Abstract
It has long been recognized that male mating competition is responsible for the evolution of weaponry for mate acquisition. However, when females mate with more than one male, competition between males can continue after mating in the form of sperm competition. Theory predicts that males should increase their investment in sperm production as sperm competition is increased, but it assumes that males face a trade-off between sperm production and other life-history traits such as mate acquisition. Here, we use a genus of horned beetle, Onthophagus, to examine the trade-off between investment in testes required for fertilizations and investment in weapons used to obtain matings. In a within-species study, we prevented males from developing horns and found that these males grew larger and invested relatively more in testes growth than did males allowed to grow horns. Among species, there was no general relationship between the relative sizes of horns and testes. However, the allometric slope of horn size on body size was negatively associated with the allometric slope of testes size on body size. We suggest that this reflects meaningful evolutionary changes in the developmental mechanisms regulating trait growth, specifically in the degree of nutrition-dependent phenotypic plasticity versus canalization of traits. Finally, we show how this resource allocation trade-off has influenced the evolutionary diversification of weapons, revealing a rich interplay between developmental trade-offs and both pre- and postmating mechanisms of sexual competition.
Keywords: secondary sexual traits, sperm competition, testes size, beetle horns
Competition among males for mating opportunities has long been recognized as a potent selective pressure shaping the evolution of exaggerated secondary sexual weapons, such as deer antlers and beetle horns (1). However, when females mate with more than one male, competition between males can continue after copulation so that sperm from different males must compete to fertilize eggs (2). Theory predicts that sperm competition should favor increased male allocation to sperm production (3), and there is accumulating empirical support for the basic prediction of this theory, that sperm competition can favor increased male expenditure on sperm production. Thus, among species from a broad range of taxa, positive evolutionary associations have been found between male investment in gametogenic tissue (testes size) and estimates of the strength of selection generated by sperm competition (4–8).
Sperm competition theory rests on the fundamental assumption that males have a limited resource pool with which to invest in reproduction and that they must trade off resources between gaining matings and fertilizations (3). This underlying assumption of a trade-off between investment in testes and investment in other life-history traits has received less attention from empiricists than have the predicted outcomes of sperm competition theory. Nonetheless, there is some evidence to suggest that energetically expensive traits such as immunity can trade off against testis size and ejaculate quality (9, 10), and across species of fruit flies in the genus Drosophila, species developing larger testes require longer periods of sexual maturation (11, 12). Finally, a recent comparative study of bats revealed a trade-off between brain size and testes size, two metabolically expensive organs (13).
But do males face a trade-off between investments in competing for fertilizations and competing for access to mates? Studies of species in which males follow alternative life histories that are associated with alternative mating tactics suggest that they might. For example in salmon, anadromic males invest heavily in body growth and in weapons for competing for spawning females. In contrast parr mature at a small body size and invest more in their testes, producing sperm with greater levels of ATP activity, motility, and fertilization capacity (14, 15).
Beetles in the genus Onthophagus are notable for the size and diversity of their horns. Horns can comprise a significant proportion of male body size and can project from any of five locations on the head or thorax. Horns can be expressed in both sexes but in most species are expressed in males only (sexual dimorphism) or in just the largest males (male dimorphism) (16). Males use their horns to block the entrance to breeding tunnels containing females, and males with larger horns are competitively superior (17, 18) and attain a higher reproductive success than their short-horned conspecifics (19). The horns of onthophagines thereby represent typical sexually selected weapons that increase the number of matings obtained.
Sperm competition can also be an important selective pressure in onthophagine mating systems. In male dimorphic species, minor or hornless males adopt the alternative mating tactic of sneaking copulations with females that are guarded by major or horned males (17, 18, 20). In the species that have been studied, a male's fertilization success depends on the amount of sperm inseminated relative to other males (21, 22), an assumption made in sperm competition game theory (3), and the proportion of sneak males in a population is associated with both the relative sizes of the testes, and the numbers of sperm produced (23), suggesting that sperm competition favors large testes size in these beetles. Thus, onthophagine beetles appear to have experienced a history of selection for significant investment into both horns and testes and for this reason are ideal for testing for a developmental allocation trade-off between these traits.
Production of horns is known to come at the expense of the development of other morphological structures (24). Within natural populations of onthophagine beetles, the relative sizes of horns are often negatively correlated with the relative sizes of other traits including eyes, wings, and antennae (24), and within-species perturbation experiments have confirmed that altering allocation to one trait can impact growth of another [e.g., artificial selection for increased relative horn size reduced relative eye size (25), and ablation of genital capsules in larvae resulted in males developing horns at smaller body sizes compared with intact males (26)].
Here we use an experimental manipulation of horn development to provide evidence that male Onthophagus nigriventris trade-off investment in the weapons used to obtain matings for investment in testes required for fertilizations. We then conduct a comparative analysis across 25 species of Onthophagus to determine whether this resource allocation trade-off has influenced the evolutionary diversification of weapons in this group of beetles. Our data reveal a rich interplay between developmental trade-offs and both pre- and postmating mechanisms of sexual competition.
Results
Experimental Manipulation of Horn Development.
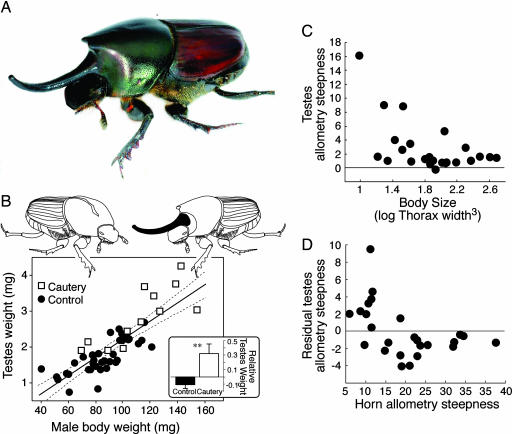
Male O. nigriventris produce a pair of thoracic horns, one of which can reach up to 40% of the male's total body length (Fig. 1A). We experimentally engineered males not to invest in horn growth by cauterizing in late instar larvae the area of proliferating cells that would otherwise become the principal thoracic horn. Cauterized beetles grew larger (112.4 ± 7.0 mg) than uncauterized beetles (87.3 ± 3.9 mg) (t47 = 2.93, P = 0.005), and they failed to produce a thoracic horn (Fig. 1B). An ANCOVA with treatment (cauterized versus control) as the main effect and log body weight as the covariate explained 71% of the variance in log testes weight (F2,45 = 55.53, P < 0.0001). The interaction between the covariate and main effect was not significant (F1,44 = 0.15, P = 0.704) indicating homogeneity of slopes, and was removed from the model before evaluating the independent partial effect of treatment (27). Testes weight increased with body weight (F1,45 = 12.98, P = 0.0008; common slope 0.89 ± 0.14), but cauterized males developed disproportionately larger testes than did control males (F1,45 = 52.84, P < 0.0001) (Fig. 1B).
Fig. 1.
Evidence for an allocation trade-off between weapons (horns) and testes in the beetle genus Onthophagus (Coleoptera: Scarabaeidae). (A) Within the species O. nigriventris, large males produce two horns on the thorax. (B) Males engineered to be hornless (□) eclosed into larger adults with testes that were disproportionately large compared with control males that developed horns (●). The common regression line is shown with its 95% confidence limits. (B Inset) The residual testes weights for cauterized and control beetles after controlling for body weight. (C and D) In a comparative analyses of 25 congeneric species, the steepness of the allometric slope of log testes weight on log body weight declined across species as both body size (C) and the steepness of the scaling relationship between horn length and body size (D) increased. Photograph by O. Helmy.
Comparative Analyses.
We looked for evidence of an evolutionary trade-off between investment in horns and testes across 25 species of Onthophagus (Table 1, which is published as supporting information on the PNAS web site). If the phenotypic trade-off found in O. nigriventris has affected long term patterns of either horn or testes evolution, then we might expect species in which males invest heavily in horn development for mate acquisition to have a lower investment in testes growth.
We first used a general linear model to test whether testes weight was negatively correlated with horn size, including species mean body size as a covariate in the analysis (all variables were log transformed for analysis). The full model explained 92% of the variation in testes weight (F2,22 = 130.47, P < 0.001). Not surprisingly, larger species had larger testes (effect estimate 0.64 ± 0.06, F1,22 = 119.46, P < 0.001). Contrary to our prediction however, we detected a nonsignificant trend toward a positive relationship between the relative sizes of these primary and secondary sexual structures (effect estimate 0.05 ± 0.02, F1,22 = 3.31, P = 0.083), suggesting that developmental allocation trade-offs may not have been important in shaping the evolution of relative trait sizes.
We also looked at a second pattern. We used the slopes of the trait-size body size scaling relationships (allometry) as a measure of the species-specific pattern of trait development (28, 29), and we tested for an evolutionary relationship between these allometric slopes. Because among-individual variation in body size in these beetles is largely determined by the nutritional environment encountered by larvae as they develop (30, 31), the steepness of a trait allometry reflects the magnitude of nutrition-dependent phenotypic plasticity in the growth of that trait. Traits that are nutrition-sensitive (plastic) have steep allometric slopes, whereas traits that are more tightly canalized developmentally do not.
We used a general linear model to look for variation in the within-species plasticity of testis growth (testes allometry) that might be explained by our measures of within-species plasticity in horn growth (horn allometry), again including species mean body size in the analysis. The model explained 49% of the within species variance in plasticity in testes growth (F2,22 = 10.67, P < 0.001). Larger species had more canalized testes growth (shallow allometries) (partial effect estimate –1.88 ± 0.47, F1,22 = 16.25, P < 0.001; Fig. 1C). After controlling for this effect of body size, species with the most plastic patterns of horn growth had the most canalized patterns of testes growth, and vice versa (partial effect estimate –0.16 ± 0.06, F1,22 = 8.27, P = 0.009; Fig. 1D).
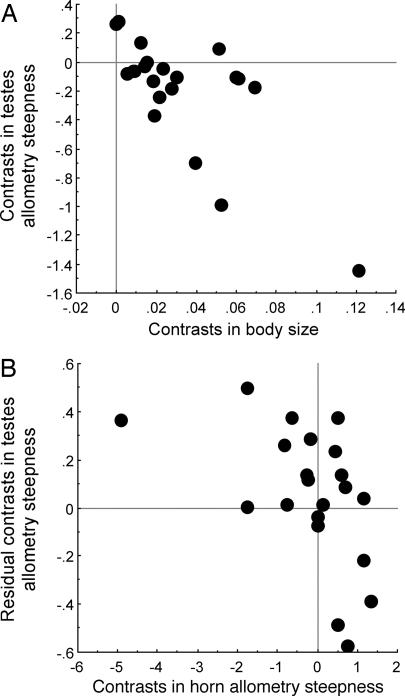
Robust hypotheses exist for the phylogenetic relationships of 22 of the 25 species in Table 1 (16, 32), and we conducted comparative analyses by using independent contrasts (33) on this reduced data set, obtaining 21 evolutionary contrasts. Regression of the evolutionary contrasts returned qualitatively similar patterns to our cross species analyses. Thus, evolutionary decreases in the plasticity of testes growth were associated with evolutionary increases in plasticity in horn growth (partial effect estimate –0.11 ± 0.04, F1,19 = 7.24, P = 0.015) and increases in body size (partial effect estimate –8.41 ± 1.32, F1,19 = 40.71, P < 0.001) (Fig. 2).
Fig. 2.
Comparative analysis of independent contrasts showing that evolutionary increases in body size were associated with evolutionary decreases in the steepness of the allometric slope of log testes weight on log body weight. After controlling for evolutionary increases in body size (A), evolutionary increases in horn allometry steepness were associated with evolutionary decreases in testes allometry steepness (B).
Finally, we looked for an evolutionary effect of the phenotypic trade-off between horns and testes by exploring patterns of evolutionary gains/losses of specific horn types (head versus thorax). One pattern that has emerged from the within-species studies of Onthophagus spp. is that trade-offs are often more pronounced for morphological structures that are physically nearest to the developing horn (16, 24). For example, in species with horns positioned at the rear of the head, the negative relationship between horn size and eye size is greater than that between horn size and either antennae or wing size (24). These data are consistent with a developmental model in which there is a stronger trade-off between structures that compete for resources locally within a larva. Based on these studies, we predicted that allocation trade-offs between horns and testes would be most pronounced if the horns developed from the thorax (as opposed to the head) because these horns develop in closer proximity to the testes. Thus, we predicted that if trade-offs associated with allocation to testes growth have influenced patterns of horn evolution, these effects should be more closely associated with the evolution of horns on the thorax than with horns on the head.
We still know very little regarding levels of sperm competition in natural beetle populations. However, we do know that sperm competition is likely to be a particularly important selection pressure in male-dimorphic taxa, where minor males sneak copulations with females guarded by major males (23). We can predict therefore, that sperm competition may be higher, on average, in male-dimorphic taxa than in male-monomorphic taxa, which lack sneaking minor males. If the origin of novel horns has been constrained by sperm competition, then we might expect gains of thorax horns to be concentrated on lineages of the tree lacking minor males.
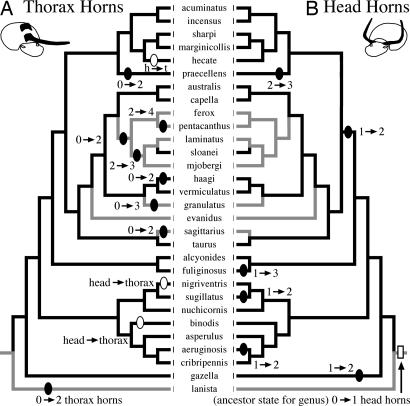
The genus Onthophagus exhibits striking evolutionary lability in the location and expression of male horns and is suitable for testing this prediction. The ancestral character state for this genus is thought to be one in which males were monomorphic for a single horn at the back or vertex of the head, but there have been repeated evolutionary gains of additional horns elsewhere on the head or on the thorax (16). Across the phylogeny of onthophagines in our data set, there were 14 separate increases in the number of horns, with horns added to the thorax in 8 lineages, and horns added to the head in 6 (Fig. 3). Male dimorphism in horn expression appears to have arisen once early in the history of this genus, but there is evidence for four subsequent losses and one evolutionary regain of male dimorphism, resulting in a mixture of monomorphic and dimorphic taxa (16, 32) (Fig. 3).
Fig. 3.
Phylogeny for 30 Onthophagus species from refs. 16 and 32 showing the presence/absence of male-dimorphism (black branches, dimorphic; gray branches, monomorphic) and evolutionary gains of novel horns on the thorax (A) and the head (B). The ancestor of this genus is reconstructed to have been monomorphic for a single head horn (open rectangle in B) (32). New horns were added as compliments to the ancestral horn 14 times (filled ovals) and as a replacement of the ancestral horn three times (open ovals). Character mappings performed in MacClade 4 (36).
Additions of new horns on the thorax were significantly concentrated on branches of the tree lacking hornless minor males (i.e., male-monomorphic lineages predicted to have lower levels of sperm competition; concentrated changes test: 6/8 increases in thorax horn number, P < 0.001), whereas increases in the number of head horns were not (Fig. 3). All six additions of head horns occurred in male-dimorphic lineages containing hornless minor males (concentrated changes test: 6/6 gains of head horns, P = 0.150). Thus, among-species patterns of thoracic horn diversity seem to be correlated with apparent differences in sperm competition inferred from the occurrence of discrete sneak phenotypes, suggesting that trade-offs between thoracic horns and testes may have constrained the evolutionary diversification of these weapons of sexual selection.
Discussion
We provide experimental and comparative data from horned beetles that point to a resource allocation trade-off between male investments in weapons used in contests for access to females, and investment in sperm production. These data thereby provide support for the fundamental assumption that underlies sperm competition game theory: a trade-off exists between investment in acquiring matings and investment in acquiring fertilizations (3).
Our data for O. nigriventris show that males engineered not to invest in horn growth allocate more resources to testes growth. These data complement those for O. taurus, where males engineered not to invest in genitalia allocated resources to horn growth at a smaller body size (26). Correlational support for a phenotypic trade-off in this genus also comes from studies of O. binodis where major males that produce a large bilobed thoracic horn have absolutely and relatively smaller testes than do minor males who do not produce the horn (23). Collectively, these studies provide evidence of a phenotypic trade-off between investment in horns and in testes growth.
Our data also show that males engineered not to produce horns put more resources into general body growth. Studies of both O. acuminatus (17) and O. taurus (18) show that body size contributes to male competitive success in onthophagines, independently of horn size, and the same is true for at least one species in a second genera of dung beetle, Euoniticellus intermedius (34). Our data for O. nigriventris thus suggest that males face a resource allocation trade-off between two traits that contribute to success in premating male contest competition, body size and horn size, as well as between these traits and testes.
But are these developmental resource allocation trade-offs relevant to longer-term patterns of morphological evolution? Our comparative analyses suggest that they may be, but not in the manner one might initially predict. In a comparative analysis of 25 Onthophagus species, we failed to detect the most obvious pattern: relative horn and testes sizes were not negatively correlated across taxa. One possibility suggested by these findings is that resource allocation trade-offs associated with horn and testes growth have not affected long-term patterns of beetle evolution. Certainly this is plausible; all but perfect genetic correlations are predicted to be transient sources of constraint to the independent evolution of traits (37–39), and it may be that the time scales involved with the evolutionary diversification of this beetle genus were sufficient to permit horns and testes to evolve relatively independently. Furthermore, accumulating evidence suggests that developmental trade-offs associated with horn growth, although widespread, are not universal. In particular, they seem to occur primarily when environmental conditions are stressful (C. Allen, O. Helmy, and D.J.E., unpublished results; see also refs. 40 and 41). If true, then the transient nature of these trade-offs may minimize their impact on character evolution. However, we detected two additional evolutionary patterns that suggest that trade-offs may indeed have influenced the evolution of morphology in these beetles.
First, we found a significant negative relationship between the steepness of horn allometry slopes and the steepness of testes allometry slopes: species with the steepest horn allometries had the shallowest testes allometries, and vice versa. In insects, the scaling of trait size with body size (allometry) results from developmental mechanisms that couple trait growth with nutrition (28, 29, 42). Traits with nutrition-sensitive patterns of growth (phenotypic plasticity) have steeper allometric slopes than traits with nutrition-insensitive patterns of growth (developmentally canalized). Moreover, because the marginal costs of increased trait size are smaller for large individuals, secondary sexual traits that are subject to strong sexual selection have been shown to evolve greater phenotypic plasticity and steeper allometric slopes (43, 44).
Developmental trade-offs between growing insect structures likely result from competition either for stored nutrients and/or for circulating growth factors or other signals that are associated with nutrition (29, 42, 45). Insensitivity of a particular trait (e.g., testes) to these circulating physiological signals would lead to nutrition-insensitive or canalized patterns of growth (46), and would protect that structure from competition with surrounding traits. If true, then secondary sexual structures such as ornaments or weapons may truly be secondary in the sense that their growth may only be cost-effective when it does not impact allocation to primary sexual structures such as testes. This may also help to explain why genitalia have shallow allometric slopes in such a breadth of animal taxa (35, 47, 48). Our data suggest that such a situation may have characterized the evolutionary history of onthophagines, so that extant species under the strongest selection for increased allocation to the weapons of sexual selection have also evolved the most protected patterns of testes development.
The second pattern we observed involves the evolution of horn location. Previous studies of this genus have shown how trade-offs between developing horns and adjacent body structures can account for the evolutionary diversification of weapons. For example, the allocation of resources to the growth of horns at the back of the head comes at a cost of reduced allocation to eye development (24), and evolutionary losses of head horns appear to be correlated with evolutionary shifts from diurnal to nocturnal flight behavior, presumably because enlarged eyes are more important for visual acuity at night (16). We predicted that a trade-off between investment in testes growth and horn growth could impose similar constraints on the origin of novel horns in lineages with intense sperm competition from alternative male phenotypes that sneak copulations with females guarded by horned males. Although we found that gains of novel thoracic horns were restricted to lineages that lacked sneaker male phenotypes, gains of head horns were statistically independent of the presence of sneak males. This pattern is consistent with previous within-species studies which showed that negative phenotypic associations between horns and body structures such as eyes, antennae or wings, increase in magnitude with increasing proximity of the developing horns to adjacent body structures (24). Thus, it might be that sperm competition constrains the origin of novel thoracic horns but not head horns, because thoracic horns are more likely to draw from the same resource pool during development.
In conclusion, our data reveal the trade-off between male investment in gaining matings and fertilizations explicit in evolutionary models of ejaculate expenditure. They suggest that sperm competition can play an important, although not always intuitive, role in shaping the evolution of secondary sexual traits used in conventional contest competition, and thereby contribute to the evolutionary diversification of male morphology.
Methods
Experimental Manipulation of Horn Development.
We looked for a trade-off between horn growth and testes growth in O. nigriventris by experimentally manipulating horn development. Experimental O. nigriventris were derived from adult beetles collected from pastures of the Parker Ranch on the island of Hawaii. Mated females were established in plastic breeding chambers (8 cm diameter × 20 cm deep) in the laboratory (University of Montana) and maintained in growth chambers at 25°C with unlimited access to cow dung. Females build a series of individual brood balls, each of which provides the resources for the development of a single offspring from hatching until adult emergence. Brood balls were sieved from breeding chambers and placed in shallow soil-filled containers in the same growth chambers. Individual broods were opened and larvae checked for their stage of development. Toward the end of the third larval instar, during the gut purge, the area of proliferating cells that would otherwise become the principal thoracic horn was cauterized by application of a hyphrecator. The larvae were held in place with forceps during cauterization and care was taken not to discolor the larval cuticle. The larvae were then returned to their individual brood balls and allowed to continue their development. Emerging adult beetles were checked for horn growth. No cauterized beetles developed horns. A series of controls were established in which larvae were removed from their broods, handled in a similar manner to cauterized beetles, and then returned to their broods. Seven days after adult emergence (after testes have matured), beetles were weighed and then dissected, and their testes were removed and weighed to an accuracy of 0.01 mg. Weights were log transformed before statistical analyses.
Comparative Analyses.
We collected data on thorax width (as a measure of body size), testes weight, body weight, and horn morphology from 25 species of Onthophagus. For all species beetles were sampled from the field, collected from fresh animal dung. Beetles were returned to the laboratory and maintained at 25°C with access to fresh dung for 7 days. Before dissection, beetles were held without access to dung or water for 24 h. This procedure was adopted to ensure that beetles were sexually mature, of equivalent recent mating history, and that they had purged their guts before weight determination. Thorax width was cubed to convert it to the same scale as our weight measures.
The genus exhibits complex patterns of variation in horn morphology, developing horns at one or several of five locations on the head and thorax (16, 32). We used two metrics of horn morphology (Table 1). First we measured the length of the largest or most exaggerated horn and again cubed this value to achieve the same scale as our weight measures. Second, we examined the degree of developmental plasticity in horn growth by calculating the scaling relationship between horn size and body size. For many species of Onthophagus, the scaling relationship of horn size on body size deviates from linearity because of the occurrence of minor males that in some species are hornless, whereas in others develop only rudimentary horns (32). We therefore fitted data for each species to the following equation:
where y0 is equal to the minimum horn length, a describes the range of horn lengths present in the sample, b the maximum rate of increase in horn size per unit increase in body size, c represents the body size at the point of inflection of the sigmoid, and x represents our measure of body size, thorax width. We used the software package SigmaPlot (SYSTAT) to estimate the parameter b from this equation and used this value as our measure of horn allometry for each species. Where species possessed both head and thoracic horns, we calculated the scaling relationship for the largest or most exaggerated horn.
Again, in addition to measuring a species' absolute testes weight, we quantified each species' degree of developmental plasticity in testes growth as the allometric slope of log testes weight on log body weight. We calculated the major axis slope (MA in Table 1) because we are interested in the true relationship between these two variables, both were measured with error, and both were measured on the same scale (49).
Of the species listed in Table 1, all but O. blackwoodensis, O. nodulifer, and O. rupicapra were included in a recent phylogeny, based on regions of four nuclear and three mitochondrial genes (3,315 bp total; 837 parsimony-informative) from 48 Onthophagus species and three outgroups (16, 32). Based on this phylogeny, we used the software package CAIC (33) to calculate phylogenetically independent contrasts. We also used the phylogeny to test for correlated evolutionary changes between gains of novel horns (on the head; on the thorax) and changes in the level of sperm competition as estimated by the presence/absence of hornless minor (sneak) males (male-dimorphism; male-monomorphism; see results for justification). For these analyses, we used the concentrated changes test (50) as implemented in MacClade 4 (36).
Supplementary Material
Acknowledgments
We thank everyone who sent beetles from their travels and Quenna Szafran for conducting the cauteries. The work was funded by grants from the Australian Research Council and the University of Western Australia Small Grant Scheme (to L.W.S.) and by National Science Foundation Grant IOB PECASE 0092873 (to D.J.E.) At the University of Montana, beetles were housed under U.S. Department of Agriculture Animal and Plant Health Inspection Service Permit 45534.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Darwin C. The Descent of Man and Selection in Relation to Sex. London: John Murray; 1871. [Google Scholar]
- 2.Parker GA. Biol Rev. 1970;45:525–567. [Google Scholar]
- 3.Parker GA. In: Sperm Competition and Sexual Selection. Birkhead TR, Møller AP, editors. London: Academic; 1998. pp. 3–54. [Google Scholar]
- 4.Stockley P, Gage MJG, Parker GA, Møller AP. Am Nat. 1997;149:933–954. doi: 10.1086/286031. [DOI] [PubMed] [Google Scholar]
- 5.Hosken DJ. Proc R Soc London Ser B. 1997;264:385–392. doi: 10.1098/rspb.1997.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gage MJG. Proc R Soc London Ser B. 1994;258:247–254. [Google Scholar]
- 7.Harcourt AH, Purvis A, Liles L. Funct Ecol. 1995;9:468–476. [Google Scholar]
- 8.Byrne PG, Roberts JD, Simmons LW. J Evol Biol. 2002;15:347–355. [Google Scholar]
- 9.Hosken DJ. Curr Biol. 2001;11:379–380. doi: 10.1016/s0960-9822(01)00211-1. [DOI] [PubMed] [Google Scholar]
- 10.Simmons LW, Roberts B. Science. 2005;309:2031. doi: 10.1126/science.1114500. [DOI] [PubMed] [Google Scholar]
- 11.Pitnick S. Am Nat. 1996;148:57–80. [Google Scholar]
- 12.Pitnick S, Miller GT. Heredity. 2000;84:416–426. doi: 10.1046/j.1365-2540.2000.00679.x. [DOI] [PubMed] [Google Scholar]
- 13.Pitnick S, Jones KE, Wilkinson GS. Proc R Soc London Ser B. 2006;273:719–724. doi: 10.1098/rspb.2005.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vladic TV, Järvi T. Proc R Soc London Ser B. 2001;268:2375–2381. doi: 10.1098/rspb.2001.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gage MJG, Stockley P, Parker GA. Philos Trans R Soc London B. 1995;350:391–399. [Google Scholar]
- 16.Emlen DJ, Marangelo J, Ball B, Cunningham CW. Evolution (Lawrence, KS) 2005;59:1060–1084. [PubMed] [Google Scholar]
- 17.Emlen DJ. Behav Ecol Sociobiol. 1997;41:335–342. [Google Scholar]
- 18.Moczek AP, Emlen DJ. Anim Behav. 2000;59:459–466. doi: 10.1006/anbe.1999.1342. [DOI] [PubMed] [Google Scholar]
- 19.Hunt J, Simmons LW. Proc R Soc London Ser B. 2001;268:2409–2414. doi: 10.1098/rspb.2001.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt J, Simmons LW. J Evol Biol. 2002;15:784–795. [Google Scholar]
- 21.Tomkins JL, Simmons LW. Proc R Soc London Ser B. 2000;267:1547–1553. doi: 10.1098/rspb.2000.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons LW, Beveridge M, Krauss S. Behav Ecol Sociobiol. 2004;57:164–173. [Google Scholar]
- 23.Simmons LW, Tomkins JL, Hunt J. Proc R Soc London Ser B. 1999;266:145–150. [Google Scholar]
- 24.Emlen DJ. Science. 2001;291:1534–1536. doi: 10.1126/science.1056607. [DOI] [PubMed] [Google Scholar]
- 25.Nijhout HF, Emlen DJ. Proc Natl Acad Sci USA. 1998;95:3685–3689. doi: 10.1073/pnas.95.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moczek AP, Nijhout HF. Am Nat. 2004;163:184–191. doi: 10.1086/381741. [DOI] [PubMed] [Google Scholar]
- 27.Engqvist L. Anim Behav. 2005;70:967–971. [Google Scholar]
- 28.Emlen DJ, Nijhout HF. Annu Rev Entomol. 2000;45:661–708. doi: 10.1146/annurev.ento.45.1.661. [DOI] [PubMed] [Google Scholar]
- 29.Emlen DJ, Allen CE. Integr Comp Biol. 2004;43:617–634. doi: 10.1093/icb/43.5.617. [DOI] [PubMed] [Google Scholar]
- 30.Hunt J, Simmons LW. Evolution (Lawrence, KS) 2000;54:936–941. doi: 10.1111/j.0014-3820.2000.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 31.Emlen DJ. Proc R Soc London Ser B. 1994;256:131–136. [Google Scholar]
- 32.Emlen DJ, Hunt J, Simmons LW. Am Nat. 2005;166:S42–S68. doi: 10.1086/444599. [DOI] [PubMed] [Google Scholar]
- 33.Purvis A, Rambaut A. Comput Appli Biosci. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- 34.Pomfret JC, Knell RJ. Anim Behav. 2006;71:567–576. [Google Scholar]
- 35.Kawano K. Am Nat. 2004;163:1–15. doi: 10.1086/379796. [DOI] [PubMed] [Google Scholar]
- 36.Maddison WP, Maddison DR. Sunderland, MA: Sinauer; 1999. MacClade 4. [Google Scholar]
- 37.Via S, Lande R. Evolution (Lawrence, KS) 1985;39:505–522. doi: 10.1111/j.1558-5646.1985.tb00391.x. [DOI] [PubMed] [Google Scholar]
- 38.Schluter D. Evolution (Lawrence, KS) 1996;50:1766–1774. doi: 10.1111/j.1558-5646.1996.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 39.Lande R. Evolution (Lawrence, KS) 1979;33:402–416. doi: 10.1111/j.1558-5646.1979.tb04694.x. [DOI] [PubMed] [Google Scholar]
- 40.Messina FJ, Slade AF. Physiol Entomol. 1999;24:358–363. [Google Scholar]
- 41.Messina FJ, Fry JD. J Evol Biol. 2003;16:501–509. doi: 10.1046/j.1420-9101.2003.00535.x. [DOI] [PubMed] [Google Scholar]
- 42.Stern DL, Emlen DJ. Development (Cambridge, UK) 1999;126:1091–1101. doi: 10.1242/dev.126.6.1091. [DOI] [PubMed] [Google Scholar]
- 43.Cotton S, Fowler K, Pomiankowski A. Proc R Soc London Ser B. 2004;271:771–783. doi: 10.1098/rspb.2004.2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simmons LW, Tomkins JL. Evol Ecol. 1996;10:97–104. [Google Scholar]
- 45.Goberdhan DC, Wilson C. Dev Genes Evol. 2002;212:196–202. doi: 10.1007/s00427-002-0226-3. [DOI] [PubMed] [Google Scholar]
- 46.Shingleton AW, Das J, Vinicius L, Stern DL. PLoS Biol. 2005;3:1607. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eberhard WG, Huber BA, Rodriguez RLS, Briceño RD, Salas I, Rodriguez V. Evolution (Lawrence, KS) 1998;52:415–431. doi: 10.1111/j.1558-5646.1998.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 48.House CM, Simmons LW. J Evol Biol. 2005;18:1281–1292. doi: 10.1111/j.1420-9101.2005.00926.x. [DOI] [PubMed] [Google Scholar]
- 49.Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 50.Maddison WP. Evolution (Lawrence, KS) 1990;44:539–557. doi: 10.1111/j.1558-5646.1990.tb05937.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.