Abstract
Small molecules that activate signaling pathways used by neurotrophic factors could be useful for treating CNS disorders. Here we show that the flavonoid fisetin activates ERK and induces cAMP response element-binding protein (CREB) phosphorylation in rat hippocampal slices, facilitates long-term potentiation in rat hippocampal slices, and enhances object recognition in mice. Together, these data demonstrate that the natural product fisetin can facilitate long-term memory, and therefore it may be useful for treating patients with memory disorders.
Keywords: cAMP response element binding protein (CREB), phosphorylation, learning, polyphenol, natural product
Neurotrophic factors promote the differentiation, survival, and functional maintenance of nerve cells. Because of these properties, they have the potential to treat a variety of chronic and acute disorders of the CNS. Although there have been some successes, clinical use of classical neurotrophic factors, such as brain-derived neurotrophic factor, has been limited for technical reasons, including difficulty in crossing the blood–brain barrier (1, 2). Therefore, the identification of small molecules that mimic some or all of the properties of neurotrophic factors could have significant potential for treating CNS disorders.
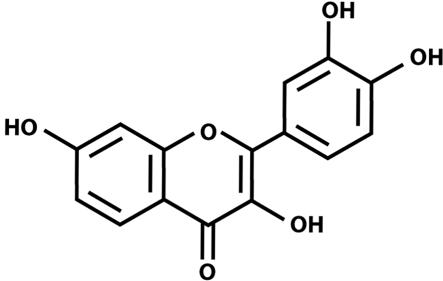
Recently, we described the ability of the flavonoid 3,7,3′,4′-tetrahydroxyflavone (fisetin; Fig. 1) to promote the differentiation of nerve cells (3). Although a wide range of flavonoids were tested in that study, most failed to induce differentiation. Of the few effective flavonoids, fisetin showed significantly greater efficacy than any of the others. The induction of differentiation by fisetin depends on the activation of the Ras–extracellular signal-regulated kinase (ERK) cascade and in particular on the activation of the ultimate kinase in this cascade, ERK. Inhibitors of both Ras and ERK activation block fisetin-induced differentiation. Not only does fisetin promote nerve cell differentiation, but in earlier studies it was shown to protect nerve cells from oxidative stress-induced death (4). Thus, fisetin has several of the properties of classical neurotrophic factors.
Fig. 1.
Structure of the flavonoid fisetin.
Although ERK was previously identified outside the CNS based on its role in cell proliferation, over the past 10 years a wide variety of studies have highlighted its importance in the CNS and in particular in synaptic plasticity and memory formation across many species, brain areas, and types of synapses (for reviews, see refs. 5 and 6). In the hippocampus, ERK can be activated through several different signaling pathways implicated in learning and memory, including NMDA receptors and brain-derived neurotrophic factor receptors. ERK activation leads to a number of cellular changes associated with the development of long-term memory, such as alterations in gene expression and protein synthesis, dendritic spine stabilization, the modulation of ion channels, and changes in receptor trafficking. Among the direct downstream targets of activated ERK is the transcription factor cAMP response element-binding protein (CREB) (for reviews, see refs. 7 and 8). CREB activation appears to be a critical step in the signaling cascade that leads to the structural changes underlying the development of long-term memory. Thus, activation of this cascade in neuronal cells by fisetin could result in the changes in the brain that form the cellular basis of memory. In the work reported here, we used biochemical, electrophysiological, and behavioral assays to test the hypothesis that fisetin treatment can stimulate signaling pathways leading to the enhancement of memory.
Results
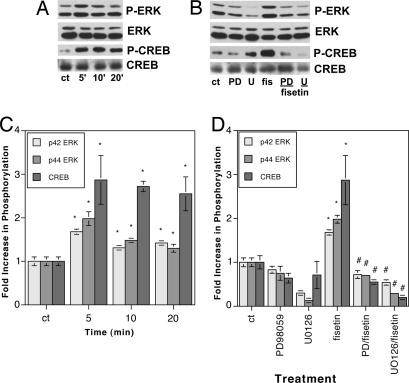
In our initial studies on the differentiation-promoting properties of fisetin, we showed that it induced the differentiation of PC12 cells in an ERK-dependent manner (3). No other flavonoid tested was as effective. To begin determining whether this effect of fisetin could translate into a facilitation of learning and memory, we asked whether fisetin could promote ERK activation in hippocampal slices. One micromolar fisetin induced rapid activation of both ERK1 (p44) and ERK2 (p42) within 5 min of treatment, which was sustained at a lower level for up to 20 min (Fig. 2A and C). The ≈2-fold increase in ERK1 and ERK2 phosphorylation seen after treatment of the hippocampal slices with fisetin is very similar to the increases in ERK phosphorylation reported after treatment of slices with either glutamate (1.5- to 2-fold) (9) or NMDA (≈2.5-fold) (10).
Fig. 2.
Fisetin activates ERK1 (p44), ERK2 (p42), and CREB in rat hippocampal slices. (A) Hippocampal slices in ACSF were treated with 1 μM fisetin for 5–20 min, and then equal amounts of protein were analyzed by SDS/PAGE and immunoblotting with antibodies to phospho-ERK and phospho-CREB along with antibodies to the unphosphorylated forms of the proteins, demonstrating no changes in overall protein levels. Similar results were obtained in two independent experiments. (B) Hippocampal slices were pretreated for 30 min with either 50 μM PD98059 (PD) or 10 μM U0126 (U) before the addition of 1 μM fisetin for 5 min. Samples were analyzed as in A. (C) The average phosphoprotein signal from the blots in A, quantified by densitometry and normalized to total protein, was plotted ±SD. The asterisks indicate a significant difference from the control (P < 0.005). (p42: 5 min, 1.68 ± 0.06; 10 min, 1.31 ± 0.05; 20 min, 1.42 ± 0.03; p44: 5 min, 1.98 ± 0.16; 10 min, 1.48 ± 0.02; 20 min, 1.30 ± 0.09; CREB: 5 min, 2.87 ± 0.56; 10 min, 2.72 ± 0.12; 20 min, 2.55 ± 0.39.) (D) The average phosphoprotein signal from the blots in B, quantified by densitometry and normalized to total protein, was plotted ±SD. ∗, significant difference from the control (P < 0.0005).#, significant difference from fisetin alone (P < 0.0001). (p42: PD98059, 0.83 ± 0.08; U0126, 0.30 ± 0.05; 5 min fisetin, 1.68 ± 0.06; fisetin + PD98059, 0.72 ± 0.09; fisetin + U0126, 0.54 ± 0.06; p44: PD98059, 0.75 ± 0.05; U0126, 0.13 ± 0.06; 5 min fisetin, 1.98 ± 0.16; fisetin + PD98059, 0.70 ± 0.10; fisetin + U0126, 0.29 ± 0.05; CREB: PD98059, 0.64 ± 0.11; U0126, 0.71 ± 0.31; 5 min fisetin, 2.87 ± 0.56; fisetin + PD98059, 0.55 ± 0.05; fisetin + U0126, 0.20 ± 0.03.) Similar results were obtained in two independent experiments.
One of the key signaling molecules activated downstream from ERK that is involved in learning and memory is the transcription factor CREB (7, 8). CREB, in turn, regulates a transcription factor cascade that eventually results in the facilitation of memory. The mechanisms that underlie the ability of CREB to enhance memory, however, are still not completely understood (8). Fisetin treatment enhances CREB activation with a time course that is similar to that seen for ERK activation (Fig. 2 A and C). The ≈3-fold increase in CREB phosphorylation seen after treatment of the hippocampal slices with fisetin is very similar to the increase in CREB phosphorylation reported after treatment of slices with glutamate (≈2.5-fold) (9). The activation of both ERK and CREB by fisetin is blocked by pretreatment with the MAPK/ERK kinase (MEK) inhibitors PD98059 and U0126, indicating that CREB activation is downstream from ERK activation (Fig. 2 B and D).
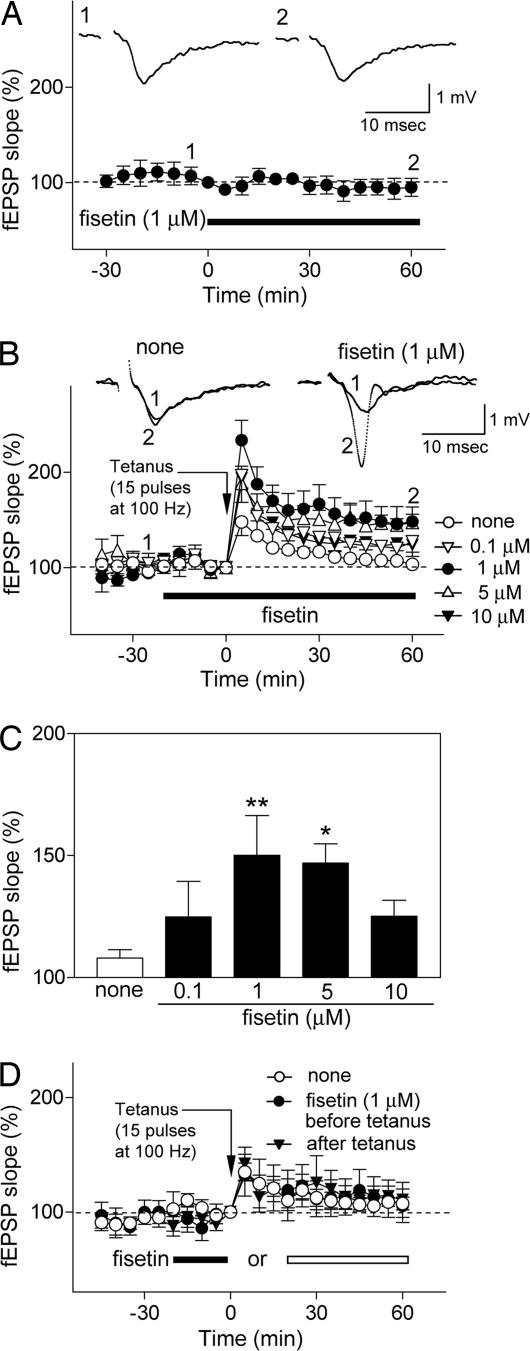
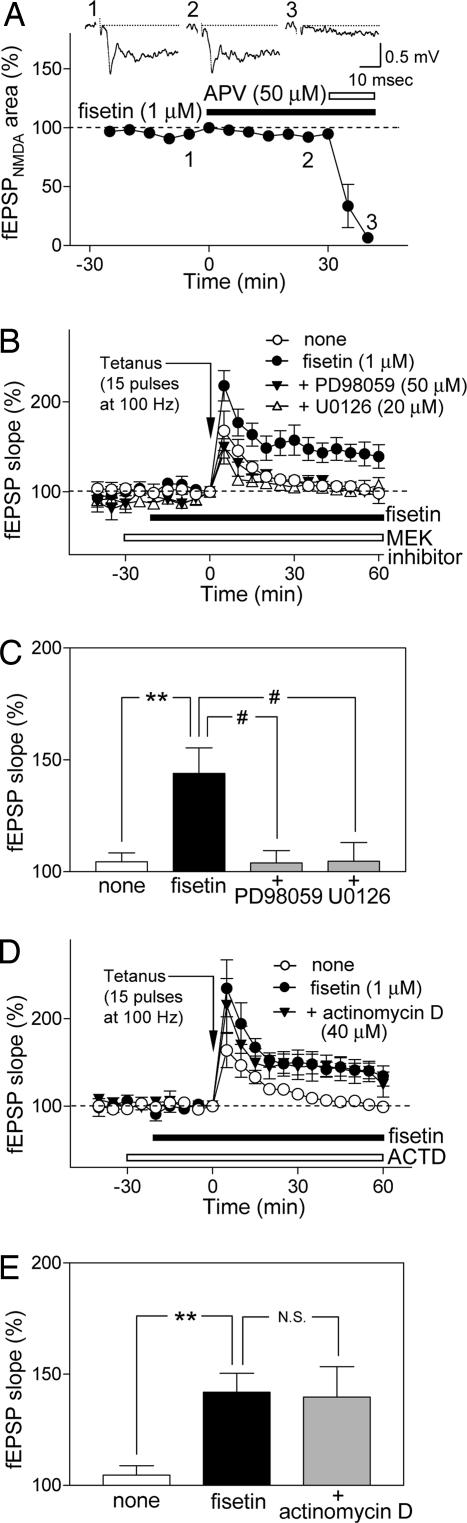
Given these results and the known associations between ERK and CREB activation and memory (6, 8), we next asked whether fisetin could affect long-term potentiation (LTP) in hippocampal slices. LTP is considered to be a good model of how memory is formed at the cellular level (11). Although fisetin had no direct effect on basal synaptic transmission in the CA1 area of rat hippocampal slices (Fig. 3A), it induced LTP in slices exposed to a weak tetanic stimulation (15 pulses at 100 Hz), which by itself failed to induce LTP (Fig. 3 B and C). The facilitation of LTP induction by fisetin was dose-dependent, with a maximal effective dose of 1 μM, and it persisted for at least 60 min (Fig. 3 B and C). Further studies demonstrated that fisetin needed to be present during the period of tetanic stimulation to promote the induction of LTP (Fig. 3D). Consistent with the effects of fisetin on ERK and CREB, it had no effect on NMDA receptor-mediated excitatory postsynaptic potentials (Fig. 4A). Furthermore, pretreatment with the MEK inhibitors PD98059 and U0126 blocked fisetin-induced facilitation of LTP, indicating a requirement for ERK activation in this process (Fig. 4 B and C). Neither PD98059 nor U0126 by itself had any effect on basal synaptic transmission-data (data not shown). Moreover, the RNA synthesis inhibitor actinomycin D did not affect the induction of LTP by fisetin (Fig. 4 D and E). This result is consistent with ERK and CREB acting upstream of mRNA and protein synthesis (5, 7, 12) and with the observation that only the latest phases of LTP (>6 h) are dependent on mRNA synthesis (11, 13).
Fig. 3.
Fisetin facilitates the induction of LTP in Schaffer collateral CA1 pyrimidal cell synapses in rat hippocampal slices. (A) Effect of fisetin (1 μM, n= 6) on basal synaptic transmission. Hippocampal slices were exposed to fisetin during the time indicated by the black bar. The field excitatory postsynaptic potential (fEPSP) slope is expressed as the percentage of the value immediately before the addition of fisetin. (A Insets) Representative records 5 min before (Inset 1) and 60 min after (Inset 2) exposure to fisetin. Fisetin does not affect basal synaptic transmission. (B and C) Fisetin facilitates the induction of LTP after a weak tetanic stimulation (15 pulses at 100 Hz), which alone does not induce LTP in control slices. The effect of fisetin is dose-dependent. The hippocampal slices were untreated (n= 14) or exposed to fisetin (0.1 μM, n= 7; 1 μM, n= 8; 5 μM, n= 6; 10 μM, n= 6) for the time indicated by the black bar, and weak tetanic stimulation was applied at time 0. The fEPSP slope is expressed as the percentage of the value immediately before the application of weak tetanic stimulation. (B) Time course of changes in the fEPSP slope. (B Insets) Representative records at −25 min (Inset 1) and + 60 min (Inset 2) in control and 1 μM fisetin-treated slices. To compare the data among the groups, the averages of the fEPSP slopes 30–60 min after tetanic stimulation were calculated as an index of LTP magnitude; they are shown in C. (C) None: 108.0 ± 3.4%; 0.1 μM fisetin: 124.8 ± 14.6%, not significant vs. none; 1 μM fisetin: 150.1 ± 16.3%, **, P < 0.01 vs. none; 5 μM fisetin: 146.9 ± 7.9%, *, P < 0.05 vs. none; 10 μM fisetin: 150.1 ± 16.3%, ∗∗, P < 0.01 vs. none; 5 μM fisetin: 146.9 ± 7.9%, ∗, P < 0.05 vs. none; 10 μM fisetin: 125.2 ± 6.5%, not significant vs. none (ANOVA followed by Dunnett's test). All data are the mean ± SEM. (D) The facilitation of LTP by fisetin depends on the timing of its addition relative to the tetanic stimulus. Fisetin must be present during the stimulus to show facilitation of LTP. The hippocampal slices were untreated (n= 9) or exposed to 1 μM fisetin before (filled bar, n= 5) or after (open bar, n= 5) application of weak tetanic stimulation (15 pulses at 100 Hz). Data are presented as in B.
Fig. 4.
Involvement of the MEK/ERK cascade in fisetin-induced facilitation of LTP in rat hippocampal slices. (A) Effect of fisetin (1 μM, n= 6) on NMDA receptor-mediated fEPSP in Mg2+-free medium. The hippocampal slices were exposed to fisetin (black bar) and the NMDA receptor antagonist 2-amino-5-phosphonovalerate (APV, white bar). The NMDA receptor-mediated fEPSP area is expressed as the percentage of the value immediately before addition of fisetin. (A Insets) Representative records 5 min before (Inset 1) and 25 min after (Inset 2) exposure to fisetin (Inset 2) and 10 min after exposure to APV (Inset 3). Fisetin has no effect on NMDA receptor-mediated synaptic responses. (B and C) MEK inhibitors PD98059 and U0126 block fisetin-dependent facilitation of LTP. The hippocampal slices were untreated (n= 12) or exposed to 1 μM fisetin alone (n= 7) or to a weak tetanic stimulation applied at time 0 after a 10-min pretreatment with PD98059 (50 μM, n= 6) or U0126 (20 μM, n= 5). (B) Time course of changes in the fEPSP slope. To compare the data among the groups, the averages of the fEPSP slopes 30–60 min after tetanic stimulation were calculated as an index of LTP magnitude and are shown in C. (C) None: 104.5 ± 4.0%; fisetin: 143.9 ± 11.4%, ∗∗, P < 0.01 vs. none; fisetin + PD98059: 103.9 ± 5.5%, , P < 0.05 vs. fisetin; fisetin + U0126: 104.7 ± 8.3%, , P < 0.05 vs. fisetin (ANOVA followed by Tukey–Kramer test). All data are mean ± SEM. (D and E) Effect of actinomycin D on fisetin-induced facilitation of LTP. The hippocampal slices were untreated (n= 10) or exposed to 1 μM fisetin alone (n= 7) or after a 10-min pretreatment with 40 μM actinomycin D (ACTD, n= 6) and weak tetanic stimulation was applied at time 0. The presentation of the data are as in B and C. ACTD does not block fisetin-dependent facilitation of LTP. [None: 104.6 ± 4.3%; fisetin: 141.9 ± 8.6%, ∗∗, P < 0.01 vs. none; fisetin + ACTD 139.7 ± 13.6%, not significant vs. fisetin (ANOVA followed by Tukey–Kramer test).] All data are the mean ± SEM.
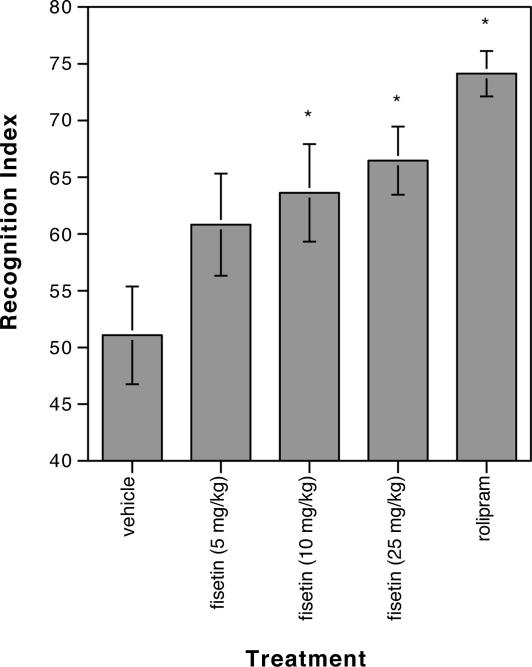
To determine whether the biochemical and electrophysiological effects of fisetin seen in hippocampal slices translate into alterations in memory in the animal, fisetin was tested in mice by using an object-recognition task (14). Among the alternatives available for testing memory, this assay has proven very effective for measuring CREB-dependent functions (7, 15, 16). In this test, during the training period mice are presented with two identical objects, which they explore for a fixed time period. To test for memory, the mice are presented 1 day later with two different objects, one of which was presented previously during the training and is thus familiar to the mice; the other object is new to them. The better the mice remember the familiar object, the more time they will spend exploring the novel object. To test the effects of fisetin in this memory task, it was administered orally to the mice before the start of the training period. Rolipram, a phosphodiesterase (PDE4) inhibitor that potentiates memory in this assay (16), requires i.p. injection, and it was used as a positive control. As shown in Fig. 5, three doses of fisetin were tested in the object-recognition task, and significant effects were seen at both 10 and 25 mg/kg. Higher doses were not tested.
Fig. 5.
Fisetin enhances long-term memory in mice, and the effect of different oral doses of fisetin on object recognition over a 10-min test period is shown. Rolipram, injected i.p. at 0.1 mg/kg, served as a positive control. Data represent the mean ± SEM of 10 mice per treatment group. Data were analyzed by one-way ANOVA followed by post hoc comparisons with Fisher's test. ∗, significant difference from vehicle control (P < 0.02). Vehicle, 51.072 ± 4.293; fisetin 5 mg/kg, 60.820 ± 4.521; fisetin 10 mg/kg, 63.628 ± 4.332; fisetin 25 mg/kg, 66.461 ± 2.984; rolipram, 74.132 ± 2.041. Similar results were obtained in two independent, blinded experiments done by PsychoGenics.
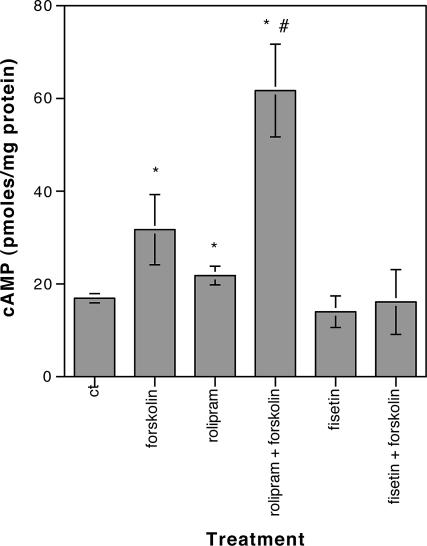
To date, PDE4 inhibitors such as rolipram are the only compounds that have been shown both to increase CREB phosphorylation and to enhance memory (7). These PDE4 inhibitors activate CREB by increasing the levels of the CREB activator, cAMP, through inhibition of its breakdown. To determine whether fisetin works through a mechanism similar to that of rolipram, hippocampal slices were treated with fisetin by using the conditions where maximal stimulation of CREB phosphorylation and facilitation of LTP were seen, and then the slices were assayed for cAMP. Forskolin, an activator of adenylyl cyclase and rolipram, was used as a positive control. As shown in Fig. 6, and in agreement with published data (17), 3 μM rolipram alone modestly increased cAMP levels in the slices and significantly potentiated the effect of forskolin on cAMP levels. In contrast, fisetin had no effect on cAMP levels by itself, nor did it potentiate the effect of forskolin. These data are consistent with earlier results obtained using PC12 cells which showed that fisetin treatment does not result in the activation of the cAMP target protein kinase A (3) and indicated that fisetin activates CREB through a mechanism that is distinct from that of rolipram.
Fig. 6.
Fisetin does not increase cAMP levels in hippocampal slices. Hippocampal slices in ACSF were treated with 1 μM fisetin for 5 min (fisetin) or 3 μM rolipram for 30 min (rolipram) and then either immediately frozen or treated with 5 μM forskolin for an additional 15 min (rolipram + forskolin; fisetin + forskolin) before freezing. Additional slices were treated only with 5 μM forskolin for 15 min (forskolin). The levels of cAMP in the slices were measured by using a scintillation proximity assay, and they are presented as pmol/mg protein ± SD. *, significant difference from control (P < 0.05);#, significant difference from forskolin or rolipram alone (P < 0.005).
Discussion
The above data demonstrate that the flavonoid fisetin can activate signaling pathways in hippocampal slices that are implicated in the development of long-term memory. This activation translates into the facilitation of the induction of LTP in hippocampal slices and an increase in long-term memory in mice. Thus, this report supports the hypothesis that natural products such as fisetin can have functional effects on nerve cells both in vitro and in vivo, acting not only to increase nerve cell survival (4) and differentiation (3) but also to enhance long-term memory.
The consequences of CREB activation on LTP facilitation and long-term memory by fisetin are consistent with studies in which the activity of CREB was manipulated by using genetic techniques. CREB loss-of-function mutants have impairments in long-term memory, whereas CREB gain-of-function mutants show enhanced long-term memory. Thus, CREB appears to function as a rate-limiting “molecular switch,” which makes it ideally suited to pharmacological intervention (7).
We show that the activation of CREB by fisetin is mediated through stimulation of ERK phosphorylation and not by the inhibition of PDEs. The rapid stimulation of ERK by fisetin in hippocampal slices appears to account for its ability to enhance LTP because the inhibition of ERK activation by two different inhibitors of the upstream kinase, MEK, completely blocks both fisetin-induced ERK and CREB phosphorylation and the facilitation of LTP. Previously, we showed that fisetin activates the full Ras–ERK cascade (3). However, it is possible that fisetin activates additional signaling pathways that contribute to the development of long-term memory.
In addition to enhancing long-term memory as shown in this study, fisetin has a number of other effects on nerve cells, indicating that it might also be neuroprotective in the CNS. For example, fisetin can maintain the levels of glutathione, the major intracellular antioxidant, in nerve cells exposed to a variety of toxic insults (4, 18). In addition, several studies have shown that fisetin can induce the activity and expression of phase II detoxification proteins, part of the endogenous cellular antioxidant defense mechanism which provides long-term protection of cells against oxidative stress (19–21). Finally, fisetin is a potent inhibitor of β-amyloid fibril formation in vitro (22), and it can also decrease myelin phagocytosis by macrophages (23), suggesting that it might be able to limit both amyloid toxicity and demyelination. Together these data show that fisetin has a diverse collection of biological properties that may be of clinical interest for the treatment of CNS disorders.
Although several studies have demonstrated effects of mixtures of flavonoids, including ginkgo biloba (24–26), soy isoflavones (27, 28), or green tea polyphenols (29), on memory, the specific flavonoids producing this effect were not determined. Although studies with flavonoid mixtures have used biochemical, electrophysiological, or psychological assays to demonstrate effects on cognitive function, we are not aware of any study that has shown activity of a single flavonoid at all three levels, thereby providing an underlying mechanism for the observed action of the flavonoid.
In recent years, there has been a great deal of effort expended on developing drugs to enhance long-term memory, and a few drugs have been made that facilitate LTP and enhance the CREB-dependent phase of memory (7, 30–33). Because fisetin promotes CREB activation, it appears to fall into this class of memory-enhancing compounds. However, unlike many of the compounds in this group, such as rolipram, fisetin is not a PDE inhibitor, and fisetin is effective when given orally. Rolipram and other PDE4 inhibitors also have dose-limiting side effects, including nausea and vomiting (34), which have severely restricted their therapeutic use.
Fisetin, in fact, is present in a number of commonly eaten foods, such as strawberries (35). Consistent with our results, older rats fed a diet enriched in strawberry extract for 8 weeks showed an enhancement of cognitive performance in the Morris water maze relative to rats fed a control diet (36). Thus, the effects of fisetin on learning and memory as well as its additional neuroprotective activities, coupled with its presence in commonly eaten foods, suggest that it could have long-term beneficial effects on memory with little cost or side effects.
Materials and Methods
Chemicals.
Fisetin was from Indofine Chemical Co. (Hillsborough, NJ). U0126 and PD98059 were from Promega (Madison, WI). Rolipram was from Calbiochem (San Diego, CA). All other chemicals were from Sigma (St. Louis, MO).
Immunoblotting.
Rat hippocampal slices (400 μm) were prepared from male Wistar rats (5–7 weeks old) and maintained in a chamber at 30°C, where they were continuously perfused with artificial CSF (ACSF) consisting of 124 mM NaCl/3.0 mM KCl/2.2 mM CaCl2/1.4 mM MgSO4/1.24 mM KH2PO4/25.0 mM NaHCO3/10 mM glucose, bubbled with 95% O2/5% CO2. The slices were allowed to recover for 2 h, and they were then treated with 1 μM fisetin either alone or in the presence of 10 μM U0126 or 50 μM PD98059. After treatment, the slices were immediately frozen in PBS containing 1% Nonidet P-40, 0.5% deoxycholate, 0.1% SDS, 1 mM Na3VO4, and 0.01 volume of Sigma protease inhibitor mixture. For analysis of protein phosphorylation, the slices were defrosted and solubilized by sonication, and equal amounts of protein were analyzed by SDS/PAGE and immunoblotting. Equal loading and transfer of the samples were confirmed by staining the nitrocellulose with Ponceau S. Transfers were blocked for 2 h at room temperature with 5% (wt/vol) nonfat milk in TBS/0.1% Tween 20, and then they were incubated overnight at 4°C in the primary antibody (phospho-p44/42 MAPK, phospho-CREB, and total ERK) diluted in 5% (wt/vol) BSA in TBS/0.05% Tween 20 or for 1 h at room temperature in 5% (wt/vol) nonfat milk in TBS/0.1% Tween 20 (total CREB). The primary antibodies used were: phospho-p44/42 MAPK antibody (9101, 1/1,000), phospho-CREB (9196, 1/1,000), and total ERK antibody (9102, 1/1,000) from Cell Signaling (Beverly, MA) and total CREB (1/2,000) from Marc Montminy (The Salk Institute). The transfers were rinsed with TBS/0.05% Tween 20 and incubated for 1 h at room temperature in horseradish peroxidase/goat anti-rabbit or goat anti-mouse (Bio-Rad, Hercules, CA), diluted 1/5,000 in 5% (wt/vol) nonfat milk in TBS/0.1% Tween 20. The immunoblots were developed with the Super Signal reagent (Pierce, Rockford, IL). Blots were scanned and quantified using Image software (National Institutes of Health, Bethesda, MD). Results were analyzed with an unpaired Student's t test.
LTP Experiments.
Slice preparation and field potential recording were made as described (37). Briefly, hippocampal slices (400 μm) were prepared from male Wistar rats (5–7 weeks old) and maintained in a chamber at 30°C, where they were continuously perfused with ACSF bubbled with 95% O2/5% CO2. Schaffer collaterals were stimulated by a bipolar tungsten electrode positioned in the stratum radiatum of the CA1 region near the CA2/CA1 border, and the evoked fEPSPs were recorded from the stratum radiatum of the CA1 region. The stimulus intensity was adjusted in the range of 25–55 μA to evoke fEPSPs of 50% of the maximum amplitude. Tetanic stimulation was applied at the same intensity with the test stimulation. The rising slope of fEPSP was measured as an index of synaptic efficacy. NMDA receptor-mediated synaptic responses were recorded in Mg2+-free ACSF supplemented with 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione, a non-NMDA receptor antagonist, and 50 μM picrotoxin, a GABAA receptor channel blocker. The area of field potentials recorded in this condition was measured as an index of NMDA receptor-mediated synaptic responses. All results are presented as the mean ± SEM of 5–14 experiments. Data were analyzed by one-way ANOVA followed by Tukey–Kramer test or Dunnett's test.
Object Recognition.
Male C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were used, and the testing was done by PsychoGenics (Tarrytown, NY). All mice were acclimated to the colony room for at least 2 weeks before testing, and they were tested at an average age of 8 weeks. Mice were randomly assigned across treatment groups with 10 mice in each group. For each dose tested, a 10× solution of fisetin was prepared in 95% ethanol, and then it was diluted with 4 volumes of polyethylene glycol 660 hydroxystearate (Solut HS15 from BASF, Florham Park, NJ) and 5 volumes of PBS. The vehicle contained the identical ratios of ethanol, Solut HS15, and PBS. All were adminstered orally 60 min before training at a volume of 10 ml/kg of body weight. Rolipram was dissolved in 10% DMSO, and it was administered i.p. at 0.1 mg/kg 20 min before training. The test was performed as described in ref. 16. Briefly, on day 1, mice were habituated to a circular open field arena for 1 h in cage groups of four. Twenty-four hours later, individual mice were placed back in the same arena, which now contained two identical objects for a 15-min training trial. On day 3, vehicle-, fisetin-, or rolipram-treated mice were individually placed back in the same arena in the presence of both the familiar object (i.e., previously explored) and a novel object. The spatial positions of the objects were counterbalanced between subjects. Each animal's test trial was recorded, and the first 10 min of each session were scored. Object recognition was computed by using the following formula: Time spent with novel object × 100. Total time spent exploring both objects. Data were analyzed by a one-way ANOVA followed by post hoc comparisons with Fisher's test. Similar results were obtained in two independent experiments.
cAMP Assays.
Rat hippocampal slices (400 μm) were prepared from male Wistar rats (5–7 weeks old) and maintained in a chamber at 30°C, where they were perfused continuously with artifical cerebrospinal fluid (ACSF) bubbled with 95% O2/5% CO2. The slices were allowed to recover for 2 h, and they were then treated with either 3 μM rolipram for 30 min or 1 μM fisetin for 5 min and then immediately frozen in liquid nitrogen. In some cases, slices were treated for an additional 15 min with 5 μM forskolin before freezing. Additional slices were treated only with 5 μM forskolin for 15 min. For analysis of cAMP, the slices were thawed and solubilized in cAMP lysis buffer (1% dodecyltrimethylammonium bromide in 0.05 M acetate buffer, pH 5.8), and cAMP levels were determined by a scintillation proximity assay (Amersham Biosciences, Piscataway, NJ) according to the manufacturer's instructions. Duplicate individual slices were assayed for each data point. Total protein was determined by using the BCA assay, and results are presented as pmol of cAMP per mg of protein. The data were analyzed by using an unpaired Student's t test.
Acknowledgments
We thank Drs. Michael Saganich and Qi Chen for help with the hippocampal slice preparations and Dr. Charles Stevens for critical reading of the manuscript and helpful suggestions.This work was supported by U.S. Public Health Service Grants NS28121 and AG025337 (to P.M.) and by MEXT.HAITEKU (2004–2008) (to K.A.).
Abbreviations
- ACSF
artificial cerebrospinal fluid
- CREB
cAMP response element-binding protein
- fEPSP
field excitatory postsynaptic potentials
- LTP
long-term potentiation
- MEK
MAPK/ERK kinase
- PDE4
phosphodiesterase 4.
Footnotes
The authors declare no conflict of interest.
References
- 1.Levy YS, Gilgun-Sherki Y, Melamed E, Offen D. BioDrugs. 2005;19:97–127. doi: 10.2165/00063030-200519020-00003. [DOI] [PubMed] [Google Scholar]
- 2.Fumagalli F, Racagni G, Riva MA. Pharmacogen J. 2006;6:95–104. doi: 10.1038/sj.tpj.6500360. [DOI] [PubMed] [Google Scholar]
- 3.Sagara Y, Vahnnasy J, Maher P. J Neurochem. 2004;90:1144–1155. doi: 10.1111/j.1471-4159.2004.02563.x. [DOI] [PubMed] [Google Scholar]
- 4.Ishige K, Schubert D, Sagara Y. Free Radic Biol Med. 2001;30:433–446. doi: 10.1016/s0891-5849(00)00498-6. [DOI] [PubMed] [Google Scholar]
- 5.Adams JP, Sweatt JD. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- 6.Sweatt JD. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Tully T, Bourtchouladze R, Scott R, Tallman J. Nat Rev Drug Discov. 2003;2:267–277. doi: 10.1038/nrd1061. [DOI] [PubMed] [Google Scholar]
- 8.Carlezon WA, Duman RS, Nestler EJ. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Vanhoutte P, Barnier J-V, Guibert B, Pages C, Besson M-J, Hipskind RA, Cabouche J. Mol Cell Biol. 1999;19:136–146. doi: 10.1128/mcb.19.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.English JD, Sweatt JD. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 11.Bliss TVP, Collingridge GL. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed T, Frey JU. Neuropharmacol. 2005;49:477–492. doi: 10.1016/j.neuropharm.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Pang PT, Lu B. Ageing Res Rev. 2004;3:407–430. doi: 10.1016/j.arr.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Ennaceur A, Delacour J. Behav Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 15.Prickaerts J, Van Staveren WCG, Sik A, Markerink-Van Ittersum M, Niewohner U, Van der Staay FJ, Blokland A, De Vente J. Neuroscience. 2002;113:351–361. doi: 10.1016/s0306-4522(02)00199-9. [DOI] [PubMed] [Google Scholar]
- 16.Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, Scott R, Tully T. Proc Natl Acad Sci USA. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Proc Natl Acad Sci USA. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maher P. Free Radic Res, in press. 2006 doi: 10.1080/10715760600672509. [DOI] [PubMed] [Google Scholar]
- 19.Fiander H, Schneider H. Cancer Lett. 2000;156:117–124. doi: 10.1016/s0304-3835(00)00368-2. [DOI] [PubMed] [Google Scholar]
- 20.Hou D-X, Fukuda M, Johnson JA, Miyamori K, Ushikai M, Fujii M. Int J Oncol. 2001;18:1175–1179. doi: 10.3892/ijo.18.6.1175. [DOI] [PubMed] [Google Scholar]
- 21.Maher P, Hanneken A. Invest Ophthalmol Visual Sci. 2005;46:4796–4803. doi: 10.1167/iovs.05-0397. [DOI] [PubMed] [Google Scholar]
- 22.Kim H, Park B-S, Lee K-G, Choi CY, Jang SS, Kim Y-H, Lee S-E. J Agric Food Chem. 2005;53:8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 23.Hendricks JJ, de Vries HE, van der Pol SM, van den Berg TK, van Tol EA, Dijkstra CD. Biochem Pharmacol. 2003;65:877–885. doi: 10.1016/s0006-2952(02)01609-x. [DOI] [PubMed] [Google Scholar]
- 24.DeFeudis FV, Drieu K. Curr Drug Targets. 2000;1:25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- 25.Gertz HJ, Kiefer M. Curr Pharm Des. 2004;10:261–264. doi: 10.2174/1381612043386437. [DOI] [PubMed] [Google Scholar]
- 26.Williams B, Watanabe CMH, Schultz PG, Rimbach G, Krucker T. Neurobiol Aging. 2004;25:955–962. doi: 10.1016/j.neurobiolaging.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Lephart ED, West TW, Weber KS, Rhees RW, Setchell KD, Adlercreutz H, Lund TD. Neurotoxicol Teratol. 2002;24:5–16. doi: 10.1016/s0892-0362(01)00197-0. [DOI] [PubMed] [Google Scholar]
- 28.Lee YB, Lee HJ, Sohn HS. J Nutr Biochem. 2005;16:641–649. doi: 10.1016/j.jnutbio.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 29.Kim HK, Kim M, Kim S, Kim M, Chung JH. Biosci Biotechnol Biochem. 2004;68:1977–1979. doi: 10.1271/bbb.68.1977. [DOI] [PubMed] [Google Scholar]
- 30.Lynch G. Nat Neurosci. 2002;5:1035–1038. doi: 10.1038/nn935. [DOI] [PubMed] [Google Scholar]
- 31.Marshall E. Science. 2004;304:36–38. doi: 10.1126/science.304.5667.36. [DOI] [PubMed] [Google Scholar]
- 32.Cooke SF, Bliss TVP. Curr Opin Invest Drugs. 2005;6:25–34. [PubMed] [Google Scholar]
- 33.Lynch G. Curr Opin Pharmacol. 2006;6:82–88. doi: 10.1016/j.coph.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Dyke HJ, Montana JG. Expert Opin Investig Drugs. 2002;11:1–13. doi: 10.1517/13543784.11.1.1. [DOI] [PubMed] [Google Scholar]
- 35.Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. J Nutr. 2000;130:2243–2250. doi: 10.1093/jn/130.9.2243. [DOI] [PubMed] [Google Scholar]
- 36.Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, Bickford PC. J Neurosci. 1999;19:8114–8121. doi: 10.1523/JNEUROSCI.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abe K, Kimura H. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]