Abstract
Mast cells play a pivotal role in inflammatory and immediate-type allergic reactions by secreting a variety of potent inflammatory mediators, including sphingosine-1-phosphate (S1P). However, it is not known how S1P is released from cells. Here, we report that S1P is exported from mast cells independently of their degranulation and demonstrate that it is mediated by ATP binding cassette (ABC) transporters. Constitutive and antigen-stimulated S1P release was inhibited by MK571, an inhibitor of ABCC1 (MRP1), but not by inhibitors of ABCB1 (MDR-1, P-glycoprotein). Moreover, down-regulation of ABCC1 with small interfering RNA, which decreased its cell surface expression, markedly reduced S1P export from both rat RBL-2H3 and human LAD2 mast cells. Transport of S1P by ABCC1 influenced migration of mast cells toward antigen but not degranulation. These findings have important implications for S1P functions in mast cell-mediated immune responses.
Keywords: secretion, sphingolipids, multidrug resistance, allergic responses, sphingosine kinase
Sphingosine-1-phosphate (S1P) is a pleiotropic lipid mediator that has been implicated in cancer, immunity, and allergy (1–4). S1P is a ligand for a family of five specific G protein-coupled receptors, designated S1P1–5 (collectively referred to as S1PRs) (5), through which it regulates many different biological responses, including growth, survival, differentiation, cytoskeleton rearrangements, motility, angiogenesis, vascular maturation, lymphocyte trafficking, and mast cell functions (reviewed in refs. 1–3, 5, and 6). Moreover, like its precursors, sphingosine (Sph) and ceramide, S1P may also have intracellular functions (1, 2, 7).
S1P is produced in cells by phosphorylation of Sph, the backbone of all sphingolipids, catalyzed by two closely related Sph kinase (SphK) isoenzymes. SphK1 activity is enhanced by numerous external stimuli, including growth factors, ligands for G protein-coupled receptors, and proinflammatory cytokines, and by cross-linking of Ig receptors (reviewed in refs. 1 and 6). To date, only EGF (8) and cross-linking of Ig receptors (4) have been shown to stimulate SphK2.
Although S1P secretion has been demonstrated in only a few types of cells (platelets, astroglial cells, and mast cells) activation of SphK1 involves its translocation to the plasma membrane where its substrate Sph resides (9–11). Increased S1P formation, in turn, activates S1P receptors on the same and/or neighboring cells in an autocrine or paracrine manner. It has been demonstrated that this type of “inside-out” signaling by S1P is critical for migratory responses of fibroblasts and smooth muscle cells toward PDGF (9, 12) and human breast cancer cells toward EGF (8). Similarly, S1P secreted by mast cells is important for migration of mast cells toward antigen (Ag) and might be involved in the movement of mast cells to sites of inflammation (13). In addition, S1P provokes human airway smooth muscle contraction and may promote inflammation and airway remodeling in asthma (14). Because S1P's levels are significantly elevated in bronchoalveolar lavage fluid of asthmatics after allergen challenge, it has been suggested that secretion of S1P from mast cells perpetuates inflammation and allergic responses (13, 14).
It is still a mystery how S1P produced inside cells by two SphKs can reach its receptors on the cell surface. Membrane lipids do not spontaneously exchange across lipid bilayers as the polar head groups do not readily traverse the hydrophobic interior of the membrane, and cells have many special transporter proteins for this purpose. Sph is an exception and when added to cells or produced intracellularly spontaneously translocates to intracellular membranes. An important question that this article is focused on is: how is S1P transported out of cells?
Results
S1P Secretion Is Independent of Degranulation.
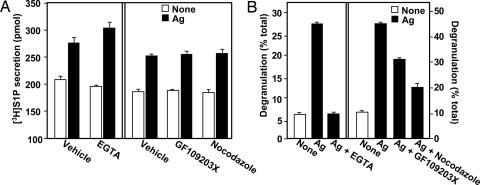
Cross-linking of the high-affinity IgE receptor, FcεR1, enhanced S1P production and secretion from RBL-2H3 mast cells (Fig. 1A), in agreement with previous studies (4, 13, 15, 16). Mast cells contain preformed inflammatory mediators that are packaged in secretory granules, and it is possible that at least some of the S1P is stored within these granules and released during degranulation. However, the calcium chelator EGTA, which blocks extracellular calcium influx, significantly inhibited Ag-induced degranulation (Fig. 1B), as expected (17), but had no effect on Ag-stimulated S1P secretion (Fig. 1A). The highly specific protein kinase C inhibitor GF109203X and the microtubule depolymerizer nocodazole, which block exocytosis (17, 18), also significantly decreased degranulation (Fig. 1B), and like EGTA, had no effect on S1P secretion (Fig. 1A). These results suggest that S1P secretion and degranulation are unrelated events.
Fig. 1.
Ag-induced S1P release from mast cells is independent of degranulation. (A) RBL-2H3 cells were sensitized with anti-dinitrophenyl (DNP) IgE overnight and treated without or with EGTA (2.5 mM), nocodazole (1 μM), or GF109203X (1 μM) for 15 min. Cells were then labeled for 30 min at 37°C with [3H]Sph (1.5 μM, 0.45 μCi) in serum-free medium and stimulated without (open bars) or with DNP-HSA (Ag, 100 ng/ml, filled bars). Secretion of [3H]S1P was determined by scintillation counting after differential extraction. Data are means ± SD. (B) Duplicate cultures of IgE-sensitized RBL-2H3 cells were preincubated without or with EGTA, nocodazole, or GF109203X for 15 min at 37°C and then stimulated without (open bars) or with DNP-HSA (100 ng/ml, filled bars) for 30 min. Degranulation was assessed by measuring release of β-hexosaminidase.
ABCB1 Is Not Involved in Secretion of S1P.
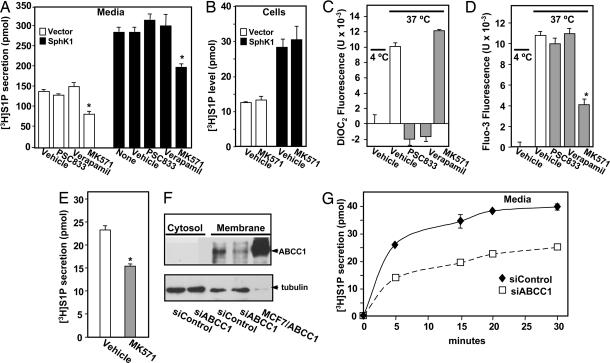
Studies originally related to multidrug resistance in cancer cells identified several ATP-binding cassette (ABC) transporters that in addition to amphiphilic drugs, catalyze the movement of lipids from the inner to the outer leaflet of the plasma membrane (reviewed in ref. 19). In particular, ABCB1 (previously called MDR-1 and P-glycoprotein) is a promiscuous transporter of hydrophobic substrates including a broad variety of short-chain analogues of phosphatidylcholine, glucosylceramide, and sphingomyelin, as well as platelet-activating factor, from the cytosol to the extracellular environment (20, 21). To investigate the role of ABCB1 in S1P transport, RBL-2H3 mast cells were pretreated with the ABCB1 inhibitors verapamil and PSC833, and secretion of S1P was determined. These inhibitors had no effect on [3H]S1P secretion even after overexpression of SphK1, which enhanced S1P secretion as described (16) (Fig. 2A). Moreover, they had no effects on the levels of cell-associated S1P (data not shown). To assess the functional activity of these inhibitors, cells were loaded with a fluorescent transport substrate DiOC2, which is transported by ABCB1 more efficiently than by other ABC transporters (22), and fluorescence secreted from the cells was measured (23). There was increased fluorescence at 37°C, indicating transport of the dye from the intracellular to the extracellular compartment, whereas cells held at 4°C exported DiOC2 to a much lesser extent. As shown in Fig. 2C, verapamil and PSC833, but not MK571 (an ABCC1 inhibitor), significantly inhibited DiOC2 efflux from the cells, confirming that these inhibitors effectively blocked efflux by ABCB1. Taken together, these data suggest that ABCB1 does not mediate release of S1P.
Fig. 2.
Involvement of ABC transporters in S1P secretion. (A and B) RBL-2H3 cells stably expressing vector (open bars) or SphK1 (filled bars) were preincubated for 1 h at 37°C with vehicle or 15 μM verapamil, PSC833, or MK571 as indicated. Cells were then incubated for 30 min with [3H]Sph (1.5 μM, 0.45 μCi) and [3H]S1P in media (A) and cells (B) differentially extracted and quantified by scintillation counting. ∗, P < 0.01. Data are means ± SD. (C and D) RBL-2H3 cells were loaded with DiOC2 (C) or Fluo3-AM (D), washed, and then kept at 4°C or treated with vehicle or 15 μM PSC833, verapamil, or MK571 for 1 h at 37°C, and DiOC2 (C) and Fluo3 (D) fluorescence in the medium was measured with a FLUOstar OPTIMA fluorescence plate reader. Data are means ± SD of fluorescence measurements at 37°C minus those at 4°C. (E–G) Involvement of ABCC1 in release of S1P by LAD2 cells. (E) LAD2 cells were preincubated for 1 h at 37°C with vehicle or MK571 (15 μM) and incubated with [3H]Sph for 30 min at 37°C, and [3H]S1P secretion was measured. (F) LAD2 cells transfected with siControl or siABCC1 were lysed, and membrane and cytosol fractions were prepared by centrifugation at 100,000 × g. Equal amounts of proteins (70 μg) from cytosol and membrane fractions were separated by SDS/PAGE and immunoblotted with anti-ABCC1. Lysate (10 μg) from MCF7 cells overexpressing ABCC1 (45) was included as a positive control. Blots were stripped and reprobed with antitubulin to confirm equal loading. (G) LAD2 cells transfected with siControl (⧫) or siABCC1 (□) were incubated with [3H]Sph, and [3H]S1P secretion was measured at the indicated times.
Inhibitors of ABCC1 Decrease S1P Export.
Another ABC family member, ABCC1, selectively transports C6(N-6[7-nitro-2,1,3-benzoxadiazol-4yl] aminohexanoyl) (NBD)-glucosylceramide and C6-NBD-sphingomyelin (24). To explore ABCC1's potential role in the export of S1P, RBL-2H3 cells were treated with MK571, an ABCC1-specific inhibitor (25, 26). MK571 inhibited S1P secretion by vector and SphK1 transfected RBL-2H3 cells (Fig. 2A), whereas it did not affect uptake and intracellular conversion of [3H]Sph to S1P (Fig. 2B). To confirm that MK571 inhibited ABCC1-mediated transport, cells were loaded with Fluo-3AM, a fluorescent dye that is transported out of the cell by ABCC1 (22, 25). In agreement with other reports (22, 25), only MK571, and not PSC833 or verapamil, inhibited Fluo-3 efflux (Fig. 2D).
Involvement of ABCC1 in Export of S1P from Human Mast Cells.
We next examined S1P secretion by human LAD2 mast cells that express functional cell surface FcεRI and KIT, the receptor for stem cell factor (SCF). MK571 also inhibited S1P export by LAD2 cells (Fig. 2E). Previous studies have implicated the 190-kDa integral plasma membrane glycoprotein ABCC1 in ATP-dependent export of LTC4 from human mast cells (27). LAD2 mast cells express ABCC1 on the plasma membrane (Fig. 2F), which was specifically down-regulated after transfection with siRNA targeted to human ABCC1 (Fig. 2F). Down-regulation of ABCC1 markedly reduced S1P export (Fig. 2G), without affecting uptake of Sph or its intracellular metabolism (data not shown).
Role of ABCC1 in Ag-Stimulated Release of S1P.
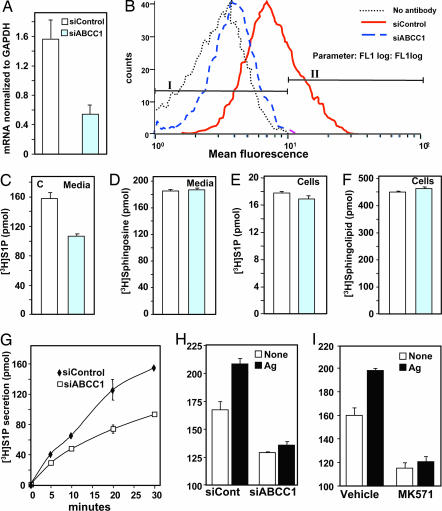
Similarly, down-regulation of ABCC1 in RBL-2H3 cells not only reduced ABCC1 mRNA, as measured by quantitative PCR, by >60% (Fig. 3A), it also markedly reduced its expression on the cell surface as determined by flow cytometry (Fig. 3B). Similar to LAD2 cells, reduction of ABCC1 expression significantly decreased S1P release compared with cells transfected with scrambled siRNA (Fig. 3 C and G). ABCC1 siRNA, however, did not affect uptake of [3H]Sph (Fig. 3D) or its intracellular conversion to complex sphingolipids (Fig. 3F) or S1P (Fig. 3E). Importantly, small interfering ABCC1 (siABCC1) not only reduced constitutive S1P secretion by RBL-2H3 cells, it also markedly reduced Ag-stimulated secretion (Fig. 3H). In agreement, the ABCC1 inhibitor MK571 also blocked Ag-stimulated release of S1P (Fig. 3I).
Fig. 3.
Down-regulation of ABCC1 decreases S1P secretion. RBL-2H3 cells were transfected with control siRNA or siRNA targeted to ABCC1. (A) RNA was isolated, and mRNA levels of ABCC1 and GAPDH were determined by quantitative real-time PCR. (B) In duplicate cultures, ABCC1 expression on the surface was determined by flow cytometry. Percentage of cells staining above background in fluorescence intensity gates I and II are: no antibody, 99.1 and 0.1; siControl, 73.1 and 26.8; siABCC1, 94.8 and 4.7, respectively. (C–H) RBL-2H3 cells transfected with siControl (open bars) or siABCC1 (gray bars) were labeled with [3H]Sph (1.5 μM, 0.45 μCi) for 30 min (C–F) or the indicated times (G) in serum-free medium. Lipids were differentially extracted from media (C, D, and G) and cells (E and F) into aqueous (C, E, and G) and organic fractions (D and F) and quantified by scintillation counting. Data are the means ± SD. (H) RBL-2H3 cells transfected with siControl or siABCC1 were sensitized with anti-DNP IgE overnight, labeled with [3H]Sph for 30 min, and stimulated without (open symbols) or with DNP-HSA (Ag, 100 ng/ml, filled symbols) for 30 min, and [3H]S1P released into the media was quantified. (I) RBL-2H3 cells were sensitized with anti-DNP IgE overnight, pretreated with vehicle or 15 μM MK571 for 1 h, labeled with [3H]Sph for 30 min, and stimulated without (open symbols) or with (filled bars) Ag for 30 min, and [3H]S1P released into the media was quantified. Data are the means ± SD.
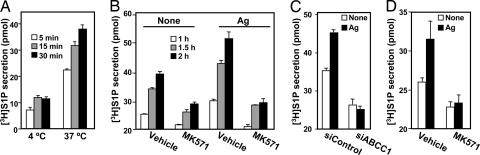
To further substantiate the role of ABCC1 in export of S1P, mast cells were extensively washed to eliminate [3H]S1P potentially bound to the cell surface followed by incubation in fresh medium, and kinetics of secretion of [3H]S1P was determined. Relatively little secretion of S1P was detected when cells were incubated at 4°C compared with 37°C (Fig. 4A), confirming that S1P secretion is an energy-dependent process. Similar to the LAD2 and RBL-2H3 mast cell lines, primary human skin-derived mast cells and murine bone marrow-derived mast cells also secrete S1P in a time-dependent manner, which is enhanced by FcεRI cross-linking with Ag (Fig. 6 A and B, which is published as supporting information on the PNAS web site).
Fig. 4.
Inhibition of ABCC1 blocks constitutive and Ag-induced S1P secretion. (A) RBL-2H3 cells were labeled with [3H]Sph (1.5 μM, 0.45 μCi) for 10 min, washed extensively, and incubated in 1 ml of serum-free medium for the indicated times at 4°C or 37°C, and secretion of [3H]S1P was determined. (B) RBL-2H3 cells were sensitized with anti-DNP IgE overnight, labeled with [3H]Sph for 10 min, washed extensively, incubated in fresh medium in the absence or presence of MK571, and stimulated without or with Ag. [3H]S1P release was measured at the indicated times. (C) RBL-2H3 cells transfected with siControl or siABCC1 were sensitized with anti-DNP IgE overnight, labeled with [3H]Sph for 10 min, washed extensively, and incubated in fresh medium without (open bars) or with (filled bars) Ag for 30 min, and [3H]S1P release was measured. (D) Human skin mast cells were sensitized with anti-DNP IgE overnight, labeled with [3H]Sph for 10 min, washed extensively, incubated in fresh medium in the absence or presence of MK571 for 1 h, and then stimulated without (open bars) or with (filled bars) Ag (30 ng/ml, hatched bars), and [3H]S1P release was measured after 1 h. Data are means ± SD.
MK571 markedly suppressed constitutive and Ag-stimulated S1P secretion (Fig. 4B) and secretion from both vector and SphK1-transfected RBL-2H3 cells (Fig. 6C). In concordance, down-regulation of ABCC1 also reduced constitutive S1P secretion and blocked Ag-stimulated secretion (Fig. 4C). Similarly, MK571 also inhibited constitutive and Ag-stimulated S1P secretion from skin-derived mast cells (Fig. 4D). Collectively, our results suggest that ABCC1 is involved in export of S1P from rodent and human mast cells.
Transport of S1P by ABCC1 Influences Migration of Mast Cells Toward Ag but Not Degranulation.
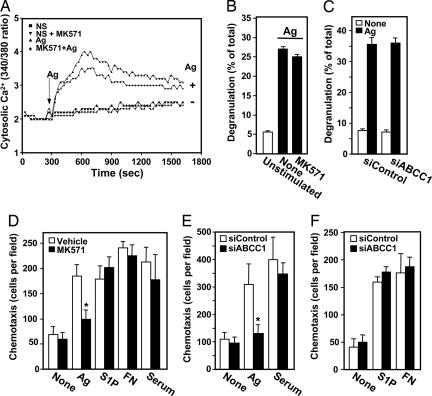
It has been suggested that intracellular S1P formed by activation of SphK after FcεRI triggering mobilizes calcium from internal stores in a unique manner independent of inositol trisphopshate (7, 28, 29). Decreasing S1P secretion with MK571 had only a minimal effect on Ag-stimulated calcium mobilization (Fig. 5A). Moreover, degranulation of mast cells induced by Ag was not affected by inhibiting S1P secretion with MK571 or down-regulating ABCC1 (Fig. 5 B and C), further supporting the notion of an intracellular role for S1P in calcium mobilization and degranulation (7, 15, 28, 29).
Fig. 5.
Inhibition of ABCC1 affects Ag-induced chemotaxis but not degranulation or calcium mobilization. (A) IgE-sensitized RBL-2H3 cells were loaded with the calcium indicator Fura-2AM, pretreated without or with MK571 (15 μM), and then stimulated without or with Ag (100 ng/ml), as indicated. Changes in intracellular free calcium were determined by the ratio of fluorescence emission at 510 nm after excitation at 340 and 380 nm. (B) IgE-sensitized RBL-2H3 cells were pretreated with vehicle or MK571 (15 μM) for 1 h, followed by stimulation with DNP-HSA (Ag, 100 ng/ml, filled bars) for 30 min, and degranulation was measured. (C) IgE-sensitized LAD2 cells transfected with siControl or siABCC1 were stimulated without (open bars) or with (filled bars) Ag for 30 min, and degranulation was measured. (D) IgE-sensitized RBL-2H3 cells were treated without (open bars) or with (gray bars) 15 μM MK571 for 2 h and then allowed to migrate toward vehicle (None), Ag (30 ng/ml), S1P (10 nM), fibronectin (20 μg/ml), or serum (2%) for 4 h, and chemotaxis was measured as described (13). (E and F) RBL-2H3 cells transfected with siControl (open bars) or siABCC1 (filled bars) were sensitized with anti-DNP IgE overnight and allowed to migrate toward vehicle, Ag, serum, S1P, and fibronectin for 4 h. Results are expressed as means ± SD of triplicate determinations.
However, we recently reported that chemotaxis of mast cells toward Ag, which plays an important role in immune responses at inflammation sites, requires inside-out signaling of S1P and subsequent activation of its cell surface receptors (13). Interestingly, inhibition of ABCC1 by MK571 or its down-regulation with siRNA, which both decreased S1P secretion, also markedly reduced chemotaxis of RBL-2H3 cells toward Ag (Fig. 5 D and E). These effects appeared to be specific, as migration toward S1P and serum or haptotactic migration toward fibronectin were not significantly altered (Fig. 5 D–F).
To conclusively demonstrate that these effects were mediated by S1P and not by cysteinyl leukotrienes (Cys-LTs), which are also released during activation of mast cells by the same ABCC1 transporters (30, 31), mast cells were treated with MK886, a potent and specific leukotriene biosynthesis inhibitor (32). MK886, in contrast to MK571, had no effect on Ag-stimulated S1P secretion (Fig. 7A, which is published as supporting information on the PNAS web site) or Ag-induced chemotaxis of mast cells (Fig. 7B); yet as expected (31), it blocked Ag-induced Cys-LT formation (Fig. 7C). MK571 can also function as a Cys-LT1-selective antagonist (31). However, the potent Cys-LT1 receptor antagonist Montelukast, in contrast to MK571, had no significant effect on S1P secretion (Fig. 7D).
Discussion
S1P is a recent addition to the many bioactive compounds produced and released by mast cells (7, 13, 15, 29). Previous studies with human bone marrow-derived mast cells (29) and RBL-2H3 mast cells (13, 16) have shown that SphK1 is primarily cytosolic and is rapidly translocated to the plasma membrane by Ag. FcεRI cross-linking activates both SphK1 and SphK2 and requires the Src protein tyrosine kinases Lyn (33) and Fyn (4). The finding that SCF, an important growth factor required for mast cell survival and differentiation, also activates SphKs (4) further emphasizes the importance of S1P in mast cells. Yet it was not clear from any of these studies how S1P generated intracellularly is released from these mast cells or reaches its cell surface receptors.
In this study, using pharmacological and molecular approaches, we showed that ABCC1 is involved in transport of S1P out of rodent and human mast cells, especially after Ag stimulation. Activation and translocation of both isoforms of SphK to the plasma membrane after FcεRI cross-linking, serum, and SCF (4) and therefore to their substrate Sph and the subsequent synthesis of S1P at the plasma membrane in close proximity to ABCC1 could account for the abundant constitutive and stimulated secretion of S1P by Ag in mast cells. Similarly, overexpressed SphK1 is already localized to the plasma membrane (16) from which S1P export required ABCC1 activity.
Constitutive secretion of S1P might also be partially independent of ABCC1 as it is inhibited to a lesser extent than Ag-stimulated S1P secretion. Mast cells express multiple ABC transporters that also could contribute to basal S1P secretion. Moreover, production of S1P in mast cells has grown even more complex with the recent demonstration that the kinetics and mechanisms of activation of SphK1 and SphK2 in mast cells by Ag, SCF, and IL-3 are distinct (4).
Little is known of the expression of ABC family transporters on human mast cells, although it has been shown that ABCB1 (34) and ABCC1, but not ABCC2 or ABCC3 (35), are present on rodent mast cells. Mice deficient in ABCC1 display impaired inflammatory responses attributed to decreased secretion of LTC4 from leukotriene-synthesizing cells (30). It is tantalizing to speculate that the impaired inflammatory responses might be partly caused by impaired secretion of S1P, which acts not only in an autocrine manner to regulate mast cells functions (4, 7, 13, 15, 16), but in a broader manner to promote inflammation by recruiting and activating other cells involved in allergic and inflammatory responses (6, 13, 14). Interestingly, intracellular S1P was first linked to the initial rise in mast cell calcium induced by Ag and its mobilization from internal stores independently of inositol trisphosphate (7, 29). Our results are consistent with an intracellular role for S1P in calcium mobilization and degranulation (7, 15, 28, 29). More recently, it was shown that secretion of S1P from mast cells and activation of its S1P1 receptor plays an important role in chemotaxis (13). Discovery of an active transport system for mast cell secretion of S1P further supports the notion that S1P secretion might regulate migration of mast cells toward Ag and their arrival at sites of inflammation.
The observation that S1P can be secreted by ABCC1 has important implications not only for mast cells and inflammatory responses, but perhaps for other physiological and pathological processes regulated by S1P. For example, clinical studies have documented expression of ABCC1 in a range of solid and hematological cancers and, in some cases, have correlated its expression with negative responses to treatment and poor disease outcome (36). SphK1 is also up-regulated in many types of cancer (37, 38). Moreover, a monoclonal antibody that binds S1P with extremely high affinity and specificity significantly slows tumor progression and associated angiogenesis in several animal models of human cancer (39). The ability of S1P to act in an autocrine or paracrine manner, combined with its actions on angiogenesis and vascular maturation, which are also critical for tumor progression, suggest that increased secretion of S1P by cancer cell, perhaps by ABC transporters, caused by up-regulation of SphK1 or the transporter could contribute to tumorigenesis.
Materials and Methods
Cell Culture and Transfection.
RBL-2H3 cells and human LAD2 mast cells were cultured as described (13, 40). Highly purified human skin mast cells (41) and bone marrow-derived mast cells (13) were prepared and cultured as described.
Secretion of S1P.
Mast cells (106 cells) cultured in six-well plates were incubated with [3H]Sph (1.5 μM, 0.45 μCi) for 10 or 30 min, as indicated. The medium was then removed, and the cells were washed with cold PBS. Alkaline chloroform-methanol extraction was used to isolate lipids from the medium, and cells and phases were separated as described (42). In this differential extraction procedure, S1P partitions into the aqueous phase at alkaline pH with high recovery, while Sph, as well as ceramide and other sphingolipids, remains in the organic phase. In agreement with previous studies (42, 43), >95% of the radioactivity in the aqueous phase was [3H]S1P as determined by TLC. Data are expressed as pmol S1P released per million cells.
Degranulation.
Mast cell degranulation was measured by the release of the granule marker β-hexosaminidase (13).
Chemotaxis.
Chemotaxis was determined with a modified Boyden chamber exactly as described (13).
Calcium Measurements.
Cytosolic free Ca2+ was measured in Fura2-loaded RBL-2H3 cells as described (44).
Transporter Efflux Assays.
Cells were loaded with DiOC2 or Fluo-3AM, which are transported by ABCB1 and ABCC1, respectively, and fluorescence secreted from the cells was measured (23).
siRNA Transfection.
RBL-2H3 cells were transfected in OptiMem (Invitrogen, Carlsbad, CA) with siRNA (50 nM) targeted to ABCC1 (CGUCAGAAGAAGUGGUACCTT and GGUACCACUUCUUCUGACGTG; Ambion, Austin, TX) or a scrambled siRNA by using Oligofectamine (Invitrogen) (13). Quantitative PCR was performed with premixed primer-probe sets by using the ABI 7800 (Applied Biosystems, Foster City, CA). LAD2 cells were transfected with 200 nM sequence-specific siRNA for human ABCC1 (GGAAGGGAGUUCAGUCUUCTT and GAAGACUGAACUCCCUUCCTC; Ambion) or a scrambled siRNA in Stem-Pro-34 medium by using Lipofectamine 2000 (Invitrogen).
Flow Cytometry.
RBL-2H3 cells (106 per ml) were washed and incubated with antibodies in PBS containing 1% BSA at 4°C essentially as described (22).
Further details are available in Supporting Text, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank Dr. C. S. Morrow (Wake Forest University, Winston-Salem, NC) for MCF-7 cells overexpressing ABCC1, Drs. D. D. Metcalfe and A. S. Kirshenbaum (National Institutes of Health) for LAD2 cells, Dr. J. Ryan (Virginia Commonwealth University) for bone marrow-derived mast cells, and Amgen (Thousand Oaks, CA) for SCF. This work was supported by National Institutes of Health Grant RO1AI50094 (to S.S.), by the National Institute of Mental Health Intramural Research Program (S.M.), and in part by the National Heart, Lung, and Blood Institute Intramural Research Program (M.A.B.).
Abbreviations
- Ag
antigen
- ABC
ATP-binding cassette
- DNP
dinitrophenyl
- SCF
stem cell factor
- Sph
sphingosine
- S1P
Sph-1-phosphate
- SphK
Sph kinase
- siABCC1
small interfering ABCC1.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Spiegel S, Milstien S. Nat Rev Mol Cell Biol. 2003;4:397–407. doi: 10.1038/nrm1103. [DOI] [PubMed] [Google Scholar]
- 2.Saba JD, Hla T. Circ Res. 2004;94:724–734. doi: 10.1161/01.RES.0000122383.60368.24. [DOI] [PubMed] [Google Scholar]
- 3.Rosen H, Goetzl EJ. Nat Rev Immunol. 2005;5:560–570. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 4.Olivera A, Urtz N, Mizugishi K, Yamashita Y, Gilfillan AM, Furumoto Y, Gu H, Proia RL, Baumruker T, Rivera J. J Biol Chem. 2006;281:2515–2525. doi: 10.1074/jbc.M508931200. [DOI] [PubMed] [Google Scholar]
- 5.Anliker B, Chun J. J Biol Chem. 2004;279:20555–20558. doi: 10.1074/jbc.R400013200. [DOI] [PubMed] [Google Scholar]
- 6.Olivera A, Rivera J. J Immunol. 2005;174:1153–1158. doi: 10.4049/jimmunol.174.3.1153. [DOI] [PubMed] [Google Scholar]
- 7.Choi OH, Kim J-H, Kinet J-P. Nature. 1996;380:634–636. doi: 10.1038/380634a0. [DOI] [PubMed] [Google Scholar]
- 8.Hait NC, Sarkar S, Le Stunff H, Mikami A, Maceyka M, Milstien S, Spiegel S. J Biol Chem. 2005;280:29462–29469. doi: 10.1074/jbc.M502922200. [DOI] [PubMed] [Google Scholar]
- 9.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 10.Johnson KR, Becker KP, Facchinetti MM, Hannun YA, Obeid LM. J Biol Chem. 2002;277:35257–35262. doi: 10.1074/jbc.M203033200. [DOI] [PubMed] [Google Scholar]
- 11.Pitson SM, Xia P, Leclercq TM, Moretti PA, Zebol JR, Lynn HE, Wattenberg BW, Vadas MA. J Exp Med. 2005;201:49–54. doi: 10.1084/jem.20040559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goparaju SK, Jolly PS, Watterson KR, Bektas M, Alvarez S, Sarkar S, Mel L, Ishii I, Chun J, Milstien S, Spiegel S. Mol Cell Biol. 2005;25:4237–4249. doi: 10.1128/MCB.25.10.4237-4249.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jolly PS, Bektas M, Olivera A, Gonzalez-Espinosa C, Proia RL, Rivera J, Milstien S, Spiegel S. J Exp Med. 2004;199:959–970. doi: 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ammit AJ, Hastie AT, Edsall LC, Hoffman RK, Amrani Y, Krymskaya VP, Kane SA, Peters SP, Penn RB, Spiegel S, Panettieri RA., Jr FASEB J. 2001;15:1212–1214. doi: 10.1096/fj.00-0742fje. [DOI] [PubMed] [Google Scholar]
- 15.Prieschl EE, Csonga R, Novotny V, Kikuchi GE, Baumruker T. J Exp Med. 1999;190:1–8. doi: 10.1084/jem.190.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jolly PS, Bektas M, Watterson KR, Sankala H, Payne SG, Milstien S, Spiegel S. Blood. 2005;105:4736–4742. doi: 10.1182/blood-2004-12-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozawa K, Szallasi Z, Kazanietz MG, Blumberg PM, Mischak H, Mushinski JF, Beaven MA. J Biol Chem. 1993;268:1749–1756. [PubMed] [Google Scholar]
- 18.Oka T, Hori M, Ozaki H. J Immunol. 2005;174:4584–4589. doi: 10.4049/jimmunol.174.8.4584. [DOI] [PubMed] [Google Scholar]
- 19.van Meer G, Lisman Q. J Biol Chem. 2002;277:25855–25858. doi: 10.1074/jbc.R200010200. [DOI] [PubMed] [Google Scholar]
- 20.van Helvoort A, Smith AJ, Sprong H, Fritzsche I, Schinkel AH, Borst P, van Meer G. Cell. 1996;87:507–517. doi: 10.1016/s0092-8674(00)81370-7. [DOI] [PubMed] [Google Scholar]
- 21.Raggers RJ, Vogels I, van Meer G. Biochem J. 2001;357:859–865. doi: 10.1042/0264-6021:3570859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honig SM, Fu S, Mao X, Yopp A, Gunn MD, Randolph GJ, Bromberg JS. J Clin Invest. 2003;111:627–637. doi: 10.1172/JCI16200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhary PM, Roninson IB. Cell. 1991;66:85–94. doi: 10.1016/0092-8674(91)90141-k. [DOI] [PubMed] [Google Scholar]
- 24.Raggers RJ, van Helvoort A, Evers R, van Meer G. J Cell Sci. 1999;112:415–422. doi: 10.1242/jcs.112.3.415. [DOI] [PubMed] [Google Scholar]
- 25.Prechtl S, Roellinghoff M, Scheper R, Cole SP, Deeley RG, Lohoff M. J Immunol. 2000;164:754–761. doi: 10.4049/jimmunol.164.2.754. [DOI] [PubMed] [Google Scholar]
- 26.Dallas S, Zhu X, Baruchel S, Schlichter L, Bendayan R. J Pharmacol Exp Ther. 2003;307:282–290. doi: 10.1124/jpet.103.054304. [DOI] [PubMed] [Google Scholar]
- 27.Bartosz G, Konig J, Keppler D, Hagmann W. Biol Chem. 1998;379:1121–1126. doi: 10.1515/bchm.1998.379.8-9.1121. [DOI] [PubMed] [Google Scholar]
- 28.Kinet JP. Annu Rev Immunol. 1999;17:931–972. doi: 10.1146/annurev.immunol.17.1.931. [DOI] [PubMed] [Google Scholar]
- 29.Melendez AJ, Khaw AK. J Biol Chem. 2002;277:17255–17262. doi: 10.1074/jbc.M110944200. [DOI] [PubMed] [Google Scholar]
- 30.Wijnholds J, Evers R, van Leusden MR, Mol CA, Zaman GJ, Mayer U, Beijnen JH, van der Valk M, Krimpenfort P, Borst P. Nat Med. 1997;3:1275–1279. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]
- 31.Mellor EA, Austen KF, Boyce JA. J Exp Med. 2002;195:583–592. doi: 10.1084/jem.20020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouzer CA, Ford-Hutchinson AW, Morton HE, Gillard JW. J Biol Chem. 1990;265:1436–1442. [PubMed] [Google Scholar]
- 33.Urtz N, Olivera A, Bofill-Cardona E, Csonga R, Billich A, Mechtcheriakova D, Bornancin F, Woisetschlager M, Rivera J, Baumruker T. Mol Cell Biol. 2004;24:8765–8777. doi: 10.1128/MCB.24.19.8765-8777.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wein D, Gupta S. Cancer Lett. 1996;101:241–246. doi: 10.1016/0304-3835(96)04146-8. [DOI] [PubMed] [Google Scholar]
- 35.Candussio L, Crivellato E, Rosati AM, Klugmann FB, Granzotto M, Giraldi T, Decorti G. Histochem J. 2001;33:259–266. doi: 10.1023/a:1017920922500. [DOI] [PubMed] [Google Scholar]
- 36.Kruh GD, Belinsky MG. Oncogene. 2003;22:7537–7552. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 37.Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, Wargovich MJ, Reddy BS, Hannun YA, Obeid LM, Zhou D. FASEB J. 2005;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 38.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN, Eberly JL, Yun JK, Smith CD. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 39.Visentin B, Vekich JA, Sibbald BJ, Cavalli AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, et al. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 40.Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Leuk Res. 2003;27:677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- 41.Oskeritzian CA, Zhao W, Pozez AL, Cohen NM, Grimes M, Schwartz LB. J Immunol. 2004;172:593–600. doi: 10.4049/jimmunol.172.1.593. [DOI] [PubMed] [Google Scholar]
- 42.Edsall LC, Spiegel S. Anal Biochem. 1999;272:80–86. doi: 10.1006/abio.1999.4157. [DOI] [PubMed] [Google Scholar]
- 43.Paugh SW, Payne SG, Barbour SE, Milstien S, Spiegel S. FEBS Lett. 2003;554:189–193. doi: 10.1016/s0014-5793(03)01168-2. [DOI] [PubMed] [Google Scholar]
- 44.Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Blood. 2004;104:2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- 45.Paumi CM, Wright M, Townsend AJ, Morrow CS. Biochemistry. 2003;42:5429–5437. doi: 10.1021/bi027347u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.