Abstract
T cell receptor (TCR) signaling mediates cell fate decisions throughout the life of a T cell. The earliest biochemical events during antigen-stimulated TCR signaling include activation of the Src-family protein tyrosine kinase, p56Lck (Lck), which is an integral component of the TCR signaling complex by its association with the cytoplasmic tails of CD8 or CD4. CD8 and Lck are obligatory during thymic selection of CD8+ T cells. What remain unknown are when and with what stringency Lck is required for effective TCR-mediated activation and function throughout the life of a mature CD8+ T cell. Using mice that express an inducible Lck transgene in T cells, we have investigated the temporal importance of Lck-mediated TCR signaling in antigen-specific CD8+ T cell responses during acute viral infections. We show that Lck deficiency induced in naive mice abrogated the antigen-specific activation and clonal expansion of CD8+ T cells during a primary response to acute viral infections. Moreover, the magnitude of primary CD8 T cell expansion depended on the duration of Lck-dependent TCR signaling. Quite unexpectedly, however, Lck was dispensable for enhanced functional avidity, maintenance, and reactivation of memory CD8+ T cells in vitro and in vivo. These observations suggest that the TCR signaling apparatus is rewired from an Lck-dependent state in naive CD8+ T cells to an Lck-independent state in memory CD8+ T cells. Less stringent requirements for antigen-specific TCR signaling to activate memory CD8+ T cells could, in part, account for their unique hyperreactivity to antigen, which contributes to accelerated immune control during secondary infections.
Keywords: differentiation, lymphocytes, signaling, naive
Signaling via the T cell receptor (TCR) is known to play a critical role in the selection and survival of T cells during thymic development, as well as maintenance of naive T cells in the periphery (1, 2). It is also established that TCR–MHC interactions are necessary for homeostatic proliferation of naive T cells under lymphopenic conditions (3). During an immune response, TCR signaling induced by T cell encounters with the cognate MHC–foreign peptide complex on antigen-presenting cells triggers a program of clonal expansion and differentiation into effector and memory cells. Although survival of memory T cells may or may not require signaling via the TCR (2), the secondary expansion of memory T cells upon antigen reencounter does require TCR signaling. Thus, T cells are governed by TCR signaling-mediated control throughout their life.
The most proximal events of TCR-mediated signal transduction include activation of the Src-family tyrosine kinases p56Lck (Lck) and p59Fyn (Fyn), which are associated with the coreceptors CD4 and CD8 (4). Activated Lck and/or Fyn in turn phosphorylate the immunoreceptor tyrosine-based activation motifs (ITAMs) located within the CD3 and ζ chains of the TCR complex itself. The Lck-phosphorylated ITAMs serve as docking sites for adapter phosphoproteins, such as ZAP-70, which are also substrates for Lck. Lck-phosphorylated ZAP-70 in turn phosphorylates T cell-specific adapters, LAT and SLP-76, which generate the second messenger effectors of T cell activation. Thus, the activities of Lck are known to be fully integrated into the defining steps of TCR signaling. What remain unknown in the life of a mature T cell are when and with what stringency Lck is required for effective TCR-mediated activation and function.
Studies conducted in vitro by using T cell lines established the crucial importance of the Src-family kinases Lck and Fyn in mediating TCR signal transduction in clonal lymphocytes (5). As important as these findings have been in advancing our understanding of TCR signaling in T cell function, it has nevertheless remained a significant challenge to extend these studies in vivo. It was anticipated that development of Lck-deficient (Lck−/−) mice would fill this technical hiatus. However, profound defects observed in the thymic development of Lck−/− mice also effected severe reduction in the size of the peripheral T cell population (6). Moreover, this residual of peripheral T cells also exhibited significant phenotypic abnormalities (6). Although their development was a major accomplishment, the Lck−/− mice could therefore not be used as a suitable model with which to investigate Lck signaling in TCR-regulated responses in vivo. However, the subsequent development of conditional transgenic mice (Lck1ind) that express Lck by a T cell-specific inducible mechanism has indeed bridged the gap in our understanding of T cell biology (7). This transgenic model has been fully characterized in breakthrough studies elucidating Lck-mediated regulation of CD4+ T cell homeostasis. Lck is constitutively expressed throughout normal mammalian development, but in these transgenic mice, T cell-specific Lck expression can be turned on and off (7). This flexible control allows the temporal importance of Lck-dependent regulation of antigen-specific polyclonal CD8+ T cell responses to be explored during viral infections under physiological conditions.
Our studies with the Lck1ind mice have documented that (i) Lck is required for activation and expansion of naive CD8+ T cells; (ii) the duration of Lck-dependent TCR signaling determines the magnitude of clonal expansion during the primary CD8 T cell response; (iii) Lck is dispensable in the maintenance of memory CD8+ T cells; and (iv) Lck expression is not essential for responses of memory CD8+ T cells to secondary antigenic stimulation in vivo or in vitro. In addition to unraveling a fundamental difference in the activation requirements of naive versus memory T cells, these findings have significant implications in the development of targeted immunotherapy to suppress T cell responses in transplantation and treatment of autoimmune diseases.
Results and Discussion
Lck Is Required for Activation and Expansion of Virus-Specific CD8+ T Cells During a Primary Response.
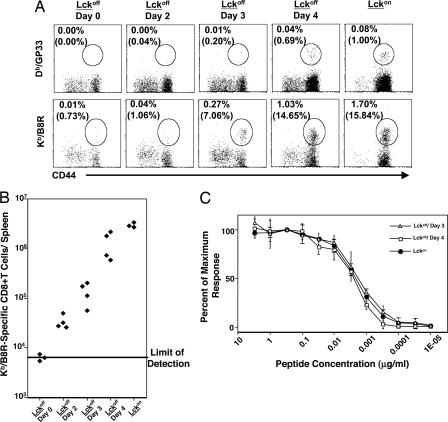
It has been shown that Lck-deficient T cells and T cells expressing a dominant negative form of Lck exhibit impaired proliferative responses in vitro (8). Here we investigated whether Lck is necessary for activation and expansion of virus-specific CD8+ T cells in vivo during acute infection of mice with recombinant vaccinia virus, VV-GP, which expresses the glycoprotein (GP) of lymphocytic choriomeningitis virus (LCMV). To accomplish this, we have used Lck1ind transgenic mice, in which Lck transgene expression can be controlled by the administration of doxycycline in drinking water (7). Mice fed with doxycycline express the Lck transgene in T cells; withdrawal of doxycycline will result in loss of Lck gene expression in T cells in ≈3 days (3). Two groups of Lck1ind mice were infected with VV-GP. Whereas one group of mice, Lckon, continued to receive doxycycline in their water after VV-GP infection, Lck expression in T cells was turned off in the second group of mice (Lckoff) by withdrawing doxycycline at the time of infection. In control experiments, we verified that the CD8+ T cell response of transgenic Lckon mice to VV-GP was comparable with that of nontransgenic C57BL/10 mice (Fig. 6, which is published as supporting information on the PNAS web site). On the eighth day after VV-GP infection, the activation and expansion of CD8+ T cells were quantitated in the spleen by flow cytometry. As illustrated in Fig. 1A, doxycycline withdrawal for 7 days had a minimal effect on CD8+ T cells in uninfected mice. However, striking differences in CD8+ T cell activation were noted when the spleens of VV-GP-infected Lckon and Lckoff mice were compared; the number of activated CD8+ T cells (CD44hi) in the spleens of Lckon mice was ≈40-fold higher compared with Lckoff mice (Fig. 1 A and C). We also quantitated CD8+ T cells that are specific to the LCMV GP33 epitope in the spleens and livers of VV-GP-infected mice by using MHC I tetramers. Data in Fig. 1 B and C illustrate robust activation of LCMV GP33-specific CD8+ T cells in the spleen and liver of Lckon mice. In striking contrast, GP33-specific CD8+ T cells were hardly detected in Lckoff mice. Next, we investigated whether Lck was necessary for activation of CD8+ T cells during an acute LCMV infection. As described above, Lck1ind transgenic mice were infected with LCMV, and Lck expression was turned off in the Lckoff mice at the time of infection. On the eighth day after LCMV infection, LCMV GP33-specific CD8+ T cells were readily detected only in the spleens of LCMV-infected Lckon mice, not of Lckoff mice (Fig. 1D). Taken together, these data provide compelling evidence that Lck activity was essential for primary activation and expansion of CD8+ T cells during acute infection of mice with VV-GP and LCMV. Previous work has shown that homeostatic proliferation of naive T cells in lymphopenic environments is dependent on Lck-mediated TCR signal transduction (3). Although the consequences of homeostatic proliferation and antigen-driven proliferation are not the same, our findings (Fig. 1) indicate that TCR-mediated signaling events that drive lymphopenia-induced (3) and antigen-driven proliferation of naive T cells both require Lck.
Fig. 1.
Lck is required for activation of CD8+ T cells during a primary response to vaccinia virus and LCMV. Lck1ind mice were infected with VV-GP or LCMV. At the time of infection, Lck expression was turned off in the Lckoff group by withdrawal of doxycycline in drinking water, whereas the Lckon group continued to get doxycycline during the course of the experiment. On the eighth day after VV-GP (A–C) or LCMV (D) infection, the activation of CD8+ T cells was assessed by staining splenocytes or mononuclear cells from the liver with anti-CD8, anti-CD44, and MHC I tetramers Db/GP33. The dot plots in A are gated on total splenocytes and the numbers are the percentages among splenocytes. The dot plots in B and D are gated on total CD8+ T cells and the numbers are the percentages among CD8+ T cells. The symbols in C represent individual mice, and these data are representative of results from three independent experiments. Data in D are representative of two independent experiments with three mice per group.
Elegant work by Ahmed's and Schoenberger's groups has shown that naive CD8+ T cells undergo programmed antigen-independent clonal expansion after transient exposure to antigens (9, 10). An unexplored extension of these studies is the effect of limiting the duration of antigenic stimulation on the magnitude of the primary CD8+ T cell response. Here, using the Lck1ind mice, we investigated the effect of limiting the duration of Lck-dependent TCR signaling on the magnitude of the clonal expansion during the primary response to vaccinia virus. Lck1ind mice were infected with VV-GP, and doxycycline treatment was stopped on day 0, 2, 3, or 4, after infection or was not stopped. On the eighth day after infection, we quantitated the number of LCMV GP33-specific and VV epitope B8R-specific CD8+ T cells in the spleen by using MHC I tetramers (Fig. 2A and B). Compared with Lckon mice, withdrawal of doxycycline on day 0 (Lckoff/D0) abrogated the activation of LCMV GP33- and VV B8R-specific CD8+ T cells on day 8, which is consistent with data presented in Fig. 1. However, the time course of rising CD8+ T cell responses on day 8 after infection in VV-GP-infected Lckoff/D0, Lckoff/D2, Lckoff/D3, Lckoff/D4, and Lckon mice showed that the magnitude of expansion of virus-specific CD8+ T cells changed as a function of the length of doxycycline treatment after infection; the magnitude of the response was therefore limited by and directly linked to the duration of maximally stimulated Lck gene expression after infection (Fig. 2B). Based on the schedule of doxycycline withdrawal in our experiments, we concluded that Lck must be induced continuously by doxycycline treatment for no less than 4 days after infection for CD8+ T cells to achieve at least half-maximal clonal expansion during primary response to VV-GP, whereas treatment of less than 3 days after infection would provide a subthreshold regimen. On day 8 after infection, FACS-sorted CD44hi (Lckoff/D3) and B8R-specific (Lckoff/D4) CD8+ T cells contained measurable levels of Lck mRNA (quantitative PCR data not shown). These data suggest that there were subpopulations (albeit small) of CD8+ T cells in which doxycycline induced sufficient levels of Lck mRNA to support TCR signaling activity minimally required to activate T cells and sustain clonal expansion after VV-GP infection. Although withdrawal of doxycycline on days 3 and 4 after infection blunted the clonal expansion of B8R-specific CD8+ T cells on day 8 after infection (Fig. 2 A and B), it had no detectable effect on the functional avidity of these CD8+ T cells measured by intracellular cytokine staining ex vivo (Fig. 2C). In summary, our findings strongly indicate that the magnitude of CD8+ T cell clonal expansion depends on the duration of uninterrupted suprathreshold levels of Lck-dependent TCR signaling. A recent report by Bevan's group conducted in a very different model system reached the same conclusion (11).
Fig. 2.
The magnitude of clonal expansion of CD8+ T cells is controlled by the duration of Lck-dependent TCR signaling. Lck1ind mice were infected with VV-GP, and doxycycline was stopped on day 0 (Lckoff/Day 0), 2 (Lckoff/Day 2), 3 (Lckoff/Day 3), or 4 (Lckoff/Day 4) after infection; Lckon mice received doxycycline throughout the course of the study. (A and B) On the eighth day after infection, the activation of CD8+ T cells specific to the LCMV epitope, GP33, and the vaccinia virus epitope, B8R, was quantitated by using Db/GP33 and Kb/B8R MHC I tetramers, respectively. The dot plots in A are gated on total CD8 T cells, and the numbers are percentages of tetramer-binding cells among splenocytes; numbers in parentheses are the percentages of tetramer-binding cells among total CD8 T cells. The data in A and B are representative of three independent experiments. (C) Splenocytes were stimulated with the indicated concentrations of B8R peptide for 6 h, and the number of B8R-specific IFNγ-producing CD8+ T cells was assessed by intracellular cytokine staining. The data are expressed as a percentage of maximum response attained at a peptide concentration of 0.3 μg of B8R peptide per ml for each mouse. The data in C are the mean ± SD of four mice per group and represent one of three independent experiments.
Lck Is Not Required for Maintenance of Virus-Specific Memory CD8+ T Cells.
Previous studies have shown that virus-specific memory CD8+ T cells can persist indefinitely in MHC-deficient mice, suggesting that TCR–MHC interactions are not required for maintenance of memory CD8+ T cells (12). However, these studies did not exclude the possibility that spontaneous ligand-independent TCR signaling might be required for memory CD8 T cell survival. For our studies, this is not an idle consideration, because Polic et al. (13) have demonstrated, using induced TCR ablation in vivo, that TCR-deficient but not TCR-sufficient memory phenotype (CD44hi) CD8+ T cells decline slowly over time. We have therefore asked whether TCR sufficiency includes a requirement for Lck-dependent TCR signaling. Is Lck required for maintenance of virus-specific memory CD8+ T cells in vivo? Lck1ind mice were infected with VV-GP, and 45–60 days after infection, Lck gene expression was switched off in one group of mice (Lckoff) for the ensuing 4 weeks, whereas Lck gene expression was sustained continuously in the other group (Lckon). Four weeks after turning off Lck expression, we quantitated the number of CD44hi CD8+ T cells and GP33-specific memory CD8 T cells in the spleens of Lckon and Lckoff mice. As shown in Fig. 3, the spleens of VV-GP-immune Lckon and Lckoff mice contained comparable numbers of CD44hi and GP33-specific memory CD8+ T cells. Not only did GP33-specific memory CD8+ T cells in both Lckon and Lckoff mice express normal levels of the cell surface CD44 (Fig. 3) and IL-7 receptor (data not shown), but turning off Lck expression for up to 8 weeks still had no significant effect on the maintenance of memory CD8+ T cells (data not shown). Slow proliferation, termed proliferative renewal, is responsible for maintenance of a stable number of memory CD8+ T cells (14). Studies of BrdU incorporation in vivo showed that the proliferative renewal of memory phenotype (CD44hi) CD8+ T cells was unaffected by turning off Lck expression for up to 6 weeks (data not shown). Thus, Lck-dependent TCR signal transduction does not appear to be required for maintenance of the memory CD8+ T cell compartment. In this same mouse model, it has previously been shown that the survival of memory phenotype CD4+ T cells does not require expression of Lck (15). Moreover, inducible TCR ablation or double deficiency of Lck and Fyn did not significantly affect the long-term survival of memory CD4+ T cells, which suggested that TCR signaling is not essential for maintenance of memory CD4+ T cells (15). However, it has been reported that long-term maintenance of CD8+ memory T cells but not CD4+ memory T cells might have a requirement for TCR (13). Yet, here in our studies, Lck-dependent TCR activity is not necessary for normal CD8+ T cell memory homeostasis. One explanation for this apparent discrepancy in Lckoff mice is that the missing Lck activity can in some cases be replaced by the redundant activity of the closely related Src family kinase Fyn (16, 17). This issue of whether Lck and Fyn possess redundant functions in the long term maintenance of memory CD8+ T cells requires further investigation.
Fig. 3.
Normal maintenance of memory CD8+ T cells in the absence of Lck. Lck1ind mice were infected with VV-GP, and 45–60 days after infection, Lck expression was turned off in the Lckoff mice for 4 weeks by not adding doxycycline to the drinking water; Lck expression was maintained in the other group of Lckon mice by continuous feeding of doxycycline. Four weeks after doxycycline withdrawal, the number of GP33-specific memory CD8+ T cells was quantitated by staining splenocytes with anti-CD8, anti-CD44, and Db/GP33 MHC I tetramer. (A) The dot plots in Upper are gated on total splenocytes, and the numbers are the percentages of CD44hi CD8 T cells among splenocytes. The dot plots in A Lower are gated on total CD8+ T cells, and the numbers represent percentages of GP33-specific CD8+ T cells in total CD8+ T cells. (B) Each symbol represents the total number of GP33-specific memory CD8+ T cells in individual mice from one of two independent experiments.
Antigen-Induced Cytokine Production by Virus-Specific Memory CD8+ T Cells in Vitro Does Not Require Lck.
Compared with naive T cells, memory T cells exhibit hyperreactive sensitivity and kinetics to antigenic stimulation (18). The increased responsiveness of memory CD4+ T cells has been attributed to alterations in the TCR signal transduction machinery (19, 20), whereas Kersh et al. (21) showed that the phosphorylation content of lipid rafts in memory CD8+ T cells is higher than in naive T cells. Kersh et al. also reported that TCR signaling-induced phosphorylation of MAP kinases was enhanced in memory CD8+ T cells. Furthermore, the enhanced responses of effector and memory CD8+ T cells were associated with higher Lck expression compared with naive T cells (22). Although the hyperreactivity of memory T cells has been ascribed to augmented TCR signaling resulting from localization of Lck molecules targeted to the CD8 molecules in the plasma membrane (23), the role of Lck in the activation of antigen-specific memory CD8+ T cells has not been studied. Here, we investigated the importance of Lck in antigen-induced cytokine production by virus-specific memory CD8+ T cells ex vivo. Lck1ind mice were infected with VV-GP, and ≈45 days later, doxycycline-dependent Lck gene expression was turned off in the cohort of Lckoff mice for 4 weeks; doxycycline was maintained in the water of other group of Lckon mice. Loss of Lck protein expression in thymic T cells from Lckoff mice but not from Lckon mice was confirmed by Western blotting (Fig. 4A). We also confirmed the loss of Lck gene expression in FACS-sorted antigen-specific memory CD8 T cells, by comparing Lck mRNA levels between VV epitope B8R-specific memory CD8+ T cells from the spleen of Lckon and Lckoff mice by PCR (Fig. 4B). Four to 6 weeks after cessation of doxycycline treatment, splenocytes from Lckon and Lckoff mice were cultured with the GP33 and B8R peptides in vitro for 6 h in medium with doxycycline. Cytokine production by memory CD8+ T cells specific to the LCMV epitope GP33 and VV epitope B8R is illustrated in Fig. 4C. In response to peptide stimulation, memory CD8+ T cells from both Lckon and Lckoff mice produced readily detectable levels of IFNγ in medium containing doxycycline. Because Lck transgene could be induced within ≈4 h after exposure to doxycycline (7), we also conducted this assay without doxycycline. IFNγ production by memory CD8+ T cells isolated from Lckon and Lckoff mice was not different whether cells were tested in medium with or without doxycycline (Fig. 4C). Because we know from PCR analyses (Fig. 4B) that CD8+ T cells from Lckoff mice were not expressing detectable Lck mRNA, we have concluded that virus-specific memory CD8+ T cells can be stimulated to produce cytokine in the absence of Lck expression.
Fig. 4.
Lck is not required for virus-specific memory CD8+ T cells to produce IFNγ in vitro. Lck1ind mice were infected with VV-GP, and 45 days after infection Lck expression was turned off for 4 weeks in Lckoff mice by cessation of doxycycline treatment. Lck expression in T cells was sustained in Lckon mice by continuous feeding of doxycycline. (A) Four weeks after withdrawal of doxycycline, the expression of Lck protein in the thymocytes of Lckon and Lckoff mice was analyzed by Western blot. (B) Lck mRNA expression in Kb/B8R-specific CD8 T cells from the spleens of Lckon and Lckoff mice four weeks after doxycycline cessation. (C) Four weeks after cessation of doxycycline treatment, in vitro cytokine production by memory CD8+ T cells (specific to the epitopes GP33 and B8R) was assessed by intracellular cytokine staining in vitro. For induction of cytokine production, splenocytes were stimulated with the antigenic peptides (0.3 μg/ml) in media with or without doxycycline. The dot plots in C are gated on total splenocytes, and the numbers are percentages of IFNγ-producing CD8+ T cells among total splenocytes; numbers in parentheses are percentages of IFNγ-producing cells of total CD8+ T cells. (D) Dose-response of memory CD8+ T cells in Lckon and Lckoff mice. Four weeks after doxycycline cessation, splenocytes from Lckon or Lckoff mice were stimulated with the indicated concentrations of B8R peptide for 6 h in media with doxycycline and without doxycycline, respectively. The number of IFNγ-producing CD8+ T cells was quantitated by intracellular staining. The data are expressed as a percent of maximum response attained at a peptide concentration of 10 μg of B8R per ml. The data are the mean ± SD of five mice per group and represent one of three independent experiments.
The requirement for Lck in the activation of memory CD8+ T cells might be dependent on the strength of antigenic stimulation; strong stimulation of the TCR might overcome the requirement for Lck during activation of CD8+ T cells. To address this issue, we examined the full peptide dose range for activation of memory CD8+ T cells from Lckon and Lckoff mice by stimulating cells ex vivo with VV peptide, B8R. As shown in Fig. 4D, the presence or absence of Lck had no detectable effect on the functional avidity of the VV-specific memory CD8+ T cells. Taken together, data in Fig. 4 A–C demonstrate that activation of virus-specific memory CD8+ T cells can occur in an Lck-independent manner.
Lck Is Not Required for Secondary Activation of Memory CD8+ T Cells in Vivo.
Data in Fig. 4 C and D indicate that Lck might not be essential for activation of memory CD8+ T cells in vitro. Here we have determined whether Lck is required for the activation and expansion of memory CD8+ T cells during a secondary response in vivo. As described above, Lck1ind mice were infected with VV-GP to induce LCMV GP33-specific memory CD8+ T cells. Approximately 60 days after VV-GP infection, Lck gene expression in T cells was turned off in Lckoff mice by withdrawal of doxycycline treatment. Four to 6 weeks after doxycycline withdrawal, VV-GP-immune Lckon and Lckoff mice were challenged with LCMV, and GP33-specific CD8+ T cells were quantitated 5 days later. As shown in Fig. 5A, in response to a secondary challenge infection with LCMV, a significant increase in the frequencies of GP33-specific CD8+ T cells was seen in both Lckon and Lckoff mice compared with unchallenged controls, whereas the total number of GP33-specific CD8+ T cells in the spleens of Lckoff mice was comparable with that of Lckon mice (Fig. 5B). Next, we examined whether Lck deficiency affected the differentiation of memory CD8+ T cells into effector cells during the secondary response. In mice, cellular granzyme B is a reliable surrogate marker for effector CD8+ T cells because only effector, and not memory, CD8+ T cells express and store granzyme (24). The expression levels of intracellular granzyme B in GP33-specific CD8+ T cells from the spleens of Lckoff mice were comparable with those of Lckon mice (Fig. 5C). Additionally, GP33-specific CD8+ T cells from Lckon and Lckoff mice produced comparable levels of IFNγ in response to in vitro peptide stimulation; the mean fluorescence intensities of staining for IFNγ (measured by intracellular cytokine staining) from Lckon and Lckoff mice were 789 ± 196 and 794 ± 182, respectively. These data suggested that differentiation of memory CD8+ T cells into effectors was not affected by Lck deficiency. It should be noted, however, that in an F5 TCR transgenic model, there is much greater dependence on the expression of Lck for the activation of memory CD8+ T cells to reveal effector function (R.Z., unpublished data), suggesting that there is a hierarchy of Lck requirements that may be linked with TCR affinity. Clearly in our Lck1ind model described here, where the response is polyclonal and driven by viral infection, we show that Lck is either nonessential or replaceable for activation of memory CD8+ T cells during a secondary viral challenge.
Fig. 5.
Normal secondary CD8+ T cell responses in the absence of Lck. Lck1ind mice were infected with VV-GP, and 60 days after infection, doxycycline treatment was stopped in Lckoff mice for 4–6 weeks to turn off Lck expression. Lck expression was maintained in Lckon mice by continuous feeding of doxycycline. Four to 6 weeks after turning off Lck expression, VV-GP-immune mice were challenged with LCMV. On the fifth day after LCMV challenge, LCMV GP33-specific CD8+ T cells in the spleen were quantitated by staining splenocytes with anti-CD8, anti-CD44, and Db/GP33 MHC I tetramer. Unchallenged VV-GP-immune mice were also included as controls. (A) The dot plots are gated on total CD8+ T cells, and the numbers are the percentages of LCMV-specific CD8+ T cells of total CD8+ T cells. (B) Each symbol represents the total number of GP33-specific memory CD8+ T cells in individual mice from two independent experiments. (C) Granzyme expression in LCMV-specific CD8+ T cells is shown. The histograms in C are gated on Db/GP33 tetramer-binding CD8+ T cells and show intracellular staining with anti-granzyme B (thick line) and an isotype control antibody (thin line); the numbers are the mean fluorescence intensities (MFI) for granzyme staining.
In summary, the data presented in this article document differential requirements for Lck in the TCR-specific activation of CD8+ T cells during primary and secondary responses in vivo. By extension, Lck function was obligatory for optimal activation of naive CD8+ T cells, but not memory CD8+ T cells, which suggests differential signaling through TCR in naive versus memory T cells. How are memory CD8+ T cells activated in the absence of Lck? It has been reported that Fyn could compensate for Lck to a limited extent in pre-TCR signaling in thymocytes (16, 17). Although expression levels of Fyn are higher in memory CD8+ T cells than in naive CD8+ T cells (21), it remains to be shown whether Fyn can effectively substitute for Lck in memory CD8 T cell activation in vivo. Yet, here in this study, it is clear that Fyn cannot substitute for Lck during primary activation of naive CD8+ T cells. Both Lck- and Fyn-specific targets have been identified (4). Therefore, in theory, either Lck or Fyn activities could be irreplaceable in activation of naive or memory T cells, respectively. Under certain in vitro conditions, activation of naive but not effector CD4+ T cells requires Fyn, which supports our hypothesis that Lck and Fyn activities are redundant in antigen-primed T cells (25). Memory CD8+ T cells constitutively express more phosphoproteins in their lipid rafts under steady-state conditions (21), and perhaps this primed state might be able to overcome Lck deficiency during secondary activation. It was recently reported that the Lck-independent TCR signaling induced by bacterial superantigens occurs via the Gα11-dependent phospholipase C-β-mediated pathway (26). Hence it is important to examine whether TCR signaling in memory CD8+ T cells use the Gα11 pathway. Anti-CD8 antibodies have been used to assess the requirement for CD8 coreceptor in the in vitro responses of naive and memory CD8+ T cells (27, 28). These studies have shown that anti-CD8 antibodies effectively inhibit responses of naive CD8+ T cells, but not memory CD8+ T cells (27, 28). The inherent resistance of memory CD8+ T cells to blocking by anti-CD8 antibodies can be explained, at least in part, by our finding that the CD8 coreceptor-associated Lck is not essential for activation of memory CD8+ T cells.
What are the implications of the findings reported in this article? First, we provide strong evidence that memory CD8+ T cell differentiation from naive T cells is associated with rewiring of the TCR signaling machinery from an Lck-dependent state to an Lck-independent state. This information will further our understanding of the mechanism(s) underlying the intrinsic hyperreactivity of memory CD8+ T cells to antigen and will also provide additional insights into the process of memory T cell differentiation and development of protective immunity. Second, our findings have implications in the development of immunotherapy to treat T cell-dependent immunopathologies. Our results indicate that Lck might not be a good therapeutic target for development of drugs to treat established and ongoing immune disorders.
Materials and Methods
Mice.
The generation and use of inducible Lck-transgenic mice (Lck1ind) have been described (7). Briefly, mice transgenic for the tetracycline-inducible transactivator (rtTA) expressed constitutively in T cells under the control of human CD2 promoter on the endogenous Lck-deficient (Lck−/−) background (rtTA-C/Lck−/−) were intercrossed with transgenic mice expressing Lck under the control of a tetO/CMV minimal promoter also on the endogenous Lck−/− background (Lck1/Lck−/−). Breeder pairs were treated with doxycycline (Sigma, St. Louis, MO) in drinking water (0.4 mg/ml) through the pregnancy and weaning. After weaning, the Lck1+/− rtTA-C+/− Lck−/− (Lck1ind) offspring resulting from the intercross were maintained on doxycycline to maintain Lck transgene expression indefinitely, except in experiments when doxycycline was withdrawn to extinguish Lck expression. Mice were housed in sterilized cages and fed with sterile food and water. All mice were used at 6–8 weeks of age according to the strict guidelines of the University of Wisconsin School of Veterinary Medicine Institutional Animal Care and Use Committee.
Virus.
The recombinant vaccinia virus VV-GP that expresses the glycoprotein of LCMV was provided by Lindsay Whitton (Scripps Research Institute, La Jolla, CA) (29). The Armstrong strain of LCMV was provided by Rafi Ahmed (Emory University, Atlanta, GA). Mice were infected with 2 × 106 pfu of VV-GP or 2 × 105 pfu of LCMV by i.p. injection. Infectious VV-GP and LCMV was quantitated by plaque assay on CV-1 cells and Vero cells, respectively (29).
Cell Surface Staining and Flow Cytometry.
Single-cell suspensions of splenocytes were obtained by standard procedures. Mononuclear cells were isolated from livers as described (30). MHC I tetramers that are specific to the LCMV epitope GP33–41 (Db/GP33) and vaccinia virus epitope B8R (Kb/B8R) were prepared as described (31). Single-cell suspensions of splenocytes or hepatic mononuclear cells were stained with anti-CD8, anti-CD44, and MHC I tetramers Db/GP33 or Kb/B8R as described previously (31). After staining, cells were fixed in 2% paraformaldehyde and analyzed on a FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA). All antibodies were purchased from BD Pharmingen (San Diego, CA).
Intracellular Staining and Flow Cytometry.
The number of cytokine-producing CD8+ T cells was determined by intracellular staining as described previously (31). Briefly, splenocytes were stimulated with the LCMV peptide GP33–41 (KAVYNFATM) or vaccinia peptide B8R20–27 (TSYKFESV) (32) for 6 h in the presence of brefeldin A. In certain experiments, doxycycline was supplemented in the culture media at a concentration of 2 μg/ml. After culture, cells were stained for cell surface CD8 and intracellular IFNγ by using the Cytofix/Cytoperm kit from BD Pharmingen. The number of cytokine-producing CD8+ T cells was determined by using a FACSCalibur flow cytometer (BD Biosciences). To stain for granzyme B in antigen-specific CD8+ T cells, splenocytes were surface stained with anti-CD8 and Db/GP33 tetramers. After surface staining, cells were stained for intracellular granzyme with the Cytofix/Cytoperm kit from BD Pharmingen and analyzed by flow cytometry. The anti-granzyme B and isotype control antibodies were purchased from Caltag (San Francisco, CA).
Western Blot Analysis.
Total cell lysates were prepared from single-cell suspensions of thymocytes in lysis buffer (50 mM Tris/2% SDS/12.8 mM 2-mercaptoethanol). Proteins derived from 2 × 106 cells were separated by SDS/PAGE under reducing conditions and electrophoretically transferred to Immobilon poly(vinylidene difluoride) membranes (Millipore, Bedford, MA). The membranes were probed with rabbit anti-human Lck antibody (BD Pharmingen) and anti-human β-actin antibody (Abcam, Cambridge, MA). The binding of antibodies was visualized with the ECL Plus Western Blotting Detection System (GE Healthcare, Little Chalfont, U.K.).
Cell Sorting and RT-PCR.
Total T cells were purified from the spleens of Lckon and Lckoff VVGP-infected mice by using T cell enrichment columns (R & D Systems, Minneapolis, MN). T cells were stained with anti-CD8, anti-CD44, and Kb/B8R MHC I tetramers, and CD8+CD44hi or CD8+CD44hi B8R-tetramer-binding cells were sorted in a FACSVantage DiVa sorter (BD Biosciences); the purity of the sorted cells was >95%. Total RNA was extracted from the sorted cells by using an RNA extraction kit (RNAqueous; Ambion, Austin, TX), and contaminating DNA was removed by using the TURBO DNA-free kit (Ambion). RNA was reverse transcribed to cDNA by using Moloney murine leukemia virus reverse transcriptase from Invitrogen (Carlsbad, CA). Equivalent amounts of cDNA (as determined by 18S rRNA measurements by quantitative PCR) were amplified in 35 cycles of PCR with Amplitaq Gold (Applied Biosystems, Foster City, CA) by using primers designed for Lck, and products were analyzed by gel electrophoresis. Primer sets for Lck were CGCATGGTGAGACCTGACAA (forward) and TCCGAAGGTAGTCAAACGTGG (reverse). cDNA was quantitated by using the following primer sets for 18S rRNA: CGCCGCTAGAGGTGAAATTCT (forward) and CGAACCTCCGACTTTCGTTCT (reverse).
Supplementary Material
Acknowledgments
We thank Katie Skell, Erin Hemmila, and Marlese Koenlin for technical assistance. This work was supported by U.S. Public Health Service Grants AI48785 and AI59804 (to M.S.).
Abbreviations
- GP
glycoprotein
- LCMV
lymphocytic choriomeningitis virus
- TCR
T cell receptor
- VV-GP
vaccinia virus that expresses the GP of LCMV.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Zamoyska R, Lovatt M. Curr Opin Immunol. 2004;16:191–196. doi: 10.1016/j.coi.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Seddon B, Zamoyska R. Curr Opin Immunol. 2003;15:321–324. doi: 10.1016/s0952-7915(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 3.Seddon B, Legname G, Tomlinson P, Zamoyska R. Science. 2000;290:127–131. doi: 10.1126/science.290.5489.127. [DOI] [PubMed] [Google Scholar]
- 4.Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B. Immunol Rev. 2003;191:107–118. doi: 10.1034/j.1600-065x.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 5.Straus DB, Weiss A. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 6.Molina TJ, Kishihara K., Siderovski DP, van Ewijk W, Narendran A, Timms E, Wakeham A, Paige CJ, Hartmann KU, Veillette A, et al. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 7.Legname G, Seddon B, Lovatt M, Tomlinson P, Sarner N, Tolaini M, Williams K, Norton T, Kioussis D, Zamoyska R. Immunity. 2000;12:537–546. doi: 10.1016/s1074-7613(00)80205-8. [DOI] [PubMed] [Google Scholar]
- 8.Trobridge PA, Levin SD. Eur J Immunol. 2001;31:3567–3579. doi: 10.1002/1521-4141(200112)31:12<3567::aid-immu3567>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Kaech SM, Ahmed R. Nat Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Stipdonk MJB, Lemmens EE, Schoenberger SP. Nat Immunol. 2001;2:423–429. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 11.Prlic M, Hernandez-Hoyos G, Bevan MJ. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 13.Polic B, Kunkel D, Scheffold A, Rajewsky K. Proc Natl Acad Sci USA. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schluns KS, Lefrancois L. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 15.Seddon B, Tomlinson P, Zamoyska R. Nat Immunol. 2003;4:680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 16.Groves T, Smiley P, Cooke MP, Forbush K, Perlmutter RM, Guidos CJ. Immunity. 1996;5:417–428. doi: 10.1016/s1074-7613(00)80498-7. [DOI] [PubMed] [Google Scholar]
- 17.van Oers NS, Killeen N, Weiss A. J Exp Med. 1996;183:1053–1062. doi: 10.1084/jem.183.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaech SM, Wherry EJ, Ahmed R. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 19.Farber DL, Acuto O, Bottomly K. Eur J Immunol. 1997;27:2094–2101. doi: 10.1002/eji.1830270838. [DOI] [PubMed] [Google Scholar]
- 20.Farber DL, Luqman M, Acuto O, Bottomly K. Immunity. 1995;2:249–259. doi: 10.1016/1074-7613(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 21.Kersh EN, Kaech SM, Onami TM, Moran M, Wherry EJ, Miceli MC, Ahmed R. J Immunol. 2003;170:5455–5463. doi: 10.4049/jimmunol.170.11.5455. [DOI] [PubMed] [Google Scholar]
- 22.Kaech SM, Hemby S, Kersh E, Ahmed R. Cell. 2002;111:837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 23.Bachmann MF, Gallimore A, Linkert S, Cerundolo V, Lanzavecchia A, Kopf M, Viola A. J Exp Med. 1999;189:1521–1530. doi: 10.1084/jem.189.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 25.Sugie K, Jeon MS, Grey HM. Proc Natl Acad Sci USA. 2004;101:14859–14864. doi: 10.1073/pnas.0406168101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bueno C, Lemke CD, Criado G, Baroja ML, Ferguson SSG, Nur-Ur Rahman AKM, Tsoukas CD, McCormick JK, Madrenas J. Immunity. 2006;25:67–78. doi: 10.1016/j.immuni.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Bachmann MF, Sebzda E, Kundig TM, Shahinian A, Speiser DE, Mak TW, Ohashi PS. Eur J Immunol. 1996;26:2017–2022. doi: 10.1002/eji.1830260908. [DOI] [PubMed] [Google Scholar]
- 28.Cai Z, Sprent J. J Exp Med. 1994;179:2005–2015. doi: 10.1084/jem.179.6.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitton JL, Southern PJ, Oldstone MB. Virology. 1988;162:321–327. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- 30.Masopust D, Vezys V, Marzo AL, Lefrancois L. Science. 2001;291:2413–2417. [PubMed] [Google Scholar]
- 31.Murali-Krishna K, Altman JD, Suresh M, Sourdive DJ, Zajac AJ, Miller JD, Slansky J, Ahmed R. Immunity. 1998;8:177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 32.Tscharke DC, Karupiah G, Zhou J, Palmore T, Irvine KR, Haeryfar SM, Williams S, Sidney J, Sette A, Bennink JR, Yewdell JW. J Exp Med. 2005;201:95–104. doi: 10.1084/jem.20041912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.