxygen atoms. In addition to these hydrogen bonds, the positive dipole of the helix could modulate the redox potential of FAD and stabilize the anionic semiquinone. This helix dipole–flavin interaction is present in the p-hydroxybenzoate hydroxylase (24) and glutathione reductase class of flavoproteins (29) and quinol-fumarate reductase (13, 14). A water molecule is located on the same plane as the flavin ring and is hydrogen-bonded to both the N5 and O2 atoms; however, its functional role is not clear at present.
Fig. 3.
Residues in the vicinity of the redox centers. (A) Stereo diagram of the FAD-binding site. The isoalloxazine ring is located at the N terminus of helix α6. The phosphate moiety is located at the N terminus of α1-helix of the βαβ Rossmann fold of the protein. Color codes for atoms are oxygen (red), nitrogen (blue), sulfur (purple), phosphorus (brown), protein carbon (yellow), and FAD carbon (green). Hydrogen bonds are shown as dotted lines. (B) The 4Fe4S cluster-binding residues. Four cysteine residues (528, 553, 556, and 559) coordinate Fe atoms in the cluster (large dotted lines). They also make hydrogen bonds to the polypeptide chain (dotted lines). Color codes for atoms are the same as in A, except that sulfur is in green and iron is in purple. (C) The UQ-binding site. The O4 atom of the UQ ring is hydrogen-bonded to the main-chain atoms of G272 and G273. A water molecule makes hydrogen bonds to O4 and the hydroxyl of Y271. All other interactions involving UQ are of hydrophobic contacts. Color codes are the same as in B.
Strands β1, β2 of the β-sheet 2, and helix α1 form the βαβ dinucleotide-binding motif. Residues G42–G47 contain the ADP-binding sequence motif (GXGXXG), form the N terminus of helix α1, and hydrogen-bond to the pyrophosphate moiety of FAD (Fig. 3A). Like other FAD-containing proteins, the pyrophosphate moiety is neutralized by R331. The hydroxyl of S82 makes a hydrogen bond to 2′-OH of the ribityl chain of FAD and is located beneath the center of the isoalloxazine ring on the si-side with an atom-plane distance of 3.0 Å. Thus, it is possible that S82 acts as a hydrogen-bond donor and the flavin ring as the acceptor, as observed in some aromatic ring compounds (30). A similar interaction has been observed in cholesterol oxidase, in which the amide nitrogen of N485 makes a N-H…π interaction with the pyrimidine side of the FAD ring and modulates the redox potential of the oxidase (31). Thus, the hydrogen bond between S82 and the flavin ring in ETF-QO also may influence the redox potential of the bound FAD. Three residues, R331, T367 and S82, are highly conserved in the ETF-QO sequences (see the supporting information).
The 4Fe4S Cluster Environment.
The iron-sulfur cluster in ETF-QO is embedded in two loops that contain residues C528–Y533 and C553–D560. As predicted from the sequence analysis, C528, C553, C556, and C559 from the two loops coordinate the four Fe atoms in the cluster (21). Residues H503, L504, and W570 complete the binding pocket formed by the two loops (Fig. 3B). The cluster is supported further through hydrogen bonds between the Sγ atoms of the four cysteines and the polypeptide chain; C553 makes hydrogen bonds with H503, C556 bonds with the hydroxyl of T558, C559 bonds with the phenolic oxygen of Y533 and main-chain N of D560, and C528 bonds with the backbone nitrogen of A530. Two of the four cluster sulfur atoms make weak hydrogen bonds, each with the main-chain N atom of K557 or C559 (both distances, 3.4 Å). Such hydrogen bonds can modulate the redox potential of iron-sulfur clusters (32, 33). The relatively positive potential of the ETF-QO cluster, +47 mV, reflects the extensive hydrogen bonding of the cysteinyl sulfur and sulfur atoms in the cluster.
UQ Binding.
UQ and the flavin isoalloxazine ring are located on the same side of β-sheet 3. The UQ-binding pocket is made mainly of hydrophobic residues (Fig. 3C). Only one of the two carbonyl oxygen atoms in the benzoquinone ring is hydrogen-bonded to the polypeptide chain. The O4 atom of UQ makes hydrogen bonds to the backbone nitrogen of G273 and carbonyl oxygen of G272. The rest of the molecule is surrounded by mostly hydrophobic residues making van der Waal's contacts (Fig. 3C). The phenolic ring of Y271, followed by residues in β11 (G272, G273, and S274), wraps around the C5 methyl, O4 carbonyl, and C3 methoxy groups; F114, H260, and V262 contacts the O1 carbonyl and C2 methoxy groups. The isoprene tail is bent such that the second isoprene unit contacts the O1 carbonyl group. There is a water molecule that hydrogen-bonds the hydroxyl of Y271 and O4 of the benzoquinone ring. This water may act as the proton donor/acceptor during the UQ redox cycle. Residues Y271, G272, G273, S274, and H260 are highly conserved among ETF-QOs from different species (Fig. 5). In particular, G273 is absolutely conserved in all ETF-QO sequences. The absence of a bulky side chain at position G273 eliminates steric hindrance so that the UQ molecule penetrates deep into its hydrophobic binding pocket. If electron transfer to UQ involves ubisemiquinone as a transient intermediate, the semiquinone is protected from reaction with molecular oxygen. Only 5 isoprene units of the UQ tail (10 isoprene units for mammalian UQ) are visible. There is a hint of disorder even at the third isoprene unit in one of the two ETF-QO molecules in the asymmetric unit, indicating that the tail is flexible (Fig. 2). The second through the fifth isoprene units of the tail are surrounded by mostly hydrophobic residues, including F114, V125, G434, M435, T438, and G439 (Fig. 3C). Thus, the binding mode of UQ observed in ETF-QO is different from those observed in other UQ-binding proteins, e.g., succinate-UQ oxidoreductase (34) and ubiquinol oxidase (35). The binding motif found in other proteins of the respiratory and photosynthetic systems has semiconserved sequences containing a Tyr/Trp or His that make direct hydrogen bonds to O1 and/or O4 of the benzoquinone group (20).
Membrane-Binding Surface.
By the usual criteria, porcine ETF-QO is classified as an integral membrane protein (36), requiring detergent to solubilize the protein. In contrast to UQ-binding proteins of the main respiratory and photosynthetic systems, the ETF-QO polypeptide does not traverse the entire membrane. In ETF-QO, two highly hydrophobic peptide segments, F114–L131 (β3a–β4) and G427–W451 (α9-helix), are located at the surface of the molecule and surround the UQ polyisoprene chain (Fig. 1). These segments form the entrance of the UQ-binding pocket and likely form the membrane-binding surface (Figs. 1 and 4). In addition, there are three detergent molecules surrounding molecule A and two surrounding molecule B, with one molecule shared between A and B. Furthermore, these two polypeptide segments are located near the local twofold axis and are surrounded by the same hydrophobic segments of the neighboring molecule. The electrostatic potential map generated by GRASP (37) clearly shows the hydrophobic surface around the UQ isoprene tail (Fig. 4). The α9-helix interacts with the membrane with its helical axis approximately parallel to the membrane surface; thus, together with the β-hairpin (β3a–β4), forming a “hydrophobic plateau” with an approximate size 25 Å × 30 Å, similar to the ones observed in other monotopic membrane proteins (36), including prostaglandin-H synthase (38) and squalene-hopene cyclase (39). The positively charged basic residues near the membrane-associated residues probably play a role in interacting with the phospholipid head groups. These residues include R113, R268, H269, H313, R423, and K454. Assuming these residues interact with the phospholipid head groups, the benzoquinone head group is penetrating the ETF-QO molecule ≈8 Å into the matrix side of the mitochondria (Fig. 1).
Fig. 4.
Electrostatic potential surface of ETF-QO viewed from the membrane side. Entrance to the UQ-binding site (dashed circle) and the UQ polyisoprene tail (green sticks) are shown. The surrounding positively charged groups (blue patches) probably are involved in interacting with the negatively charged membrane phospholipid heads. The size of the entrance (dashed circle) is ≈10 Å × 6 Å and that of the hydrophobic plateau (blue parallelogram) is ≈24 Å × 30 Å. Color codes are blue for positive (+8 kT), white for neutral, and red for negative (−8 kT).
Electron Transfer Pathway.
It is clear that ETF-QO catalyzes the reduction of UQ by ETF (1), but details of the reaction remain uncertain. ETF semiquinone (ETF1e−) is the product of the oxidative half-reaction of the acyl-CoA dehydrogenases; however, it is not possible to monitor reduction of UQ by ETF1e− in vitro because ETF1e− directly reduces the water-soluble UQ, Q1, with a second-order rate constant of ≈1,300 M−1 s−1 (9). UQ is compartmentalized in the membrane phase, which precludes the ETF-QO-independent reduction of UQ. ETF1e− can serve as the direct reductant of ETF-QO, because ETF-QO catalyzes the intermolecular oxidation-reduction of ETF1e− in a novel disproportionation of ETF1e− that is kinetically competent to participate in the overall electron transfer from an acyl-CoA dehydrogenase to a water-soluble UQ analog, Q1 (9). Physiological disproportionation of ETF1e− would effectively increase the driving force for the reduction of ETF-QO, because the potential of the hydroqinone/semiquinone couple is ≈50 mV more negative than that of the semiquinone/oxidized couple (40). On the other hand, when the reaction is run in the opposite direction (i.e., when ETF is reduced anaerobically by NADH via ETF-QO in submitochondrial particles in the presence of an inhibitor of the bc1 complex), the anionic ETF1e− is generated (41). The principle of microscopic reversibility suggests that ETF1e− reduces ETF-QO and that the disproportionation reaction may be an artifact of the soluble system.
The redox potentials of ETF-QO flavin are +28 mV for transfer of the first electron and −6 mV for the second electron. The potential of the 4Fe4S cluster is +47 mV (42). The redox potentials of the ETF flavin are +4 mV for oxidized/semiquinone and −50 mV for semiquinone/hydroquinone (40). Thermodynamic considerations and the fact that ETF in vivo utilizes only the oxidized/semiquinone couple prompted Paulsen et al. (42) to propose a model for electron transfer from ETF to ETF-QO to UQ: (i) the ETF1e− reduces ETF-QO one electron at a time, first to the FAD of ETF-QO, then from FAD to the cluster, forming the two-electron reduced state, i.e., FAD semiquinone and reduced iron-sulfur cluster, and (ii) both of these electrons transfer to UQ through the cluster in one-electron transfer steps to form ubiquinol (FAD → 4Fe4S → UQ). Because the cluster is an obligatory one electron donor/acceptor, the ubisemiquinone molecule must be formed, at least transiently, during catalysis. Another line of evidence suggesting that electrons enter ETF-QO from ETF at the flavin site and that the cluster is the electron donor to UQ comes from mutagenesis of C528 in human ETF-QO. Substitution of an alanine abolishes quinone reductase activity but retains the disproportionation activity (6). However, the mutant protein was expressed poorly in Saccharomyces cerevisiae, and the effects of the mutation on the cluster were not determined other than those inferred from the specific activities of ETF-QO in the assays using crude detergent-extracts of yeast mitochondria. A more quantitative experiment with purified protein is required to confirm the results of the mutation studies.
The structure of ETF-QO is not consistent with this model. The structure strongly suggests that the reductant of UQ is the flavin, not the 4Fe4S cluster. The Fe3 atom of the cluster is 11.5-Å from C8 of the isoalloxazine ring (Fig. 2). The two redox centers are separated by the backbone atoms of R331 and C556, the latter of which coordinates the cluster (Fig. 3 A and B). The shortest distance from C8 of FAD to the Sγ of C556 is ≈9.4 Å. The carbonyl oxygen of C556 is within hydrogen-bonding distance of the backbone nitrogen atom of R331, and this hydrogen bond may electronically couple the flavin and cluster (43). The distance between the cluster and UQ, measured between O2 of UQ and Fe3 of the cluster, is 18.8 Å (Fig. 2), and it is ≈16.7 Å to the cluster Sγ of C556. These cluster—UQ distances are significantly longer than the 14-Å distance over which efficient electron transfer by electron tunneling occurs (19). The long distance from the cluster to UQ makes intraprotein electron transfer from the flavin to the cluster and then to UQ problematic. Three possibilities could explain this apparent problem. (i) A conformational change could bring the cluster closer to the UQ ring. However, the three functional domains of ETF-QO are closely packed, and individual helices and strands are shared among the three domains. Thus, it is unlikely that conformational changes can occur that will significantly decrease the cluster—UQ distance. (ii) The protein could form a dimer as seen in Complex II and related enzymes, such that the 4Fe4S cluster of one monomer could be closer to UQ of the other monomer for an efficient electron transfer. However, both current crystal structure analysis and the electrophoretic studies of ETF-QO (44) suggest that the protein is monomeric. (iii) It is possible that there is a second UQ site. However, the following observations argue against this assertion. First, additional UQ cannot be soaked into the crystals, and cocrystallization with additional UQ did not reveal a second site. Second, when the UQ-free protein was titrated with bromodecyl-UQ or other quinone analogs, only a single site was detected (45, 46). Therefore, it is unlikely that the electron transfer to the quinone is from the cluster. On the other hand, the flavin ring and the benzoquinone ring are in close proximity. The distance between C6 of FAD and O3 of UQ is 8.5 Å (C8 of flavin to O2 of UQ is 9.9 Å, still much shorter than the distance from the cluster to UQ) (Fig. 3). Furthermore, the two redox centers (FAD and UQ) are at the same side of β-sheet 3, supporting the assumption that the flavin is the reductant of UQ, not the cluster. Then, two electron transfer pathways can be envisioned (Scheme 1).
Scheme 1.
Possible electron transfer paths between ETF and ETF-QO and within ETF-QO. Electrons from ETF1e enter ETF-QO via step 1 (path 1) or 1a (path 2) and are rapidly equilibrated between the two cofactors (step 2). When ETF-QO is in two-electron reduced state (one electron at each cofactor), both electrons are transferred to UQ via FAD (step 3).
The first possible pathway is that the flavin acts both as the electron acceptor from ETF1e− and the donor to UQ (steps 1 and 3 in Scheme 1). Then what is the role of the cluster? When ETF-QO is reduced by ETF, the ETF-QO flavin is reduced to semiquinone, and the cluster also is reduced (1, 8), indicating that the cluster is not only structural but also is involved in the redox reaction. This finding indicates that an incoming electron from ETF rapidly equilibrates between the two redox centers, FAD and the cluster (step 2). When the second electron is introduced, both the flavin and the cluster are in a one-electron reduced state, i.e., flavin semiquinone and [4Fe4S]1+. The enzyme is now primed for two-electron reduction of UQ to ubiquinol, one from the flavin and the other from the cluster via flavin (step 3). Thus, the cluster in ETF-QO has a redox-poising effect on the flavin (or electron storage), as in NADH-UQ oxidoreductase (Complex I). In the structure of the soluble domain of Complex I from Thermus thermophilus, the FeS cluster, N1a, is situated away from the main cluster chain and functions as an electron storage for FMN (47).
A second possibility is that the cluster is the entry point of electrons from ETF (step 1a in Scheme 1). The ETF-QO flavin potential is only 19 mV lower than that of the cluster, and there is no reason to exclude the possibility that ETF binding to ETF-QO could lower the potential of the cluster to make the two centers near isopotential, as seen in the heterodimeric periplasmic nitrate reductase. In the reductase, the potentials of the 4Fe4S cluster and heme II of the NapA subunit increase by 180 mV and 40 mV, respectively, upon binding the NapB subunit, making the heme and cluster almost isopotential (48). In addition, the ETF-QO cluster is located closer to the surface of the ETF-QO molecule than the flavin is (≈8 Å vs. >14 Å), suggesting that electron transfer from ETF flavin to the ETF-QO cluster would be more favorable. Once an electron enters the cluster, it can shuttle rapidly to the flavin, because the two redox centers are almost isopotential. The rest of the pathway is the same as the above (steps 2 and 3 in Scheme 1). If we consider other UQ-binding proteins, such as respiratory Complexes I and II, in which flavin is the electron acceptor from NADH and succinate, respectively, we may think that in ETF-QO, FAD is the entrance for electrons from ETF. However, the electron donor of ETF-QO is another protein (i.e., ETF) and is a one-electron donor as opposed to the two-electron donors, NADH and succinate in Complexes I and II, respectively. Thus, the cluster, an obligatory one-electron acceptor, could well be the electron entrance point to ETF-QO. Further experiments are required to distinguish between these two possibilities.
The relative simplicity of the ETF-QO structure permits a new viewpoint for considering the interaction of mitochondrial UQ oxidoreductases with the mitochondrial UQ pool. ETF-QO catalyzes the transfer of electrons from redox systems in the mitochondrial matrix to UQ, the mobile electron carrier in the membrane phase, and apparently does so without transmembrane segments. Thus, the structure of ETF-QO could be a paradigm for understanding how other similar proteins, such as glycerol-3-phosphate dehydrogenase and dihydroorotate dehydrogenase, which access the UQ pool from the cytosolic and matrix sides of the inner mitochondrial membrane, respectively, interact with the UQ pool. Also, ETF-QO provides yet another attractive model for understanding how lipid substrates access the active sites of monotopic membrane proteins (36).
Methods
Crystallization and Data Collection.
ETF-QO was purified by the procedure of Watmough et al. (45) which involves Triton X-100 extraction of porcine liver submitochondrial particles. Alternatively, the protein was extracted with 40 mM N,N-dimethylamine-lauryl N-oxide (LDAO) in the same buffer. In the latter protocol, all other steps were identical to those described by Watmough et al. There was no difference in activity of the protein prepared by the two methods; however, the LDAO-solubilized protein fortuitously contained UQ.
Purified ETF-QO solubilized with N,N-dimethylamine-lauryl N-oxide (LDAO) was concentrated to 15 mg/ml in 20 mM Tris·HCl (pH 8.5) and was crystallized by the hanging drop-vapor diffusion method at 4°C. Hanging drops were made by mixing 2.0 μl each of the protein and reservoir solution [14.5% polyethylene glycol 2000 monomethyl ether (PEG2KMME)/0.5 M NaCl/0.1 M Tris, pH 8.0/10% ethylene glycol] and 0.35 μl of 6.6 mM β-hexyl-d-glucopyranoside (HBG). All data sets were collected at 100 K, by using a solution containing 17% PEG2KMME and 20% ethylene glycol as cryoprotectant. Crystals belong to the space group P4212 (a = b = 154.3 Å, c = 128.5 Å, with two molecules per asymmetric unit). A 2.5-Å native data set and heavy-atom derivative data sets were collected at 1.000 Å by using the BioCars BM-14C beamline at the Advanced Photon Source (Argonne National Laboratory, Argonne, IL). Native multiple-wavelength anomalous dispersion (MAD) data sets were collected at BM-14D at Advanced Photon Source at 1.7389 Å (peak), 1.7426 Å (edge), and 1.6000 Å (remote). Data sets were processed with DENZO/SCALEPACK (49).
The UQ-free protein crystals were obtained in a similar manner by mixing a 1:1 ratio of the protein solution (17 mg/ml in 20 mM Hepes, pH 7.5/0.2% β-octyl-d-glucopyranoside) and reservoir solution [12% (vol/vol) tertiary butanol/3.5% PEG 400/0.1 M CaCl2/0.1 M Hepes, pH 7.5]. Data were collected at 4°C with a Rigaku RU200 and an R-AXIS IIc image plate (Rigaku MSC, Woodland, TX). The crystals diffracted to 2.7 Å. The UQ-free ETF-QO crystals also belong to the P4212 space group with cell dimensions a = b = 154.8 Å and c = 130.2 Å.
Structure Determination and Refinement.
The structure of ETF-QO containing UQ was solved by MIRAS (multiple isomorphous replacement with anomalous scattering) methods combined with multiple-wavelength anomalous dispersion phasing (MAD) (50). The native anomalous difference Patterson maps were generated by Xtalview (51) by using data collected at the peak wavelength (1.7389 Å). The strong peaks at the Harker sections clearly showed two iron-sulfur clusters per asymmetric unit and also confirmed the space group to be P4212. All heavy-atom refinements, phasing, electron density modification by solvent flattening, and noncrystallographic twofold averaging were done with PHASES (52). The initial experimental map permitted building a polyalanine model with TURBO-FRODO (53). The Sigma-A weighted map (54) calculated by using the MIRAS/MAD phases combined with the phases calculated from the initial model showed significant improvement and allowed the assignment of amino acid residues, FAD, and UQ. The structure of UQ-free ETF-QO was solved by difference Fourier techniques using the UQ-containing structure as the starting model. The structure refinements were done by using CNS (55) alternating with manual adjustments using TURBO-FRODO. Data collection and phasing statistics are given in Tables 1 and 2 and the refinement statistics are shown in Table 3, which are published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
This article is dedicated to Helmut Beinert and Frank Ruzicka for their seminal work on this important enzyme (1) and for Beinert's continuing contributions to the field of Fe-S chemistry. We thank the staff of the BioCARS at the Advanced Photon Source for assistance with data collection. This work was supported by National Institutes of Health Grants GM29076 (to J.-J.P.K.) and HD08315 (to F.E.F.). Use of the Advanced Photon Source is supported by the U.S. Department of Energy.
Abbreviations
- ETF
electron transfer flavoprotein
- ETF-QO
ETF-ubiquinone oxidoreductase
- UQ
ubiquinone
- ETF1e−
ETF semiquinone.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org [PDB ID codes 2GMH (UQ-bound structure) and 2GMJ (UQ-free structure)].
References
- 1.Ruzicka FJ, Beinert H. J Biol Chem. 1977;252:8440–8445. [PubMed] [Google Scholar]
- 2.Kim JJ, Miura R. Eur J Biochem. 2004;271:483–493. doi: 10.1046/j.1432-1033.2003.03948.x. [DOI] [PubMed] [Google Scholar]
- 3.Ghisla S, Thorpe C. Eur J Biochem. 2004;271:494–508. doi: 10.1046/j.1432-1033.2003.03946.x. [DOI] [PubMed] [Google Scholar]
- 4.Lenaz G. FEBS Lett. 2001;509:151–155. doi: 10.1016/s0014-5793(01)03172-6. [DOI] [PubMed] [Google Scholar]
- 5.Goodman SI, Binard RJ, Woontner MR, Frerman FE. Mol Genet Metab. 2002;77:86–90. doi: 10.1016/s1096-7192(02)00138-5. [DOI] [PubMed] [Google Scholar]
- 6.Beard SE, Goodman SI, Bemelen K, Frerman FE. Hum Mol Genet. 1995;4:157–161. doi: 10.1093/hmg/4.2.157. [DOI] [PubMed] [Google Scholar]
- 7.Gorelick RJ, Thorpe C. Biochemistry. 1986;25:7092–7098. doi: 10.1021/bi00370a050. [DOI] [PubMed] [Google Scholar]
- 8.Beckmann JD, Frerman FE. Biochemistry. 1985;24:3922–3925. doi: 10.1021/bi00336a017. [DOI] [PubMed] [Google Scholar]
- 9.Ramsay RR, Steenkamp DJ, Husain M. Biochem J. 1987;241:883–892. doi: 10.1042/bj2410883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z. Cell. 2005;121:1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 11.Yankovskaya V, Horsefield R, Tornroth S, Luna-Chavez C, Miyoshi H, Leger C, Byrne B, Cecchini G, Iwata S. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- 12.Huang LS, Sun G, Cobessi D, Wang AC, Shen JT, Tung EY, Anderson VE, Berry EA. J Biol Chem. 2006;281:5965–5972. doi: 10.1074/jbc.M511270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iverson TM, Luna-Chavez C, Cecchini G, Rees DC. Science. 1999;284:1961–1966. doi: 10.1126/science.284.5422.1961. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster CR, Kroger A, Auer M, Michel H. Nature. 1999;402:377–385. doi: 10.1038/46483. [DOI] [PubMed] [Google Scholar]
- 15.Liu S, Neidhardt EA, Grossman TH, Ocain T, Clardy J. Structure (London) 2000;8:25–33. doi: 10.1016/s0969-2126(00)00077-0. [DOI] [PubMed] [Google Scholar]
- 16.Xia D, Yu CA, Kim H, Xia JZ, Kachurin AM, Zhang L, Yu L, Deisenhofer J. Science. 1997;277:60–66. doi: 10.1126/science.277.5322.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Huang L, Shulmeister VM, Chi YI, Kim KK, Hung LW, Crofts AR, Berry EA, Kim SH. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 18.Iwata S, Lee JW, Okada K, Lee JK, Iwata M, Rasmussen B, Link TA, Ramaswamy S, Jap BK. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 19.Page CC, Moser CC, Chen X, Dutton PL. Nature. 1999;402:47–52. doi: 10.1038/46972. [DOI] [PubMed] [Google Scholar]
- 20.Fisher N, Rich PR. J Mol Biol. 2000;296:1153–1162. doi: 10.1006/jmbi.2000.3509. [DOI] [PubMed] [Google Scholar]
- 21.Goodman SI, Axtell KM, Bindoff LA, Beard SE, Gill RE, Frerman FE. Eur J Biochem. 1994;219:277–286. doi: 10.1111/j.1432-1033.1994.tb19939.x. [DOI] [PubMed] [Google Scholar]
- 22.Holm L, Sander C. J Mol Biol. 1993;233:123–138. doi: 10.1006/jmbi.1993.1489. [DOI] [PubMed] [Google Scholar]
- 23.Dauter Z, Wilson KS, Sieker LC, Meyer J, Moulis JM. Biochemistry. 1997;36:16065–16073. doi: 10.1021/bi972155y. [DOI] [PubMed] [Google Scholar]
- 24.Schreuder HA, van der Laan JM, Swarte MB, Kalk KH, Hol WG, Drenth J. Proteins. 1992;14:178–190. doi: 10.1002/prot.340140205. [DOI] [PubMed] [Google Scholar]
- 25.Bamford V, Dobbin PS, Richardson DJ, Hemmings AM. Nat Struct Biol. 1999;6:1104–1107. doi: 10.1038/70039. [DOI] [PubMed] [Google Scholar]
- 26.Taylor P, Pealing SL, Reid GA, Chapman SK, Walkinshaw MD. Nat Struct Biol. 1999;6:1108–1112. doi: 10.1038/70045. [DOI] [PubMed] [Google Scholar]
- 27.Leys D, Tsapin AS, Nealson KH, Meyer TE, Cusanovich MA, Van Beeumen JJ. Nat Struct Biol. 1999;6:1113–1117. doi: 10.1038/70051. [DOI] [PubMed] [Google Scholar]
- 28.Iverson TM, Luna-Chavez C, Schroder I, Cecchini G, Rees DC. Curr Opin Struct Biol. 2000;10:448–455. doi: 10.1016/s0959-440x(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 29.Karplus PA, Schulz GE. J Mol Biol. 1989;210:163–180. doi: 10.1016/0022-2836(89)90298-2. [DOI] [PubMed] [Google Scholar]
- 30.Perutz M. Philos Trans R Soc London A. 1993;345:105–112. [Google Scholar]
- 31.Yin Y, Sampson NS, Vrielink A, Lario PI. Biochemistry. 2001;40:13779–13787. doi: 10.1021/bi010843i. [DOI] [PubMed] [Google Scholar]
- 32.Stephens PJ, Jollie DR, Warshel A. Chem Rev. 1996;96:2491–2514. doi: 10.1021/cr950045w. [DOI] [PubMed] [Google Scholar]
- 33.Denke E, Merbitz-Zahradnik T, Hatzfeld OM, Snyder CH, Link TA, Trumpower BL. J Biol Chem. 1998;273:9085–9093. doi: 10.1074/jbc.273.15.9085. [DOI] [PubMed] [Google Scholar]
- 34.Horsefield R, Yankovskaya V, Sexton G, Whittingham W, Shiomi K, Omura S, Byrne B, Cecchini G, Iwata S. J Biol Chem. 2006;281:7309–7316. doi: 10.1074/jbc.M508173200. [DOI] [PubMed] [Google Scholar]
- 35.Abramson J, Riistama S, Larsson G, Jasaitis A, Svensson-Ek M, Laakkonen L, Puustinen A, Iwata S, Wikstrom M. Nat Struct Biol. 2000;7:910–917. doi: 10.1038/82824. [DOI] [PubMed] [Google Scholar]
- 36.Bracey MH, Cravatt BF, Stevens RC. FEBS Lett. 2004;567:159–165. doi: 10.1016/j.febslet.2004.04.084. [DOI] [PubMed] [Google Scholar]
- 37.Nichols A. GRASP. New York, NY: Columbia University; 1992. [Google Scholar]
- 38.Picot D, Loll PJ, Garavito RM. Nature. 1994;367:243–249. doi: 10.1038/367243a0. [DOI] [PubMed] [Google Scholar]
- 39.Wendt KU, Lenhart A, Schulz GE. J Mol Biol. 1999;286:175–187. doi: 10.1006/jmbi.1998.2470. [DOI] [PubMed] [Google Scholar]
- 40.Husain M, Stankovich MT, Fox BG. Biochem J. 1984;219:1043–1047. doi: 10.1042/bj2191043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frerman FE. Biochim Biophys Acta. 1987;893:161–169. doi: 10.1016/0005-2728(87)90035-1. [DOI] [PubMed] [Google Scholar]
- 42.Paulsen KE, Orville AM, Frerman FE, Lipscomb JD, Stankovich MT. Biochemistry. 1992;31:11755–11761. doi: 10.1021/bi00162a012. [DOI] [PubMed] [Google Scholar]
- 43.de Rege PJ, Williams SA, Therien MJ. Science. 1995;269:1409–1413. doi: 10.1126/science.7660123. [DOI] [PubMed] [Google Scholar]
- 44.Simkovic M, Degala GD, Eaton SS, Frerman FE. Biochem J. 2002;364:659–667. doi: 10.1042/BJ20020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watmough NJ, Loehr JP, Drake SK, Frerman FE. Biochemistry. 1991;30:1317–1323. doi: 10.1021/bi00219a023. [DOI] [PubMed] [Google Scholar]
- 46.Simkovic M, Frerman FE. Biochem J. 2004;378:633–640. doi: 10.1042/BJ20031272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sazanov LA, Hinchliffe P. Science. 2006;311:1430–1436. doi: 10.1126/science.1123809. [DOI] [PubMed] [Google Scholar]
- 48.Arnoux P, Sabaty M, Alric J, Frangioni B, Guigliarelli B, Adriano JM, Pignol D. Nat Struct Biol. 2003;10:928–934. doi: 10.1038/nsb994. [DOI] [PubMed] [Google Scholar]
- 49.Otwinowski Z, Minor W. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 50.Hendrickson WA. Science. 1991;254:51–58. doi: 10.1126/science.1925561. [DOI] [PubMed] [Google Scholar]
- 51.McRee DE. Practical Protein Crystallography. 2nd Ed. San Diego: Academic; 1999. [Google Scholar]
- 52.Furey W, Swaminathan S. Methods Enzymol. 1997;277:590–620. doi: 10.1016/s0076-6879(97)77033-2. [DOI] [PubMed] [Google Scholar]
- 53.Roussel A, Inisan AG, Knoop-Mouthuy A, Cambillau C. TURBO-FRODO. Marseille, France: Centre National de la Recherche Scientifique/Universitite Marseille; 1999. [Google Scholar]
- 54.Read RJ. Acta Crystallogr A. 1986;42:140–149. [Google Scholar]
- 55.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. Acta Crystallogr D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.