Abstract
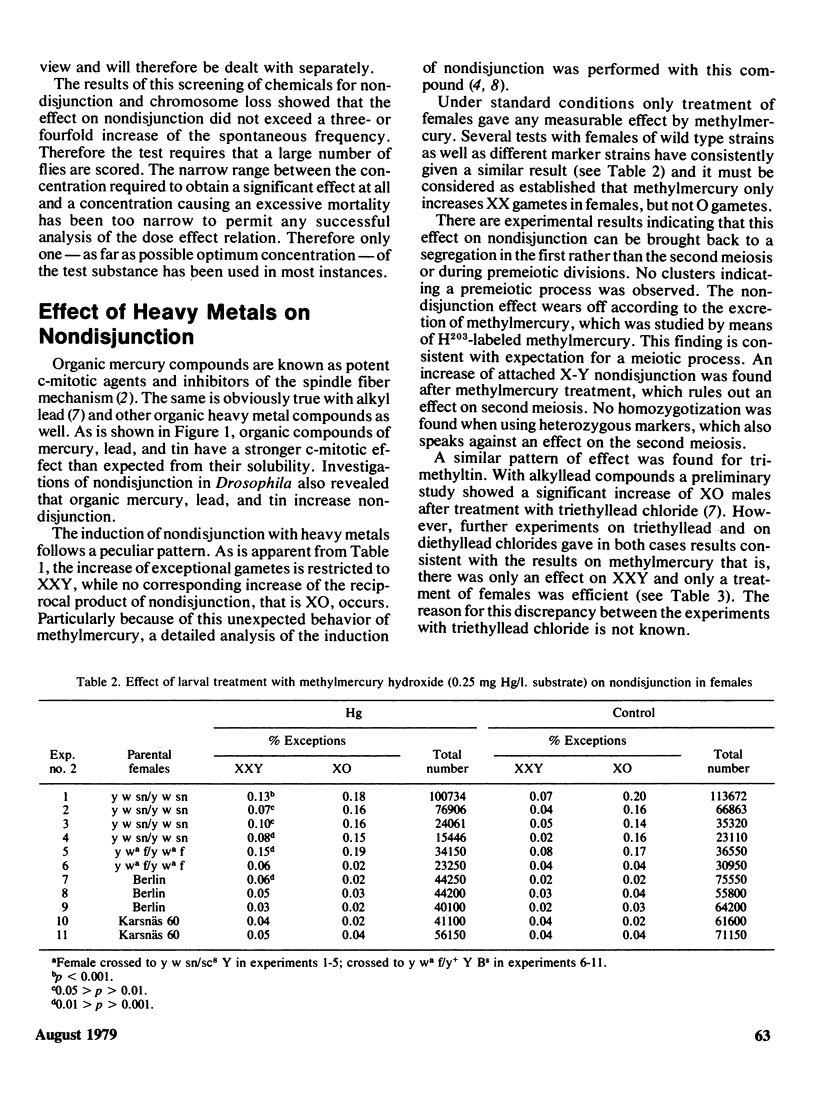
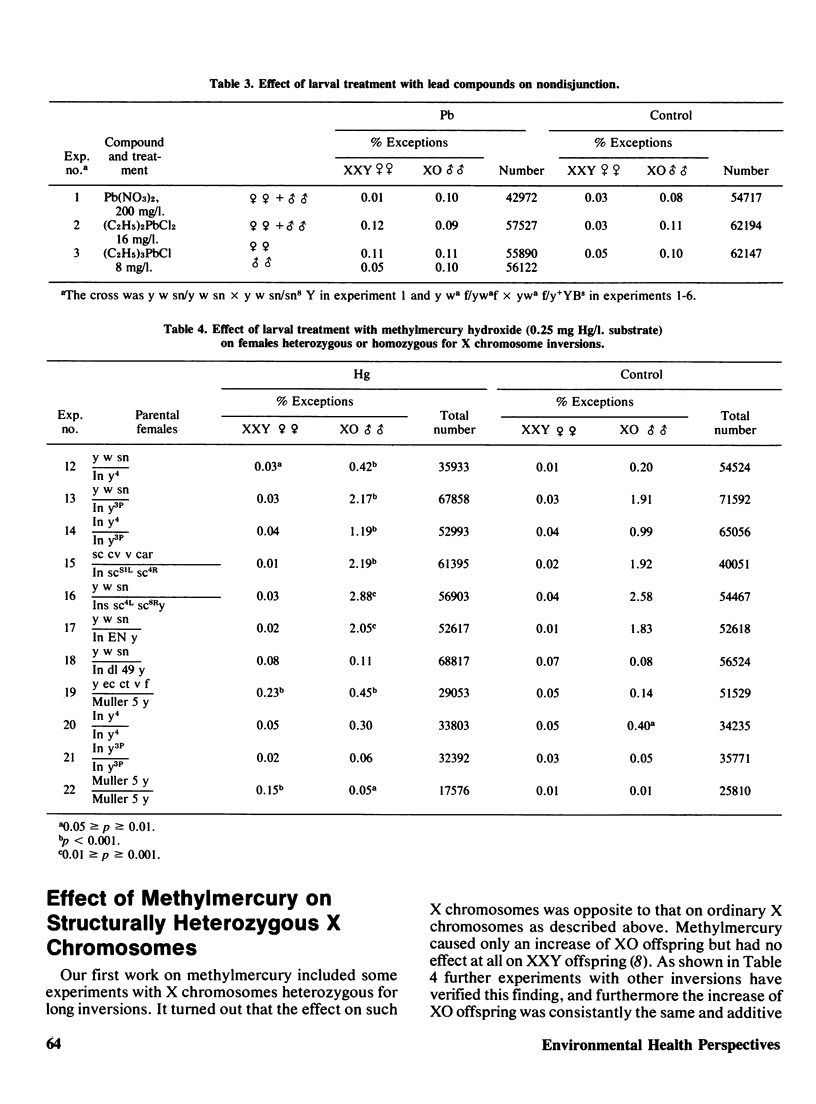
Tests for chemically induced nondisjunction and loss of the sex chromosomes in Drosophila were performed. Of 31 compounds tested four gave rise only to an increase of XO exceptions, indicating the induction of chromosome loss. Six compounds, all known spindle inhibitors (colchicine, organic mercury, lead, and tin compounds) gave rise to an increase both of XXY and XO or of only XXY. The effect by metalloorganic compounds of which methylmercury was studied particularly closely, follows a peculiar pattern. In females with structurally normal X chromosomes only an increase of XX gametes is obtained, while with X chromosomes heterozygous for long inversions only O gametes are increased. The data indicates that the effect of the metal compounds occurs at first meiosis and that the process is connected with a meiotic drive, giving rise to a preferential segregation of the two X chromosomes to the functioning pole. The increase only of O gametes with structurally heterozygous X chromosomes can tentatively be explained by a loss due to crossing over within the inversion. An increase of the effect of methyl mercury was obtained where the normal pairing of the X chromosomes was interfered with by means of autosomal inversions. Likewise a synergistic increase of nondisjunction was obtained when a temperature chock of 10 degrees C was applied together with treatment with methylmercury. It is concluded that chemical induction of nondisjunction can be studied in Drosophila, but the sensitivity of the test is rather low and large amount of material is required.
Full text
PDF
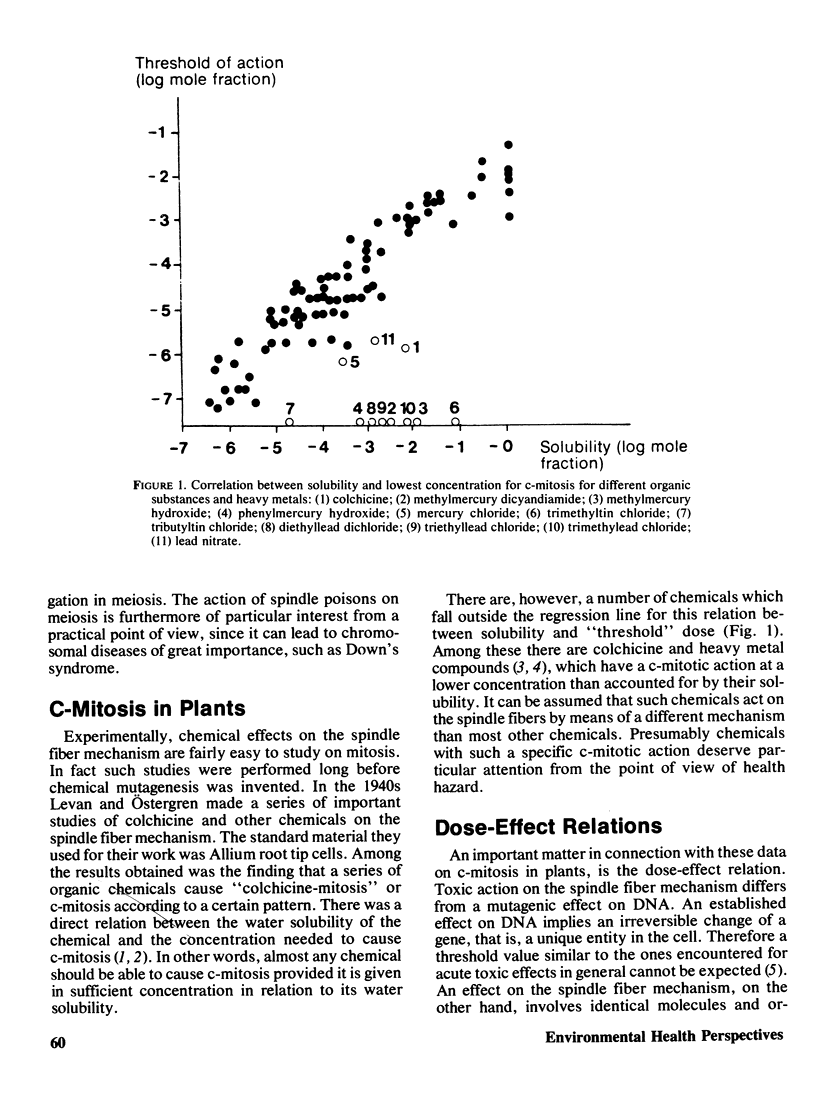
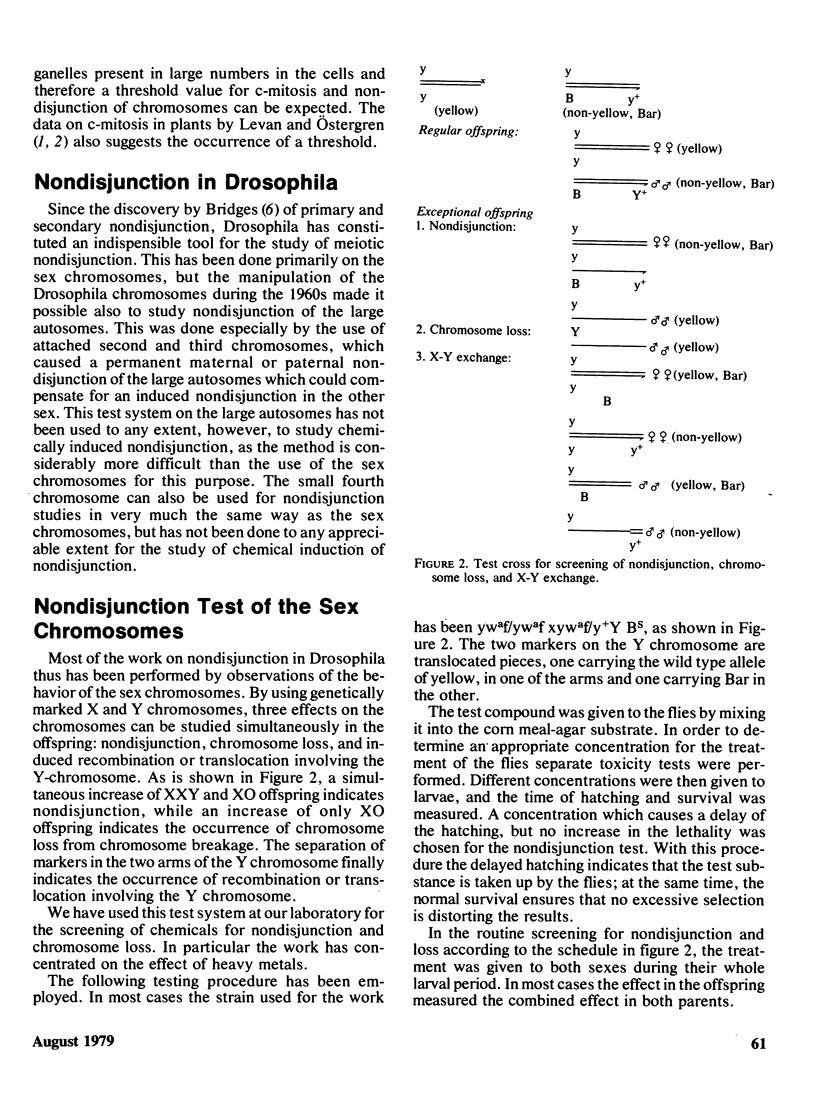
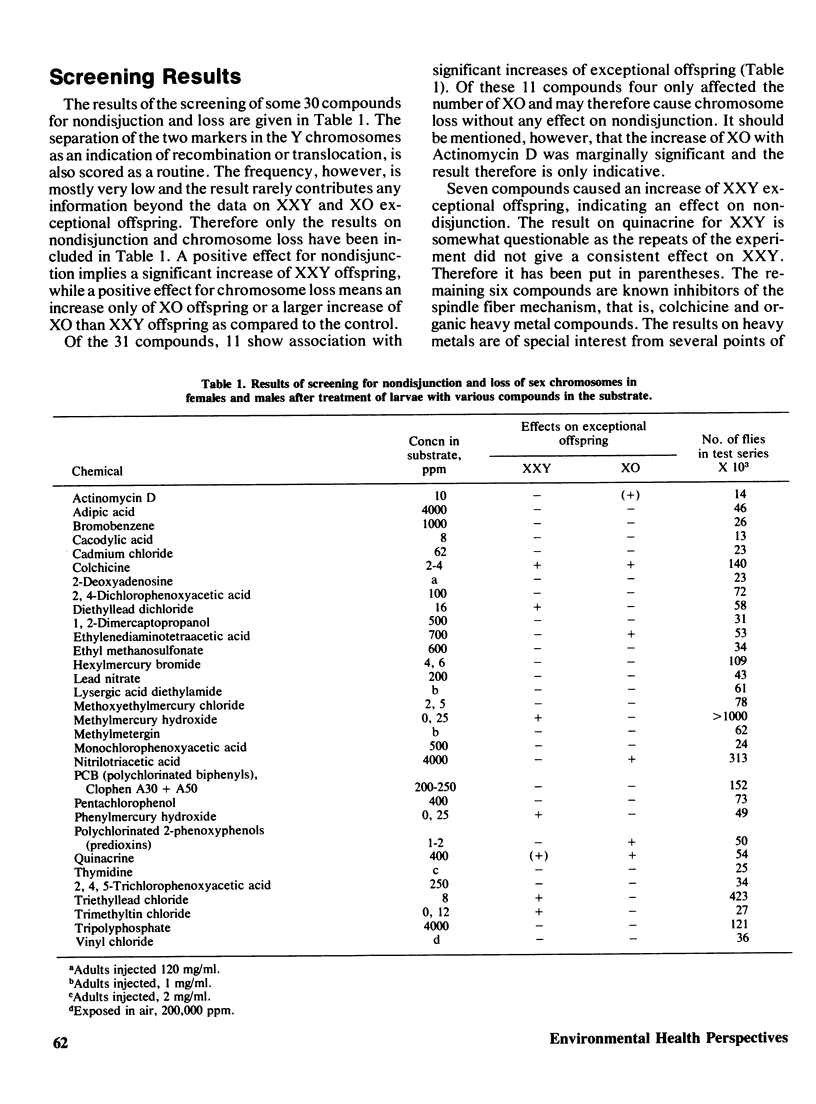


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Freese E. Thresholds in toxic, teratogenic, mutagenic, and carcinogenic effects. Environ Health Perspect. 1973 Dec;6:171–178. doi: 10.1289/ehp.7306171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVITSKI E. Genetic measures of centromere activity in Drosophila melanogaster. J Cell Physiol Suppl. 1955 May;45(Suppl 2):151–169. doi: 10.1002/jcp.1030450509. [DOI] [PubMed] [Google Scholar]
- Ramel C. Geetic effects of organic mercury compounds. I. Cytological investigations on Allium roots. Hereditas. 1969;61(1):208–230. [PubMed] [Google Scholar]
- Ramel C., Magnusson J. Genetic effects of organic mercury compounds. II. Chromosome segregation in Drosophila melanogaster. Hereditas. 1969;61(1):231–254. [PubMed] [Google Scholar]
- Tokunaga C. Aspects of low-temperature-induced meiotic nondisjunction in Drosophila females. Genetics. 1970 Dec;66(4):653–661. doi: 10.1093/genetics/66.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga C. The effects of low temperature and aging on nondisjunction in Drosophila. Genetics. 1970 May;65(1):75–94. doi: 10.1093/genetics/65.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]


