Abstract
Microbial pathogens with the ability to establish chronic infections have evolved strategies to actively modulate the host immune response. Brucellosis is a disease caused by a Gram-negative intracellular pathogen that if not treated during the initial phase of the infection becomes chronic as the bacteria persist for the lifespan of the host. How this pathogen and others achieve this action is a largely unanswered question. We report here the identification of a Brucella abortus gene (prpA) directly involved in the immune modulation of the host. PrpA belongs to the proline-racemase family and elicits a B lymphocyte polyclonal activation that depends on the integrity of its proline-racemase catalytic site. Stimulation of splenocytes with PrpA also results in IL-10 secretion. Construction of a B. abortus-prpA mutant allowed us to assess the contribution of PrpA to the infection process. Mice infected with B. abortus induced an early and transient nonresponsive status of splenocytes to both Escherichia coli LPS and ConA. This phenomenon was not observed when mice were infected with a B. abortus-prpA mutant. Moreover, the B. abortus-prpA mutant had a reduced capacity to establish a chronic infection in mice. We propose that an early and transient nonresponsive immune condition of the host mediated by this B cell polyclonal activator is required for establishing a successful chronic infection by Brucella.
Keywords: B cell mitogen, proline racemase, Brucellosis, immunomodulation
An interesting area of microbial pathogenesis is the cross-talk between the pathogen and the immune system of the host. It is currently known that certain pathogens have evolved the capacity to actively modulate the immune response of the host to avoid its bactericidal effects (1). Understanding these mechanisms can be useful not only for the development of new vaccines or therapeutic agents, but also as tools for the study of components and regulation of the host immune system.
Microorganisms capable of establishing chronic infections have not only the ability to initially escape the immune response of the host but also the capacity to persist in the host during its life span. An important member of this group of organisms is Brucella spp., the causative agent of the zoonotic disease called brucellosis. Brucellosis is an important human and animal health problem in many developing countries. It is caused by several distinct species of Brucella, which can be differentiated by their preferential host (2). The disease is characterized by an initial acute phase that, if not treated, can become chronic and persist over the life span of the host. Immunity against Brucellae requires cell-mediated mechanisms, in particular a T helper 1 immune response (3–5) characterized by the production of IL-12 and IFN-γ, which is associated with protective immunity (6–8).
Analysis of Brucella melitensis (9), Brucella suis (10), and Brucella abortus (11) genomes revealed the absence of classical virulence genes such as capsules, fimbriae, exotoxins, cytolysins, resistance forms, antigenic variation, plasmids, or lysogenic phages. The study of Brucella pathogenicity has been focused mainly on identifying factors that affect the intracellular trafficking and multiplication of the bacterium within the host cell, an essential trait of Brucella virulence. It has been found that the type IV secretion system VirB is involved in controlling the maturation of the Brucella-containing vacuole into a replication-permissive organelle (12, 13), cyclic β1–2-glucans help to prevent phagosome-lysosome fusion, allowing for bacterial intracellular replication (14), and the O-polysaccharide inhibits phagocytosis, protecting the bacteria from the phagolysosome and inhibiting host cell apoptosis (15, 16).
Although information about Brucellae intracellular replication and trafficking has notably increased in the last 10 years, little is known about the molecular factors associated with chronicity and immunomodulation of the host. Brucella LPS was described in vitro as a down-regulator of T cell activation (17), being the only molecule with immunomodulatory properties described thus far.
This work describes the identification of an immunomodulatory protein of Brucella abortus. Here, we analyze the role of a proline racemase of Brucella (PrpA), which is homologous to a proline racemase with mitogenic activity in the human protozoan parasite Trypanosoma cruzi. We show that B. abortus PrpA is a T-independent B lymphocyte mitogen and a potent IL-10 inducer required for the establishment of chronicity and the early immune suppression observed in mice after infection. The possible role of this gene in the infection process is discussed.
Results
B. abortus Has Two Genes Coding for Proteins of the Proline Racemase Family.
A comparison of the three available Brucella genomes revealed that all species have two highly conserved genes (ORFs BAB1_1800 and BAB1_0366 in B. abortus), whose products are related to the B lymphocyte mitogenic protein recently identified in T. cruzi (18). This protein belongs to a family of proline racemases (EC5.1.1.4, pfam 05544). Three motifs (MI, MII, and MIII*) were defined as patterns or signatures for proline racemases. MIII* is the most important because it includes the catalytic site (SPCGT) (19). A close examination of both ORFs revealed that the motifs MI, MII, and MIII were conserved in BAB1_1800 named prpA (proline-racemase protein A). On the other hand, MII and MIII* motifs were not conserved in BAB1_0366 named prpB (proline-racemase protein B). PrpB also presented a critical C255T replacement (SPTGT) that may render, according to this classification, an inactive enzyme (see Fig. 6, which is published as supporting information on the PNAS web site).
To determine whether these proteins are enzymatically active, both genes were cloned and the recombinant proteins were expressed and purified as described in Materials and Methods. We observed that PrpA catalyses the racemization of 22% of l-proline to d-proline, whereas no activity was detected when d-proline was used as substrate (data not shown). This result does not agree with the canonical activity described for these family of enzymes that catalyze the reversible conversion of l- and d-prolines (19, 20). As predicted by the sequence analysis, PrpB has no activity on either l- or d-proline.
To obtain further evidence that PrpA acts unidirectionally on l-proline, growth curve experiments were carried out on minimal medium with B. abortus 2308 WT and B. abortus-prpA mutant strains using d- or l-proline as the sole nitrogen source. As shown in Fig. 7, which is published as supporting information on the PNAS web site, WT and B. abortus-prpA displayed identical growth patterns on media containing l-glutamic acid as the sole nitrogen source, showing that prpA mutation does not affect bacterial metabolism. On the other hand, although the WT strain did not grow when either l-proline or d-proline was the sole nitrogen source, prpA mutants displayed significant growth on l-proline but not on d-proline-containing media. These results are in agreement with those obtained in vitro and suggest that WT bacteria converted l-proline into the nonmetabolizable d-proline by the action of PrpA, a reaction not carried out by the B. abortus-prpA mutant, thus leaving l-proline available to be used as nitrogen source. The fact that the WT strain grows normally in a medium with l-glutamic and d-proline rules out any toxic effect of the d-proline.
PrpA Is a Splenocyte Mitogen, and the Activity Depends on the Integrity of the Proline-Racemase Active Site.
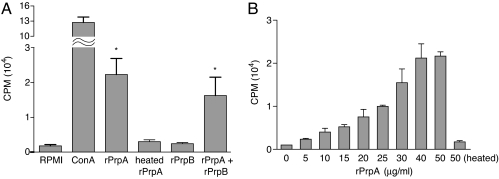
Purified recombinant rPrpA and rPrpB proteins were assayed for mitogenic activity on naive BALB/c mouse splenocytes. As shown in Fig. 1A, 50 μg/ml of rPrpA promotes 14-fold more [3H]thymidine incorporation than the control untreated cells or cells treated with either 50 μg/ml rPrpB or heat-inactivated rPrpA. Moreover, the addition of rPrpB to rPrpA has no effect on the proliferation index indicating that rPrpB has no inhibitory effect on the [3H]thymidine incorporation induced by rPrpA. On the other hand, the addition of l-proline to rPrpA significantly reduced in a dose-dependent manner its mitogenic activity (data not shown). This result suggests that either the availability of the rPrpA active site is required for such an effect or that a protein conformational change occurs in the presence of l-proline, resulting in the abolishment of the mitogenic activity.
Fig. 1.
PrpA is a polyclonal activator of naive mice splenocytes. (A) Splenocytes from naive BALB/c mice were treated for 48 h with ConA (10 μg/ml), rPrpA (50 μg/ml), rPrpB (50 μg/ml), heated-inactivated rPrpA (50 μg/ml) (15 min at 80°C), or a mix of rPrpA and rPrpB (50 μg/ml each). Cell proliferation was determined indirectly by [3H]thymidine incorporation. Results are representative of four independent experiments. Bars represent the mean ± SD of quadruplicate determinations. ∗, Statistical difference with RPMI medium 1640, rPrpB, and heated rPrpA (P < 0.01). (B) Mitogenic activity of rPrpA follows a dose-dependent response curve with saturation between 40 and 50 μg/ml. Results are representative of two independent experiments. All determinations were carried out in quadruplicate and expressed as cpm of [3H]thymidine incorporated.
As can be seen in Fig. 1B, the effect of rPrpA follows a dose-dependent response curve with saturation between 40 and 50 μg/ml. A lymphoproliferative effect caused by contaminating E. coli LPS was ruled out because heat treatment of PrpA and purified PrpB did not exert such an effect on mice splenocytes. Moreover, the same result was obtained with splenocytes from the LPS-nonresponder C3H/HeJ mice (data not shown).
As mentioned above, B. abortus PrpA SPCGT motif has a conserved C253 that has been described as an essential residue of the active site (19, 20), whereas B. abortus PrpB contains a C255T replacement in this sequence (SPTGT). It is probable that this change could account for the lack of proliferative activity. To study the role of C253 on enzymatic and mitogenic activity, PrpA (C253T) and PrpB (T255C) site-directed mutants were constructed. We observed that rPrpAC253T lost both enzymatic and mitogenic activity, while restoration of the active site in rPrpBT255C was not enough to gain any of these two activities (data not shown). This finding indicates that the cysteine present in the motif SPCGT is necessary but not sufficient for mitogenic activity. Altogether, these results indicate that B. abortus prpA codes for a protein with the capacity to induce splenocyte proliferation and that this activity depends on the integrity of the proline-racemase active site of the MIII* motif. On the other hand, B. abortus prpB codes for a protein that has no mitogenic activity even after the putative active site of the MIII* motif is reconstructed. Accordingly, PrpB was no longer included in the following studies.
PrpA Is a B Lymphocyte Mitogen.
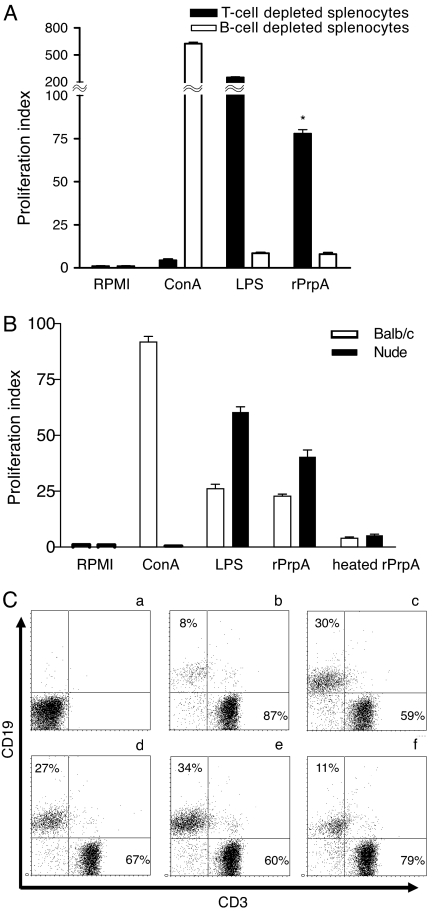
To determine whether the mitogenic activity displayed by PrpA is caused by the proliferation of B lymphocytes, T lymphocytes, or both, splenocytes from BALB/c mice were depleted of B or T lymphocytes and assayed for proliferation with purified rPrpA. Fig. 2A shows that rPrpA induces a 78-fold increment of [3H]thymidine incorporation on T cell-depleted splenocytes, whereas no mitogenic effect was exerted on B cell-depleted splenocytes. For confirmation, splenocytes obtained from nude mice were used to determine the proliferative activity of PrpA. As shown in Fig. 2B, rPrpA induces proliferation of the nude mice splenocytes, confirming that it is a B lymphocyte mitogen and that the presence of T cells is not necessary for this activity.
Fig. 2.
PrpA is a T cell-independent B cell polyclonal activator. (A) Splenocytes from naive BALB/c mice were depleted of B or T cells and treated for 48 h with ConA, E. coli LPS, or rPrpA. The T cell-depleted, but not the B cell-depleted, splenocytes proliferated in response to rPrpA. Results are representative of three experiments. Bars represent the mean ± SD of triplicate determinations. ∗, Statistical difference with respect to RPMI medium 1640-treated group (P < 0.01). (B) Splenocytes from naive BALB/c or nude mice were treated with ConA, LPS, rPrpA, or heated rPrpA. Results are representative of three independent experiments. Bars represent the mean ± SD of triplicate determinations. Results are expressed as proliferation index compared with RPMI medium 1640-treated cells. (C) A total of 106 splenocytes were recovered after 72 h of treatment and subjected to flow cytofluorometry with B lymphocyte-specific and T lymphocyte-specific markers (CD19 and CD3, respectively). (a) A control of the isotype-matching antibody. (b) Control untreated cells. (c) E. coli LPS-stimulated cells. (d and e) Cells treated with PrpA, 20 and 50 μg/ml, respectively. (f) Cells treated with 50 μg/ml rPrpAC253T. Upper-left and lower-right quadrants indicate B cell and T cell percentage, respectively.
To assess the effect of rPrpA on splenocytes, cells were recovered after 72 h of stimulation and subjected to flow cytofluorometry with B and T cell-specific markers. As shown in Fig. 2C, whereas control untreated cells contained 8% of B lymphocytes (b) and E. coli LPS-stimulated cells presented 30% of B lymphocytes (c), the PrpA-treated wells with 20 and 50 μg contained 25% and 34%, respectively (d and e). As expected, the inactive rPrpAC253T mutant had no effect (Fig. 2Cf), indicating that induction of [3H]thymidine incorporation and B lymphocyte proliferation depends on the integrity of the proline-racemase active site.
Role of PrpA on Mice Infection.
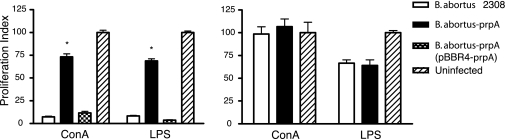
Much has been speculated about the role of immune suppression as a virulence mechanism in infectious diseases. It has been demonstrated that nonspecific proliferative responses can result in a state of immune suppression, thus facilitating infection (21–28). To study whether this mechanism is operative during Brucellae infection, the mitogenic response to E. coli LPS and ConA of splenocytes obtained from control and infected mice was studied. As shown in Fig. 3, splenocytes from mice infected with B. abortus 2308 for 3 weeks did not proliferate upon the addition of E. coli LPS or ConA, indicating that these cells are unresponsive. On the contrary, splenocytes recovered from the B. abortus-prpA mutant-infected animals responded in the same manner as the noninfected control mice. The complemented B. abortus-prpA (pBBR-prpA) displayed restoration of the WT unresponsive phenotype. This unresponsive status of the B and the T lymphocyte population of mice infected with the WT strain was reverted to normal 12 weeks after infection, suggesting that prpA is involved in a transient immune suppression exerted within a limited window of time during infection. The total numbers of B and T lymphocytes in spleens of mice infected with B. abortus WT and B. abortus-prpA were similar, ruling out the possibility of unresponsiveness caused by lack of the stimulated target cells (data not shown).
Fig. 3.
PrpA promotes a transient mice immunosuppressive status during Brucella infection. BALB/c mice were infected i.p. with B. abortus WT, B. abortus-prpA, and the complemented strain B. abortus-prpA (pBBR4-prpA). At 3 (Left) or 12 (Right) weeks after infection, spleens were removed, and splenocytes were treated for 72 h with ConA or E. coli LPS. Splenocyte proliferation was determined indirectly by [3H]thymidine incorporation. Bars represent the mean ± SD of triplicate determinations expressed as proliferation index, compared with the RPMI medium 1640-treated cells. At least three animals were used for each determination. ∗, Significant difference with uninfected or B. abortus-prpA (pBBR4-prpA)-infected mice stimulated with ConA and LPS (P < 0.01).
Persistence of the WT and the B. abortus-prpA mutant strains in BALB/c mice spleens and the intracellular replication in J774A.1 macrophage-like cell line were studied. Although no significant differences in invasion and intracellular replication were observed between B. abortus-prpA and its parental strain in J774A.1 cells (data not shown), the mutant presented an attenuated phenotype in mice. As shown in Fig. 4 no significant differences were observed between the numbers of bacteria recovered from mice infected with the WT or the mutant strain until 8 weeks after infection. Subsequently, the number of bacteria recovered from the B. abortus-prpA infected mice decreased, reaching a 100-fold reduction 12 weeks after infection. This result reveals a defect in the mutant that prevents it from establishing the chronic phase of the infection. Attempts to complement the mutant for long periods were ambiguous because of plasmid instability in mice, a phenomenon previously encountered with the pBBR1-MCS4 plasmids (29).
Fig. 4.
B. abortus-prpA displayed reduced persistence in mice. BALB/c mice were infected i.p. with B. abortus 2308 or B. abortus-prpA. At different weeks after infection, the numbers of cfu recovered from the spleens were determined by serial dilutions and plating. Results are expressed as log units. At least three animals were used for each determination. ∗ and ∗∗, Significant difference between the groups (P < 0.05).
Altogether, these results indicate that B. abortus produces a transient immunosuppressive status during the acute phase of infection. This immunosuppressive status seems to be critical for the establishment of a chronic phase of infection, because B. abortus-prpA, which is unable to produce the unresponsive phenotype, is defective in establishing a long-lasting infection.
PrpA Promotes IL-10 Secretion by Naive Splenocytes.
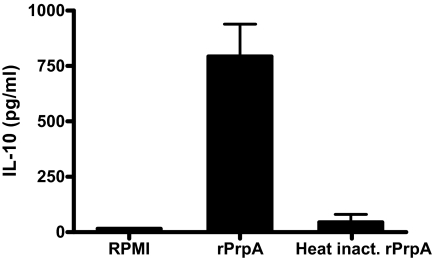
To determine whether there is a link between the nonspecific B cell proliferation induced by the recombinant PrpA and the prpA-dependent immunosuppression observed during the infection process, we evaluated the production of IL-10 by naive splenocytes treated with recombinant PrpA. The rationale behind this is that the nonspecific proliferation of B cells could be promoting the secretion of an immunosuppressive cytokine such as IL-10 (30–32). Murine naive splenocytes were stimulated 72 h with RPMI medium 1640, 50 μg/ml rPrpA or heat-inactivated rPrpA, and supernatants were subjected to ELISA for IL-10 determination. As observed in Fig. 5, PrpA induced the secretion of 750 pg/ml of IL-10. Production of the cytokine because of E. coli LPS contamination was discarded because heat-treated PrpA presented no effect. These results strongly suggest that the link between the mitogenic properties of PrpA and the prpA-dependent immune modulation could be the production of IL-10.
Fig. 5.
PrpA promotes IL-10 production by mice splenocytes. Murine naive splenocytes were treated with 50 μg/ml rPrpA or heat-inactivated rPrpA or RPMI medium 1640 as controls. Supernatants were collected at 72 h, and IL-10 production was determined by ELISA. Three animals were used for each determination.
Discussion
The cross-talk between the pathogen and the immune system of the host is a relatively unknown area of microbial pathogenesis. It is clear now that those pathogens that have had a long-lasting interaction with their host constitute an interesting group to study because they have evolved mechanisms to modulate cellular functions in a fine-tuned and reversible manner. Within this group are those with the capacity to establish a chronic infection; these are very refined pathogens that are able to persist in the host for, in some instances, the life span without inducing major disturbances in the normal homeostasis. These pathogens require a battery of virulence factors capable of modulating in a very precise manner the immune system of the host, initially avoiding the innate immunity, preventing the efficient mounting of the acquired immunity and finally establishing a chronic phase of the infection. Brucella belongs to this group of pathogens.
In past years the systematic search for virulence factors in Brucella has identified a number of genes involved in the intracellular replication or survival within the host (33). However, very little is known about how Brucella achieves a chronic state of infection. Until now, no protein involved in the immune modulation process has been identified to our knowledge.
In this article we have identified a protein, PrpA, which has the capacity to induce a T cell-independent B cell-nonspecific polyclonal activation concomitant with the secretion of IL-10. Mitogenicity caused by traces of contaminating E. coli LPS was discarded because heat-treated rPrpA or rPrpAC253T did not promote lymphoproliferation. Moreover, splenocytes from both LPS nonresponder C3H/HeJ and BALB/c mice displayed the same level of B cell proliferation upon rPrpA stimulation.
PrpA belongs to the family of proline racemaces that catalyze the reversible interconversion of l-proline and d-proline. ORFs belonging to the proline-racemase family are present in different organisms ranging from proteobacteria of the α subdivision (Agrobacterium, Rhizobium), γ subdivision (Xanthomonas and Pseudomonas), firmicutes (Streptomyces and Clostridium), and eukarya (T. cruzi and also present in human and mouse genome) (19). Our results demonstrate that PrpA has proline-racemase activity and that, in vitro and in vivo, is only capable of converting l-proline to d-proline. Notably, l-proline could inhibit in a competitive fashion the mitogenic activity of rPrpA. The most interesting, but yet puzzling, property of this protein is that its mitogenic ability depends on the integrity of the proline-racemase active site, suggesting a link between enzymatic and mitogenic activity. Indeed, the proline racemase of T. cruzi display mitogenic activity that also depends on the racemase activity (18), raising the question of whether all members of the family display both activities. Buschiazzo et al. (34) described the crystal structure of the T. cruzi proline racemase and proposed that the B cell mitogenic activity of the enzyme depends on exposed conformational epitopes by the ligand-free protein that become unavailable upon formation of the enzyme–substrate complex (34). Further experiments are needed to assess whether the mechanisms of action of the T. cruzi proline racemase and Brucella PrpA are similar.
Mitogens and super antigens are proteins that unspecifically activate B or T lymphocytes and produce polyclonal activation and proliferation. Nonspecific polyclonal stimulation is a general immunological feature after some infectious processes and is part of the strategy used by some pathogenic microorganisms to avoid the host-specific immune response, ensuring persistence by inducing a nonspecific one (35, 36). It has been demonstrated that nonspecific proliferative responses may lead to a lack of immune response, thus facilitating infection. B cell polyclonal activation induced by different virus, bacteria, and parasites has been strongly associated to immune suppression (21–28); however, in Brucellae no gene with this properties has been identified.
In this work we have shown that PrpA is a T-independent B cell mitogen that stimulates high levels of IL-10 secretion. We also show that the gene plays an important role in the modulation of the host immune response during Brucella infection. Whereas the WT and the complemented prpA mutant strains induce a temporal restricted unresponsive status in the mouse, the prpA mutant is unable to achieve this. T cell unresponsiveness to mitogens has been described in acute human brucellosis (37), supporting our results in mice and leading us to speculate that PrpA could have the same effect in humans.
B. abortus prpA mutant has a significant reduced colonization capacity at the late phase of the infection, indicating a role for PrpA in chronicity.
Based on the results of this work, we hypothesize that during the acute phase of the infection PrpA participates in stimulating B cell proliferation and IL-10 secretion, which could lead to the observed unresponsive status of the immune system. This mechanism could be allowing Brucella to manipulate the balance between the proinflammatory and antiinflammatory response in its own benefit, increasing the antiinflammatory response through IL-10 production, thus generating a transient immune suppression. The anergic state probably generates a delay of the acquired immune response that facilitates Brucella to colonize the cells/tissues in which it becomes “invisible.”
It is well known that B cells are capable of secreting IL-10 (31, 32, 38) and are involved in immune regulation (39). However, the contribution of B cells to the brucellosis pathology, besides its role as Ig producers, has not been explored in detail. IL-10 is a cytokine that plays a central role in the antiinflammatory response, down-regulating the immune system during the inflammatory process by counterbalancing the response so that the organism can clear an infection with minimal collateral damage (31). It has been shown in brucellosis (40) and other chronic infections (41–44) that reducing the level of IL-10 improves the ability of the host to efficiently control the pathogen.
We have determined that PrpA, probably in concert with a set of other virulence factors, is involved in the establishment of chronicity and in inducing a temporal anergic state in the host. Further studies are needed to determine at the molecular level how this protein participates in this immune modulation. Structural studies and the identification of the receptors involved in the mitogenic signal are needed to fully understand the molecular mechanism by which this protein induces B lymphocyte proliferation and IL-10 secretion.
Materials and Methods
Bacterial Strains and Growth Conditions.
E. coli strains were grown at 37°C in LB broth or Terrific broth (45). Brucella strains were grown at 37°C in Bacto Tryptic soy broth (BD, Sparks, MD). When necessary, media were supplemented with the appropriated antibiotics: ampicillin at 100 μg/ml for E. coli and 50 μg/ml for B. abortus and gentamicin at 20 μg/ml for E. coli and 4 μg/ml for B. abortus.
Construction of the prpA Mutant.
B. abortus 2308 prpA gene was amplified by PCR and cloned in pGEM-T vector (Promega, Madison, WI). The resulting plasmid pTprpA was digested with EcoRV, and prpA was disrupted with a gentamicin-resistance cassette. The resulting plasmid, pTprpA-Gm, was used to electroporate B. abortus 2308. Gentamicin-resistant and ampicilin-sensitive colonies were selected, and double recombination events were confirmed by PCR and Western blot. The resulting strain was designated B. abortus prpA.
Complementation of B. abortus prpA Mutant.
The B. abortus prpA gene was cloned in the KpnI site of pBBR1-MCS4, and the resulting plasmid, pBBR4-prpA, was used to electroporate B. abortus-prpA mutant. Gentamicin-resistant, ampicilin-resistant colonies were selected, complemented strain was controlled by PCR and Western blot, and the resulting complemented strain was named B. abortus prpA (pBBR4-prpA)
Infection of Mice and cfu Recovery.
Infection assays were carried out as described (46) with BALB/c female mice (60–90 days old). Briefly, female BALB/c mice were injected i.p. with 0.2 ml of PBS containing 5 × 105 cfu of B. abortus 2308 or B. abortus-prpA mutant. At different weeks after infection, animals were killed, and the spleens were removed, homogenized in sterile 150 mM NaCl solution, serially diluted, and plated onto TSB agar with the appropriate antibiotics to determine the number of cfu per spleen.
Expression of Recombinant rPrpA and rPrpB Proteins.
PCR products encoding the B. abortus 2308 prpA or prpB gene were cloned in-frame from their second codon with an N-terminal six-histidine tag into the pTrcHis expression vector (Invitrogen, Carlsbad, CA). The resulting plasmids were named pTrHprpA and pTrHprpB, respectively. Soluble recombinant proteins were produced in E. coli BL21(DE3)pLys induced with IPTG (Sigma, Steinheim, Germany) and purified to homogeneity by metal affinity chromatography through Ni2+ Hi-Trap chelating columns (Amersham Pharmacia, Uppsala, Sweden). After purification, rPrpA and rPrpB were sterilized by filtration through 0.22-μm membrane, and the protein concentration was determined by the Bradford method (47).
Enzymatic Activity.
Racemization assays were carried out on l- or d-proline as described by Reina-San-Martin et al. (18). Briefly, 3 μg of purified rPrpA or rPrpB was incubated for 60 min at 37°C with a 40 mM concentration of l- or d-proline in 0.2 M sodium acetate buffer (pH 6). The reaction was stopped by heating for 10 min at 80°C, 1 ml of water was added, and the optical rotation was determined in a optical polarizer 241 MC (Perkin–Elmer, Wellesley, MA) at a wavelength of 365 nm, in a cell with a path length of 10 cm.
Site-Directed Mutagenesis of prpA and prpB.
The C253T mutant of prpA (prpAC253T) and the prpB T255C mutant (prpBT255C) were obtained with the QuikChange method using plasmids pTrhPprA and pTrhPprB as templates. The following sense primers were used: PrpA, 5′-TCGACCGTTCGCCCACCGGCACCGGCACCT-3′ and PrpB, 5′-TCGACCGCTCGCCATGCGGCACCGGCTGCT-3′. The antisense primers used were: PrpA, 5′-AGGTGCCGGTGCCGGTGGGCGAACGGTCGA-3′ and PrpB, 5′-AGCAGCCGGTGCCGCATGGCGAGCGGTCGA-3′.
Plasmids pTrHPrpA-Mut and pTrHPrpB-Mut were obtained, and nucleotide changes were verified by sequencing.
Proliferation Assays.
Splenocytes from naive and infected mice were subjected to proliferation assays as described by Reina-San-Martin et al. (18). B or T lymphocyte cell suspensions were prepared by depleting total splenocytes with the corresponding Dyna-beads Mouse Pan T (Thy 1.2) or Pan B(B220) monoclonal immune-magnetic kit (Dynal Biotech, Oslo, Norway). Cell suspensions (50 μl) were exposed to 5–50 μg/ml rPrpA, rPrpB, rPrpAC253T, or rPrpBT255C for 48 h. ConA (10 μg/ml) and E. coli LPS (5 μg/ml) were included as controls. After incubation, cells were pulsed for 18 h with 1 μCi per well of [3H]thymidine, and [3H]thymidine uptake was determined in a liquid scintillation counter (LKB-Wallac, Turku, Finland). All determinations were repeated four times and expressed in cpm or as proliferation index. The corresponding standard deviation was calculated. Supernatants from proliferation assays were collected, and the secretion of IL-10 was determined by ELISA using the IL-10 Duoset ELISA kit (DuoSet; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Cytometry Analysis.
Spleen cells were recovered from the well after treatment with RPMI medium 1640, LPS, ConA, rPrpA, and rPrpAC253T as described above. A total of 106 cells were incubated 1 h at 4°C with 1 μg of anti-mouse CD32/16 mAb (Fc-Block; BD Pharmingen, San Diego, CA) in 50 μl of PBS with 0.01% NaN3. Afterward, cells were incubated 1 h at 4°C with 1 μg of R-phycoerythrin PE-anti-mouse-CD19 mAb and 1 μg of FITC-anti-mouse-CD3e mAb (BD Pharmingen) in the darkness and washed with 1 ml of PBS before fixing with 2% paraformaldehyde. Isotype-matched labeled mAbs were used as control. Flow cytometry was carried out in an Ortho Cytoron Absolute flow cytometer (Ortho Diagnostics, Westwood, MA) and analyzed with Win MDI 2.9 software (J. Trotter, The Scripps Research Institute, La Jolla, CA).
Statistical Analysis.
The differences between the groups were calculated by using the Student's t test for normally distributed variables and nonparametric Mann–Whitney test for non-normally distributed variables. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We thank Dr. O. Campetella (Instituto de Investigaciones Biotecnológicas) for his helpful suggestions and kind gift of reagents. This work was supported by Agencia Nacional de Promoción Científica y Tecnológica Grants PICT 01-06565 and 01-09194, Secretaria de Ciencia y Tecnologia, and Ministerio de Salud y Ambiente de la Nación Grant Beca Carrillo-Oñativia 2005 (to J.M.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS direct submission.
References
- 1.Finlay BB, McFadden G. Cell. 2006;124:767–782. doi: 10.1016/j.cell.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 2.Corbel MJ. Emerg Infect Dis. 1997;3:213–221. doi: 10.3201/eid0302.970219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Araya LN, Elzer PH, Rowe GE, Enright FM, Winter AJ. J Immunol. 1989;143:3330–3337. [PubMed] [Google Scholar]
- 4.Araya LN, Winter AJ. Infect Immun. 1990;58:254–256. doi: 10.1128/iai.58.1.254-256.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheers C. Dev Biol Stand. 1984;56:237–246. [PubMed] [Google Scholar]
- 6.Eze MO, Yuan L, Crawford RM, Paranavitana CM, Hadfield TL, Bhattacharjee AK, Warren RL, Hoover DL. Infect Immun. 2000;68:257–263. doi: 10.1128/iai.68.1.257-263.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. Immunology. 2001;103:511–518. doi: 10.1046/j.1365-2567.2001.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhan Y, Cheers C. Infect Immun. 1993;61:4899–4901. doi: 10.1128/iai.61.11.4899-4901.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DelVecchio VG, Kapatral V, Redkar RJ, Patra G, Mujer C, Los T, Ivanova N, Anderson I, Bhattacharyya A, Lykidis A, et al. Proc Natl Acad Sci USA. 2002;99:443–448. doi: 10.1073/pnas.221575398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paulsen IT, Seshadri R, Nelson KE, Eisen JA, Heidelberg JF, Read TD, Dodson RJ, Umayam L, Brinkac LM, Beanan MJ, et al. Proc Natl Acad Sci USA. 2002;99:13148–13153. doi: 10.1073/pnas.192319099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chain PS, Comerci DJ, Tolmasky ME, Larimer FW, Malfatti SA, Vergez LM, Aguero FL, Aguero ML, Ugalde RA, Garcia E. Infect Immun. 2005;73:8353–8361. doi: 10.1128/IAI.73.12.8353-8361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delrue RM, Martinez-Lorenzo M, Lestrate P, Danese I, Bielarz V, Mertens P, De Bolle X, Tibor A, Gorvel JP, Letesson JJ. Cell Microbiol. 2001;3:487–497. doi: 10.1046/j.1462-5822.2001.00131.x. [DOI] [PubMed] [Google Scholar]
- 13.Comerci DJ, Martinez-Lorenzo MJ, Sieira R, Gorvel JP, Ugalde RA. Cell Microbiol. 2001;3:159–168. doi: 10.1046/j.1462-5822.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 14.Arellano-Reynoso B, Lapaque N, Salcedo S, Briones G, Ciocchini AE, Ugalde R, Moreno E, Moriyon I, Gorvel JP. Nat Immunol. 2005;6:618–625. doi: 10.1038/ni1202. [DOI] [PubMed] [Google Scholar]
- 15.Porte F, Naroeni A, Ouahrani-Bettache S, Liautard JP. Infect Immun. 2003;71:1481–1490. doi: 10.1128/IAI.71.3.1481-1490.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Prada CM, Zelazowska EB, Nikolich M, Hadfield TL, Roop RM, II, Robertson GL, Hoover DL. Infect Immun. 2003;71:2110–2119. doi: 10.1128/IAI.71.4.2110-2119.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forestier C, Deleuil F, Lapaque N, Moreno E, Gorvel JP. J Immunol. 2000;165:5202–5210. doi: 10.4049/jimmunol.165.9.5202. [DOI] [PubMed] [Google Scholar]
- 18.Reina-San-Martin B, Degrave W, Rougeot C, Cosson A, Chamond N, Cordeiro-Da-Silva A, Arala-Chaves M, Coutinho A, Minoprio P. Nat Med. 2000;6:890–897. doi: 10.1038/78651. [DOI] [PubMed] [Google Scholar]
- 19.Chamond N, Gregoire C, Coatnoan N, Rougeot C, Freitas-Junior LH, da Silveira JF, Degrave WM, Minoprio P. J Biol Chem. 2003;278:15484–15494. doi: 10.1074/jbc.m210830200. [DOI] [PubMed] [Google Scholar]
- 20.Cardinale GJ, Abeles RH. Biochemistry. 1968;7:3970–3978. doi: 10.1021/bi00851a026. [DOI] [PubMed] [Google Scholar]
- 21.Mosier DE, Yetter RA, Morse HC., III J Exp Med. 1985;161:766–784. doi: 10.1084/jem.161.4.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minoprio PM, Eisen H, Forni L, D'Imperio Lima MR, Joskowicz M, Coutinho A. Scand J Immunol. 1986;24:661–668. doi: 10.1111/j.1365-3083.1986.tb02185.x. [DOI] [PubMed] [Google Scholar]
- 23.Minoprio P, Coutinho A, Spinella S, Hontebeyrie-Joskowicz M. Int Immunol. 1991;3:427–433. doi: 10.1093/intimm/3.5.427. [DOI] [PubMed] [Google Scholar]
- 24.Santos-Lima EC, Vasconcellos R, Reina-San-Martin B, Fesel C, Cordeiro-Da-Silva A, Berneman A, Cosson A, Coutinho A, Minoprio P. Eur J Immunol. 2001;31:634–645. doi: 10.1002/1521-4141(200102)31:2<634::aid-immu634>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 25.Ferreira P, Soares R, Ribeiro A, Arala-Chaves M. Scand J Immunol. 1988;27:549–554. doi: 10.1111/j.1365-3083.1988.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 26.Shirai A, Cosentino M, Leitman-Klinman SF, Klinman DM. J Clin Invest. 1992;89:561–566. doi: 10.1172/JCI115621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bunn-Moreno MM, Madeira ED, Miller K, Menezes JA, Campos-Neto A. Clin Exp Immunol. 1985;59:427–434. [PMC free article] [PubMed] [Google Scholar]
- 28.Galvao-Castro B, Sa Ferreira JA, Marzochi KF, Marzochi MC, Coutinho SG, Lambert PH. Clin Exp Immunol. 1984;56:58–66. [PMC free article] [PubMed] [Google Scholar]
- 29.Lavigne JP, Patey G, Sangari FJ, Bourg G, Ramuz M, O'Callaghan D, Michaux-Charachon S. Infect Immun. 2005;73:5524–5529. doi: 10.1128/IAI.73.9.5524-5529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taga K, Tosato G. J Immunol. 1992;148:1143–1148. [PubMed] [Google Scholar]
- 31.Moore KW, de Waal Malefytqq R, Coffman RL, O'Garra A. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 32.Ho AS, Moore KW. Ther Immunol. 1994;1:173–185. [PubMed] [Google Scholar]
- 33.Delrue RM, Lestrate P, Tibor A, Letesson JJ, De Bolle X. FEMS Microbiol Lett. 2004;231:1–12. doi: 10.1016/S0378-1097(03)00963-7. [DOI] [PubMed] [Google Scholar]
- 34.Buschiazzo A, Goytia M, Schaeffer F, Degrave W, Shepard W, Gregoire C, Chamond N, Cosson A, Berneman A, Coatnoan N, et al. Proc Natl Acad Sci USA. 2006;103:1705–1710. doi: 10.1073/pnas.0509010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minoprio P. Int J Parasitol. 2001;31:588–591. doi: 10.1016/s0020-7519(01)00171-0. [DOI] [PubMed] [Google Scholar]
- 36.Reina-San-Martin B, Cosson A, Minoprio P. Parasitol Today. 2000;16:62–67. doi: 10.1016/s0169-4758(99)01591-4. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez-Zapata M, Alvarez-Mon M, Salmeron I, Prieto A, Manzano L, Salmeron OJ, Carballido J. Infection. 1996;24:115–120. doi: 10.1007/BF01713314. [DOI] [PubMed] [Google Scholar]
- 38.Gieni RS, Umetsu DT, DeKruyff RH. Cell Immunol. 1997;175:164–170. doi: 10.1006/cimm.1996.1060. [DOI] [PubMed] [Google Scholar]
- 39.Duddy ME, Alter A, Bar-Or A. J Immunol. 2004;172:3422–3427. doi: 10.4049/jimmunol.172.6.3422. [DOI] [PubMed] [Google Scholar]
- 40.Fernandes DM, Baldwin CL. Infect Immun. 1995;63:1130–1133. doi: 10.1128/iai.63.3.1130-1133.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bermudez LE, Champsi J. Infect Immun. 1993;61:3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, Wynn TA, Sacks DL. J Exp Med. 2001;194:1497–1506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kane MM, Mosser DM. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 44.Reed SG, Brownell CE, Russo DM, Silva JS, Grabstein KH, Morrissey PJ. J Immunol. 1994;153:3135–3140. [PubMed] [Google Scholar]
- 45.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1989. [Google Scholar]
- 46.Comerci DJ, Pollevick GD, Vigliocco AM, Frasch AC, Ugalde RA. Infect Immun. 1998;66:3862–3866. doi: 10.1128/iai.66.8.3862-3866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradford MM. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.