Abstract
Synthetic benzamide derivatives were investigated for their ability to inhibit histone deacetylase (HDA). In this study, one of the most active benzamide derivatives, MS-27-275, was examined with regard to its biological properties and antitumor efficacy. MS-27-275 inhibited partially purified human HDA and caused hyperacetylation of nuclear histones in various tumor cell lines. It behaved in a manner similar to other HDA inhibitors, such as sodium butyrate and trichostatin A; MS-27-275 induced p21WAF1/CIP1 and gelsolin and changed the cell cycle distribution, decrease of S-phase cells, and increase of G1-phase cells. The in vitro sensitivity spectrum of MS-27-275 against various human tumor cell lines showed a pattern different than that of a commonly used antitumor agent, 5-fluorouracil, and, of interest, the accumulation of p21WAF1/CIP1 tended to be faster and greater in the cell lines sensitive to MS-27-275. MS-27-275 administered orally strongly inhibited the growth in seven of eight tumor lines implanted into nude mice, although most of these did not respond to 5-fluorouracil. A structurally analogous compound to MS-27-275 without HDA-inhibiting activity showed neither the biological effects in cell culture nor the in vivo therapeutic efficacy. These results suggest that MS-27-275 acts as an antitumor agent through HDA inhibition and may provide a novel chemotherapeutic strategy for cancers insensitive to traditional antitumor agents.
Acetylation of nuclear histones, which is regulated by acetyltransferase and deacetylase (1–4), has been supposed to play a crucial role in gene expression because transcriptionally activated genes have been found to be associated with highly acetylated loci whereas transcriptionally inactive genes have been found to be associated with hypoacetylation (5–7). Furthermore, recent molecular and genetic approaches identified histone acetyltransferases and histone deacetylases (HDA) as transcriptional coactivators and transcriptional corepressors, respectively. These observations provide a molecular basis for regulation of transcription through acetylation of histones (8–10).
Although the precise mechanism underlying cell cycle arrest or differentiation through histone acetylation has not been elucidated, sodium n-butyrate (NaBu), an HDA inhibitor (11), has been known to arrest the cell cycle and provide various differentiation phenotypes or revertant phenotypes to cancer cells, including leukemias (12, 13), colorectal cancers (14, 15), a hepatic cancer (16), breast cancers (17, 18), and fibroblasts transformed by a oncogene (19). Therefore, compounds possessing HDA-inhibiting activity have been thought to represent a novel class of agent with less toxicity, along with all-trans-retinoic acid (20), for treatment of human cancers.
Although several efforts to apply NaBu for clinical treatment have been reported (21, 22), the efficacies were very limited because of its low antiproliferative activity and short half life in blood. Therefore, several derivatives of NaBu have been studied to improve the rapid metabolism in the body (23–25). Recently, other classes of compounds such as trichostatin A, trapoxin, and depudesin, which are derived from natural products, were reported to exhibit strong HDA inhibition (26–28). Although these natural compounds display strong in vitro activity, no in vivo antitumor efficacy has been reported, presumably because of instability, low retention, or nonspecific toxicity of the compounds in the body. During our efforts to find novel agents to treat refractory malignancies, including multidrug resistance (29–31), we found a series of synthetic benzamide derivatives with HDA-inhibitory activity in vitro and in vivo (32). Here, we report the characteristic features of one of these compounds and its strong antitumor efficacy against human cancers in nude mice.
MATERIALS AND METHODS
Chemicals.
N-(2-aminophenyl)-4-[N-(pyridin-3-yl-methoxycarbonyl)aminomethyl]benzamide (MS-27-275, Fig. 1A, compound 1) and its 3′-amino derivative (Fig. 1A, compound 2) were prepared as described (32).
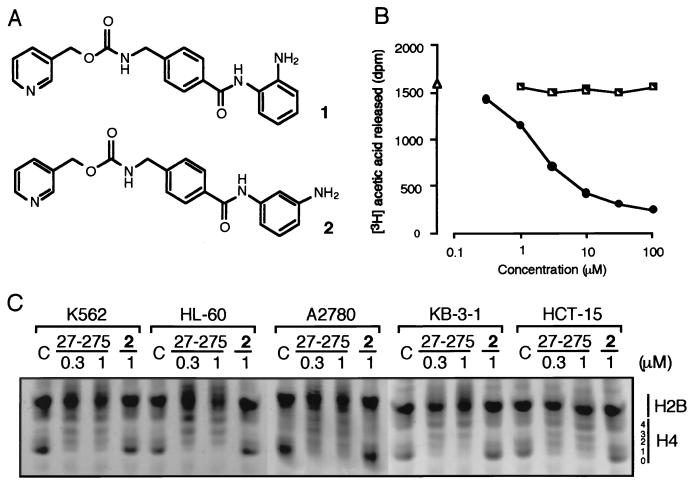
Figure 1.
Effect of MS-27-275 on HDA activity. (A) Chemical structure of MS-27-275 (1) and 3′-amino derivative (2). (B) Inhibition of human HDA by MS-27-275. HDA activity was measured either in the presence of MS-27-275 (●) or compound 2 (└) or in the absence of the agent (▵). (C) Hyperacetylation of nuclear histone (H4) by MS-27-275. Histones (40 μg) extracted from cells exposed to 0.3 μM or 1 μM MS-27-275 or 1 μM compound 2 for 24 h were separated by acid/urea/Triton X-100 gel electrophoresis. The details are described in Materials and Methods.
Cells, Animals, and Antibodies.
Human leukemia cell lines K562 and HL-60, human colorectal cancer lines COLO320DM, HT-29, and HCT-15, a human lung cancer line, Calu-3, a human ovary cancer line, SK-OV-3, and a human pancreatic cancer line, Capan-1, were obtained from the American Type Culture Collection and were maintained under the recommended conditions. A2780, a human ovary cancer line, provided by R. Ozols and T. Hamilton (National Cancer Institute), KB-3-1, a human oral cancer line obtained from I. Pastan (National Cancer Institute), and 4-1St and St-4, human gastric cancer lines established in our laboratory, were maintained as described (31). Female BALB/c-nu/nu nude mice (5 or 6 weeks old) were purchased from Charles River Breeding Laboratories. The mice were used at 7 weeks of age. Antibodies specific to p53 and p21WAF1/CIP1 were purchased from Santa Cruz Biotechnology, and retinoblastoma protein (pRb) and gelsolin were from QED Bioscience (San Diego, CA) and Sigma (St. Louis, MO), respectively.
Assay for Histone Deacetylase.
HDA was partially purified as described by Yoshida et al. (26) with slight modifications. K562 cells (2.5 × 108) were disrupted in 15 ml of HDA buffer (15 mM potassium phosphate, pH 7.5/5% glycerol/0.2 mM EDTA). Nuclei of the cells were collected by centrifugation (35,000 × g, 10 min) and were resuspended in 15 ml of HDA buffer containing 1 M (NH4)2SO4. After sonication to reduce viscosity, the supernatant was collected by centrifugation, solid (NH4)2SO4 was added to the supernatant to make the final concentration 3.5 M, and was stirred for 1 h at 0°C. The precipitates collected by centrifugation were dissolved again with 4 ml of HDA buffer and were dialyzed against 2 liters of HDA buffer. The dialysate was loaded onto MonoQ HR5/5 (Amersham Pharmacia) equilibrated with HDA buffer, and the proteins were eluted with a linear gradient of 0–1 M NaCl in 30 ml of HDA buffer. A single peak of HDA activity was eluted at 0.4 M NaCl, and the fraction was stored at −80°C until use. Nuclear histones were labeled by incubation of K562 cells (108 cells) in a 25 ml of growth medium containing 0.5 mCi/ml [3H]sodium acetate (152.8 GBq/mmol; NEN) and 5 mM NaBu at 37°C for 1 h. Histones were extracted as described (26).
HDA-inhibitory activity of the compound was estimated in 50 μl of reaction mixture containing 2 μl of the above HDA fraction, 100 μg/ml of [3H]acetylated histones, and 5 μl of the compound dissolved in HDA buffer at 37°C for 10 min. [3H]acetic acid released by the reaction was extracted with 50 μl of 1M HCl and 0.55 ml of ethyl acetate, and the radioactivity in the solvent layer was measured by liquid-scintillation counting. To assess in vivo HDA inhibition, cellular histones were extracted and examined by acid/urea/Triton X-100 PAGE followed by staining with Coomasie brilliant blue R-250, as described (26).
Northern Blot Analysis.
Total RNA was isolated by the acid guanidinium isothiocyanate-phenol-chloroform method. The RNA was separated by electrophoresis through 1% (weight/volume) agarose-formaldehyde gels, was transferred onto nylon membranes (Hybond-N+, Amersham Pharmacia), and was hybridized with a digoxigenin-labeled cRNA specific to human p21WAF1/CIP1 or gelsolin by using DIG Easy Hyb (Boehringer Mannheim) under manufacturer’s instruction. The probes were prepared by reverse transcription by using random hexamers and, after amplification, by PCR using oligonucleotides specific to human p21CIP1/WAF1 cDNA (ACTCAGAGGAGGCGCCATGT and TTCCTGTGGGCGGATTAGGG) or human gelsolin cDNA (GGAAGCCCATGATCATCTAC and TGTACCGCTTAGCAGAAGTC). The PCR products were cloned into the pGEM-T plasmid (Promega) and were confirmed by DNA sequencing. Digoxigenin-labeled cRNAs were synthesized by using a DIG RNA labeling kit (Boehringer Mannheim).
Western Blot Analysis.
Cells (2–6 × 106) were lysed with 0.3 ml of 63 mM Tris⋅HCl (pH 6.3), 2 mM EDTA, 5% 2-mercaptoethanol, 2.3% SDS, and 5% glycerol. The proteins were separated by SDS/PAGE and were electrophoretically transferred onto nitrocellulose membranes. The blots were probed with an antibody specific to each protein and were detected by using the enhanced chemiluminescence method (Amersham).
Flow Cytometric Analysis.
Unsynchronized cells were seeded at 106 per 100-mm dish and were exposed to the agent for 24 h. After fixing with 70% ethanol and treatment with 0.25 μg/ml RNase, the nuclei were stained with 50 μg/ml propidium iodide, and the relative DNA content was measured by using a fluorescence-activated cell sorter (EPICS ELITE, Coulter).
Evaluation of in Vitro Sensitivity.
Cancer cells (5 × 103) were seeded into each well of 96-well plates and were cultured with graded concentrations of the drugs for 3 days. The cells were stained with 0.1 mg/ml neutral red for 1 h in a CO2-incubator, and, after aspiration of the medium, OD540 of the neutral red solubilized with 50 μl of ethanol and 150 μl of 0.1 M Na2HPO4 was measured. The IC50 value was determined by plotting growth inhibition of the cells against the logarithm of the drug concentration.
In Vivo Antitumor Activity.
A2780 cells (9 × 106) grown in vitro were suspended in PBS and were injected subcutaneously into the flank of nude mouse. For the other tumor lines, KB-3-1, HCT-15, 4-1St, Calu-3, St-4, Capan-1, and HT-29, tumors were passaged several times before starting in vivo antitumor testing, and a tumor lump (2–3 mm in diameter) was transplanted subcutaneously into the flank of a nude mouse by using a trocar needle. Treatment (four or five mice in each experimental group) with the drugs was started after the tumors were confirmed to have grown in the body (tumor size, 20–100 mm3). MS-27-275 and compound 2, both dissolved with 0.05 N HCl, 0.1% Tween 80, and 5-fluorouracil (5-FU) (Mitsui Pharmaceuticals, Tokyo) and diluted with physiological saline, were administered orally once daily 5 days per week for 4 weeks. Tumor length and width were monitored twice weekly, and tumor volume was calculated as described (31).
RESULTS
Inhibition of Histone Deacetylase by MS-27-275.
A series of synthetic benzamide derivatives were investigated for their ability to inhibit HDA, and MS-27-275 (Fig. 1A, compound 1) was found to be one of the most active compounds. MS-27-275 inhibited HDA purified from human leukemia cells in a dose-dependent manner, and the IC50 value was estimated to be 2.0 μM (Fig. 1B) whereas trichostatin A and trapoxin were reported to inhibit HDA at nanomolar concentrations in similar assays (26, 27). The addition of MS-27-275 to cell culture resulted in the accumulation of hyperacetylated H4 molecules that produced multiple bands corresponding to histones with one, two, three, and four acetylated lysine residues on acid/urea/Triton X-100 gels (Fig. 1C). The levels of acetylation were almost identical among the cell lines examined (Fig. 1C). Of interest, compound 2, a structural analogue of MS-27-275 with a 3′-aminophenyl instead of a 2′-aminophenyl group (Fig. 1A), showed no effect on either the activity of HDA (Fig. 1B) or on the acetylation state of cellular histones (Fig. 1C), suggesting that the 2′-amino group of MS-27-275 plays an important role in binding and inhibition of the enzyme.
Induction of p21WAF1/CIP1 and Gelsolin by MS-27-275.
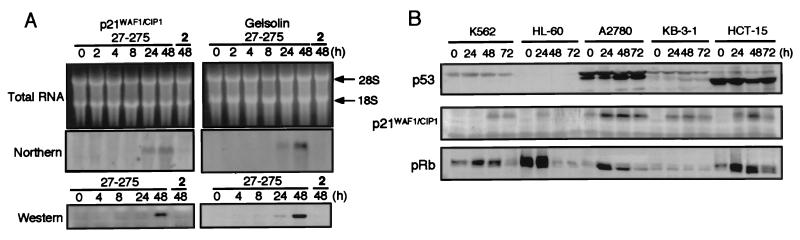
Other HDA inhibitors such as NaBu and trichostatin A were reported to transcriptionally induce p21WAF1/CIP1 (33, 34) and gelsolin (35, 36), both of which are considered to be tumor suppressors. MS-27-275 also increased the intracellular amounts of p21WAF1/CIP1 and gelsolin. In the K562 cells, mRNAs specific to both proteins were identified after 24-h exposure to MS-27-275, and the proteins were clearly accumulated after 48-h exposure (Fig. 2A). Compound 2 affected neither mRNAs nor protein expression (Fig. 2A).
Figure 2.
Effect of MS-27-275 of p21WAF1/CIP1 and gelsolin. (A) Accumulation time courses of mRNA and protein. Total RNA (10 μg) and lysate (45 μg of total protein) extracted from K562 cells exposed to 0.3 μM MS-27-275 or compound 2 for the indicated periods were used for Northern and Western blotting, respectively. (B) Western blot analysis of p53, p21WAF1/CIP1, and pRb. Lysates (45 μg of total protein) of the cells exposed to 0.3 μM MS-27-275 for the indicated periods were examined by Western blot analysis by using antibodies specific to p53, p21WAF1/CIP1, and pRb. The details are described in Materials and Methods.
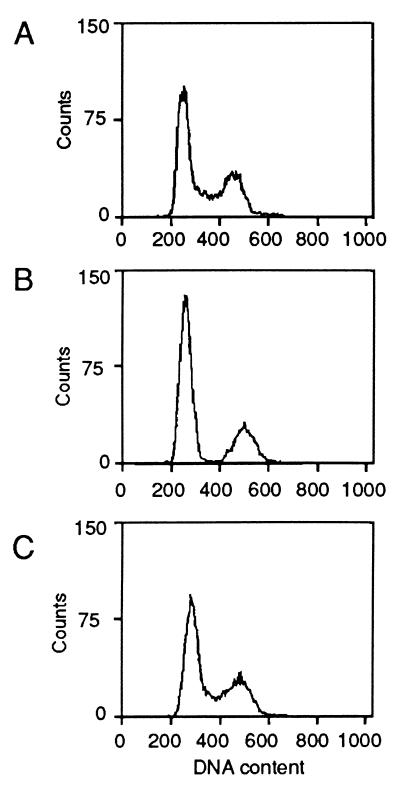
Because it is well known that p21WAF1/CIP1 inhibits cyclin-dependent kinases (37–40) and that the p21WAF1/CIP1 gene is physiologically induced by p53 (41), we checked modulation of p53, p21WAF1/CIP1, and pRb by MS-27-275 in several tumor lines. Among the tumor lines examined, K562, HL-60, KB-3-1, and HCT-15 cell lines had no intrinsic p21WAF1/CIP1 whereas A2780 had small but significant amounts of intrinsic p21WAF1/CIP1 (Fig. 2B). This observation was consistent with the previous studies that reported that K562, HL-60, and HCT-15 cell lines had dysfunctional alterations in p53 gene (42, 43) and that the A2780 line had wild-type p53 gene (44), except for KB-3-1, whose status of p53 gene has not been reported. After the exposure to MS-27-275, the cells accumulated significant amounts of p21WAF1/CIP1 irrespective of the amount of p53 and the status of p53 gene (Fig. 2B and Table 1). The induction of p21WAF1/CIP1 resulted in the reduction of hyperphosphorylated pRb molecules, which migrated more slowly than the hypophosphorylated form on SDS polyacrylamide gels (46–48) (Fig. 2B). Treatment of A2780 cells with MS-27-275 clearly decreased the population of S-phase cells and increased G1-phase cells (Fig. 3B), although compound 2, which showed neither HDA inhibition nor induction of p21WAF1/CIP1 or gelsolin, showed no effect on the cell cycle distribution (Fig. 3C). These observations suggest that, similarly to the other HDA inhibitors, MS-27-275 transcriptionally induced p21WAF1/CIP1 and gelsolin through acetylation of histones and changed the cell cycle distribution.
Table 1.
In vitro sensitivity to MS-27-275 of human tumor cell lines
| Cell line | IC50, μM
|
p53 gene status* | ||
|---|---|---|---|---|
| MS-27-275 | Compound 2 | 5-FU | ||
| A2780 | 0.0415 | 0.621 | 13.7 | wt |
| Calu-3 | 0.195 | >100 | 41.3 | - |
| HL-60 | 0.212 | 42.1 | 7.09 | mu |
| K562 | 0.589 | 58.0 | 140 | mu |
| St-4 | 0.820 | >100 | 1.63 | - |
| HT-29 | 1.29 | >100 | 153 | mu |
| KB-3-1 | 1.46 | >100 | 51.8 | - |
| Capan-1 | 1.70 | >100 | 14.2 | mu |
| 4-1St | 1.92 | >100 | 144 | - |
| HCT-15 | 4.71 | >100 | 26.3 | mu |
Figure 3.
Effect of MS-27-275 on cell cycle distribution in A2780 cells. Unsynchronized A2780 cells were grown in the absence (A) or presence of 1 μM MS-27-275 (B) or compound 2 (C) for 24 h, and the cells were harvested and examined by flow cytometry as described in Materials and Methods.
In Vitro Sensitivity and Induction of p21WAF1/CIP1 by MS-27-275 in Various Tumor Cells.
In vitro antiproliferative activity of MS-27-275 was examined in human tumor cell lines of various origins. The sensitivity spectrum of MS-27-275 against these cell lines, which showed various IC50 values ranging from 0.0415 μM (A2780) to 4.71 μM (HCT-15), was different from that of the commonly used chemotherapeutic agent 5-FU (Table 1). The antiproliferative activity of compound 2 was much weaker than that of MS-27-275 in all of the lines tested, suggesting that the antiproliferative activity is mainly caused by the HDA-inhibitory action of the compound but not by its nonspecific toxicity.
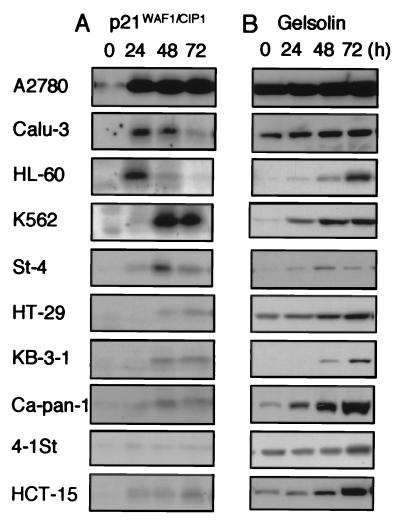
Of interest, the accumulation of p21WAF1/CIP1 in the cells exposed to MS-27-275 tended to be faster and greater in cell lines sensitive to MS-27-275, such as A2780, Calu-3, HL-60, and K562 (IC50 values were 0.0415 μM, 0.195 μM, 0.212 μM, and 0.589 μM, respectively), as compared with the others (Fig. 4A and Table 1). On the other hand, induction of gelsolin by MS-27-275 seemed to have no correlation with the sensitivities of the cells (Fig. 4B and Table 1), although gelsolin was reported to be a tumor suppressor (49, 50). From these observations, it is possible that the induction of p21WAF1/CIP1 through histone acetylation plays one of the crucial roles in the action of MS-27-275. Because the levels of acetylation were almost equal among these cell lines, it is suggested that the variations in the sensitivity to MS-27-275 and the kinetics of the induction of these proteins were derived from different genetic alterations, which might be acquired during cancer development, in the loci downstream of histone acetylation.
Figure 4.
Comparison of the accumulation of p21WAF1/CIP1 (A) and gelsolin (B) in various human tumor lines exposed to MS-27-275. Lysates (45 μg of total protein) from the cells exposed to 0.3 μM MS-27-275 for 0, 24, 48, and 72 h were analyzed by Western blotting by using specific antibodies to p21WAF1/CIP1 and gelsolin as described in Materials and Methods.
Antitumor Effect of MS-27-275 in Tumor-Bearing Nude Mice.
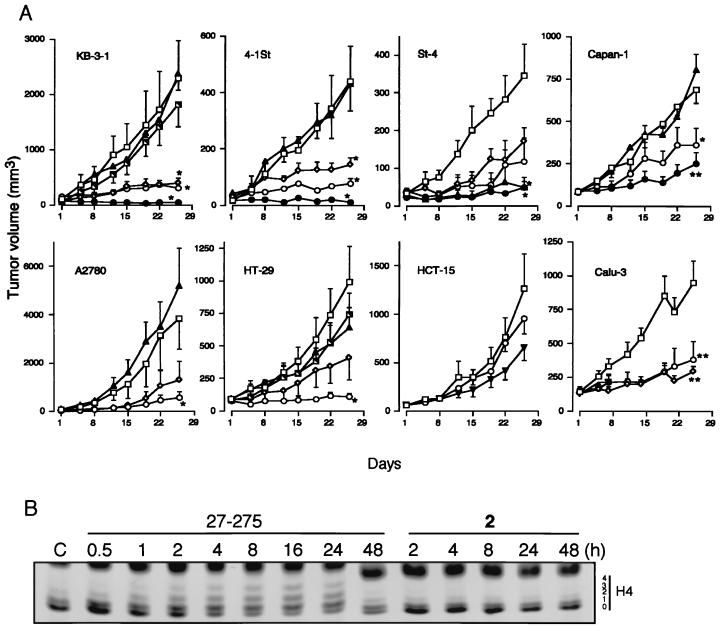
The in vivo therapeutic efficacy of MS-27-275 was examined by using the eight solid human tumor lines, which showed various sensitivities as described above (Table 1). MS-27-275 at 49 mg/kg showed marked antitumor effects against KB-3-1, 4-1St, and St-4 tumor lines, and a moderate effect against Capan-1 tumor (Fig. 5A). The drug at 24.5 mg/kg and 12.3 mg/kg also showed significant effects against these tumors. Because the dose of 49 mg/kg was the maximum tolerated dose in this administration schedule and apparent signs of toxicity such as weight loss and poor appearance were observed, we lowered the maximum dose of the drug to 24.5 mg/kg, at which no gross weight loss was observed, for the other tumors. The dose of 24.5 mg/kg was also markedly effective against A2780 and HT-29 and moderately effective against Calu-3 (Fig. 5A). Among the eight tumor lines examined, only HCT-15, relatively insensitive to MS-27-275 in vitro (Table 1), did not respond to MS-27-275 (Fig. 5A). Seven of the eight tumor lines examined responded well to MS-27-275, although only one line, St-4, responded well to 5-FU at the maximum tolerated dose (30 mg/kg). Compound 2 at 98 mg/kg, 2-fold higher than the maximum tolerated dose of MS-27-275, did not show any in vivo therapeutic activities against two tumor lines, KB-3-1 and HT-29, that were sensitive to MS-27-275 (Fig. 5A). In addition, oral administration of MS-27-275 apparently increased the level of histone acetylation in HT-29 tumor xenografts 4–24 h after the administration whereas compound 2 did not show the effect (Fig. 5B). These result suggests that the in vivo therapeutic efficacies of MS-27-275 observed in this study were derived from the HDA-inhibitory action of the compound but not from unidentified cytotoxic effects.
Figure 5.
In vivo effects of MS-27-275 against human tumor xenografts. (A) Antitumor efficacies against human tumor xenografts. The effects of MS-27-275 at doses of 12.3 (⋄), 24.5 (○) and 49 mg/kg (●), Compound 2 at 98 mg/kg (└), and 5-FU at 30 (▴) and 40 mg/kg (▾) were examined as described in Materials and Methods. □ represents the control group. Vertical bars indicate standard errors. ∗, P < 0.05; ∗∗, P < 0.01, respectively, by Dunnett’s test or Steel’s test. The discontinued line in the Calu-3 experiment was caused by death of mice in the test group. (B) Increase of histone acetylation in HT-29 tumor xenografts. MS-27-275 (49 mg/kg) and compound 2 (49 mg/kg) were administered orally to the nude mice with HT-29 tumors (tumor size average, 179 mm3). At the indicated period after the administration, tumor samples were removed, and histones were extracted and analyzed as described in Materials and Methods. A representative of three similar observations is shown.
DISCUSSION
In the present study, we demonstrated that a synthetic compound, MS-27-275, that shares no structural similarity to other HDA inhibitors such as NaBu (11), trichostatin A (26), and trapoxin (27) revealed HDA-inhibition and antitumor efficacy. Because compound 2, a structural analogue of MS-27-275 possessing a 3′-aminophenyl group instead of a 2′-aminophenyl group, showed no inhibition of HDA, it is suggested that binding of the 2′-aminophenyl group to an unidentified but specific site on HDA molecule is important for the function of MS-27-275 as an inhibitor. Furthermore, together with the structural feature, the difference in effective concentration between MS-27-275 and the other compounds suggests the differences in the binding site on HDA molecule and the mode of action between them.
As shown in studies of the biological activities of the other HDA inhibitors reported recently (33–36), MS-27-275 inhibited cell proliferation and induced p21WAF1/CIP1 and gelsolin through transcriptional activation. In all of the cell lines examined, the level of p21WAF1/CIP1 was increased in the presence of MS-27-275, irrespective of the amount of p53 and the status of p53 gene, and the accumulation of p21WAF1/CIP1 tended to be greater and faster in the cell lines sensitive to MS-27-275 whereas the accumulation of gelsolin seemed to have no correlation to the in vitro sensitivity of the cells. Because it has been reported that overexpression of gelsolin in transformed cells decreased tumorigenicity when the cells were inoculated into mice (49), gelsolin may function as a tumor suppressor in the body but not in cell culture. From these observations, it is possible that the induction of p21WAF1/CIP1 through histone acetylation plays one of the crucial roles in the action of MS-27-275.
MS-27-275 strongly inhibited the growth in seven of eight human tumor xenografts implanted into nude mice whereas 5-FU, a commonly used agent against cancers, had a marked effect on only one tumor line. It has been known that many human tumors harbor defects in p53 genes (51, 52) and that p53 monitors the integrity of the genome and halts cell proliferation through induction of p21WAF1/CIP1 in response to DNA damage induced by antitumor agent. Therefore, activation of this signaling pathway has been considered to be important for the efficacy of antitumor agents, and direct transactivation of the p21WAF1/CIP1 gene bypassing p53 can serve a novel strategy for treating cancers that are insensitive to classical antitumor agents. Recently, another tumor suppressor gene, BRCA1, which is associated with hereditary breast and ovary cancers, was reported to activate the p21WAF1/CIP1 gene in a p53-independent manner (53). Therefore, the induction of p21WAF1/CIP1 by HDA inhibition may be useful for not only cancers with defects in p53 gene but also for those harboring defects in other genes controlling the expression of p21WAF1/CIP1.
Compound 2, a structural analogue of MS-27-275 with no HDA inhibitory activity, exhibited no induction of these two proteins nor antiproliferative efficacy both in vitro and in vivo. We detected an increase in acetylation of nuclear histones in tumors implanted into mice after oral administration of MS-27-275. However, no increase in histone acetylation was observed after administration of compound 2 whereas both drugs were incorporated equally well into mouse blood by oral administration (data not shown). These results demonstrate that MS-27-275 exerts its antitumor effect through acetylation of nuclear histones. Therefore, we conclude that this compound can provide a unique way to treat cancers refractory to classical antitumor agents.
ABBREVIATIONS
- HDA
histone deacetylase
- pRb
retinoblastoma protein
- 5-FU
5-fluorouracil
References
- 1.Inoue A, Fujimoto D. Biochim Biophys Acta. 1970;220:307–316. doi: 10.1016/0005-2744(70)90015-x. [DOI] [PubMed] [Google Scholar]
- 2.Vidali G, Boffa L C, Allfrey V G. J Biol Chem. 1972;247:7365–7373. [PubMed] [Google Scholar]
- 3.Kikuchi H, Fujimoto D. FEBS Lett. 1973;29:280–282. doi: 10.1016/0014-5793(73)80038-9. [DOI] [PubMed] [Google Scholar]
- 4.Csordas A. Biochem J. 1990;265:23–38. doi: 10.1042/bj2650023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allegra P, Sterner R, Clayton D F, Allfrey V G. J Mol Biol. 1987;196:379–388. doi: 10.1016/0022-2836(87)90698-x. [DOI] [PubMed] [Google Scholar]
- 6.Hebbes T R, Thorne A W, Crane-Robinson C. EMBO J. 1988;7:1395–1403. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tazi J, Bard A. Cell. 1990;60:909–920. doi: 10.1016/0092-8674(90)90339-g. [DOI] [PubMed] [Google Scholar]
- 8.Roth S Y, Allis C D. Cell. 1996;87:5–8. doi: 10.1016/s0092-8674(00)81316-1. [DOI] [PubMed] [Google Scholar]
- 9.Wade P A, Pruss D, Wolfe A P. Trends Biochem Sci. 1997;22:128–132. doi: 10.1016/s0968-0004(97)01016-5. [DOI] [PubMed] [Google Scholar]
- 10.Pazin M J, Kadonaga J T. Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 11.Riggs M G, Whittaker R G, Neuman J R, Ingram V M. Nature (London) 1977;268:462–464. doi: 10.1038/268462a0. [DOI] [PubMed] [Google Scholar]
- 12.Cioe L, McNab A, Hubbell H R, Meo P, Curtis P, Rovera G. Cancer Res. 1981;41:237–243. [PubMed] [Google Scholar]
- 13.Hoessly M C, Rossi R M, Fischkoff S A. Cancer Res. 1989;49:3594–3597. [PubMed] [Google Scholar]
- 14.Kim Y S, Tsao D, Siddiqui B, Whitehead J S, Arnstein P, Bennet J, Hicks J. Cancer. 1980;45:1185–1192. doi: 10.1002/1097-0142(19800315)45:5+<1185::aid-cncr2820451324>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Tsao D, Morita A, Bella A, Luu P, Kim Y S. Cancer Res. 1982;42:1052–1058. [PubMed] [Google Scholar]
- 16.Ryan M P, Borenfreund E, Higgins P J. J Natl Cancer Inst. 1987;79:555–567. [PubMed] [Google Scholar]
- 17.Graham K A, Buick R N. J Cell Physiol. 1988;136:63–71. doi: 10.1002/jcp.1041360108. [DOI] [PubMed] [Google Scholar]
- 18.Guilbaud N F, Gas N, Dupont M A, Valette A. J Cell Physiol. 1990;145:162–172. doi: 10.1002/jcp.1041450122. [DOI] [PubMed] [Google Scholar]
- 19.Ryan M P, Higgins P J. J Cell Physiol. 1988;137:25–34. doi: 10.1002/jcp.1041370104. [DOI] [PubMed] [Google Scholar]
- 20.Huang M E, Ye Y C, Chen S R, Chai J R, Lu J X, Zhoa L, Gu L J, Wang Z Y. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 21.Novogradsky A, Dvir A, Ravid A, Shkolnik T, Stenzel K H, Rubin A L, Zaizov R. Cancer. 1983;51:9–14. doi: 10.1002/1097-0142(19830101)51:1<9::aid-cncr2820510104>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 22.Miller A A, Kurschel E, Osieka R, Schmidt C. Eur J Cancer Clin Oncol. 1987;23:1283–1287. doi: 10.1016/0277-5379(87)90109-x. [DOI] [PubMed] [Google Scholar]
- 23.Pouillart P, Cerutti I, Ronco G, Villa P, Chany C. Int J Cancer. 1991;49:89–95. doi: 10.1002/ijc.2910490117. [DOI] [PubMed] [Google Scholar]
- 24.Planchon P, Pouillart P, Ronco G, Villa P, Pieri F. J Pharm Sci. 1993;82:1046–1048. [PubMed] [Google Scholar]
- 25.Rephaeli A, Rabizadeh E, Aviram A, Shaklai M, Ruse M, Nudelman A. Int J Cancer. 1991;49:66–72. doi: 10.1002/ijc.2910490113. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida M, Kijima M, Akita M, Beppu T. J Biol Chem. 1990;265:17174–17179. [PubMed] [Google Scholar]
- 27.Kijima M, Yoshida M, Sugita K, Horinouchi S, Beppu T. J Biol Chem. 1993;268:22429–22435. [PubMed] [Google Scholar]
- 28.Kwon H J, Owa T, Hassig C A, Shimada J, Schreiber S L. Proc Natl Acad Sci USA. 1998;95:3356–3361. doi: 10.1073/pnas.95.7.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sato W, Fukazawa N, Nakanishi O, Baba M, Suzuki T, Yano O, Naito M, Tsuruo T. Cancer Chemother Pharmacol. 1995;35:271–277. doi: 10.1007/BF00689444. [DOI] [PubMed] [Google Scholar]
- 30.Baba M, Nakanishi O, Saito A, Miyama Y, Yano O, Shimada S, Fukazawa N, Naito M, Tsuruo T. Cancer Chemother Pharmacol. 1995;36:361–367. doi: 10.1007/BF00686183. [DOI] [PubMed] [Google Scholar]
- 31.Nakanishi O, Baba M, Saito A, Yamashita T, Sato W, Abe H, Fukazawa N, Suzuki T, Sato S, Naito M, et al. Oncol Res. 1997;9:61–69. [PubMed] [Google Scholar]
- 32.Suzuki, T., Ando, T., Tsuchiya, K., Nakanishi, O., Saito, A., Yamashita, T., Shiraishi, Y. & Tanaka, E. (1997) Eur. Patent 847992; (1998) Chem. Abstr. 29, 95402.
- 33.Nakano K, Mizuno T, Sowa Y, Orita T, Yoshino T, Okuyama Y, Fujita T, Ohtani-Fujita N, Matsuzawa Y, Tokino T, et al. J Biol Chem. 1997;272:22199–22206. doi: 10.1074/jbc.272.35.22199. [DOI] [PubMed] [Google Scholar]
- 34.Siavoshian S, Blottiere H M, Cherbut C, Galmiche J P. Biochem Biophys Res Commun. 1997;232:169–172. doi: 10.1006/bbrc.1997.6255. [DOI] [PubMed] [Google Scholar]
- 35.Higgins P J, Ryan M P. Biochem J. 1991;279:883–890. doi: 10.1042/bj2790883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoshikawa Y, Kwon H J, Yoshida M, Horinouchi S, Beppu T. Exp Cell Res. 1994;214:189–197. doi: 10.1006/excr.1994.1248. [DOI] [PubMed] [Google Scholar]
- 37.Harper J W, Hannon G J, Zhang H, Casso D, Elledge S J. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 38.Xiong Y, Hannon G J, Zhang H, Casso D, Kobayashi R, Beach D. Nature (London) 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 39.Gu Y, Turck C W, Morgan D O. Nature (London) 1993;366:707–710. doi: 10.1038/366707a0. [DOI] [PubMed] [Google Scholar]
- 40.Dulic V, Kaufmann W K, Wilson S J, Tlsty T D, Lees E, Harper J W, Elledge S J, Reed S I. Cell. 1994;76:1013–1023. doi: 10.1016/0092-8674(94)90379-4. [DOI] [PubMed] [Google Scholar]
- 41.El-Diery W S, Tokino T, Velculescu V E, Levy D B, Parsons R, Trent J M, Lin D, Mercer W E, Vogelstein B. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 42.Wolf D, Rotter V. Proc Natl Acad Sci USA. 1985;82:790–794. doi: 10.1073/pnas.82.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor P M, Jackman J, Bae I, Myers T G, Fan S, Mutoh M, Scudiero D A, Monks A, Sausville E A, Weinstein J N, et al. Cancer Res. 1997;57:4285–4300. [PubMed] [Google Scholar]
- 44.Brown R, Clugston C, Burns P, Edlin A, Vasey P, Vojtesek B, Kaye S B. Int J Cancer. 1993;55:678–684. doi: 10.1002/ijc.2910550428. [DOI] [PubMed] [Google Scholar]
- 45.Ruggeri B, Zhang S, Caamano J, DiRado M, Flynn S D, Klein Szanto A J. Oncogene. 1992;7:1503–1511. [PubMed] [Google Scholar]
- 46.DeCaprio J, Ludlow J W, Lynch D, Furukawa Y, Griffin J, Piwnica-Worms H, Huang C M, Livingston D M. Cell. 1989;58:1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- 47.Buchkovich K, Duffy L A, Harlow E. Cell. 1989;58:1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- 48.Chen P L, Scully P, Shew J Y, Wang J Y J, Lee W H. Cell. 1989;58:1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- 49.Müllauer L, Fujita H, Ishizaki A, Kuzumaki N. Oncogene. 1993;8:2531–2536. [PubMed] [Google Scholar]
- 50.Tanaka M, Müllauer L, Ogiso Y, Fujita H, Moriya S, Furuuchi K, Harabayashi T, Shinohara N, Koyanagi T, Kuzumaki N. Cancer Res. 1995;55:3228–3232. [PubMed] [Google Scholar]
- 51.Vogelstein B, Fearson E R, Hamilton S R, Kern S E, Preisinger A C, Leppert M, Nakamura Y, White R, Smits A M M, Bos J L. N Engl J Med. 1988;319:525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- 52.Vogelstein B, Kinzler K W. Cell. 1998;70:523–526. doi: 10.1016/0092-8674(92)90421-8. [DOI] [PubMed] [Google Scholar]
- 53.Somasdaram K, Zhang H, Zeng Y-X, Houvras Y, Peng Y, Zhang H, Wu G S, Licht J D, Weber B L. Nature (London) 1997;389:187–190. doi: 10.1038/38291. [DOI] [PubMed] [Google Scholar]