Abstract
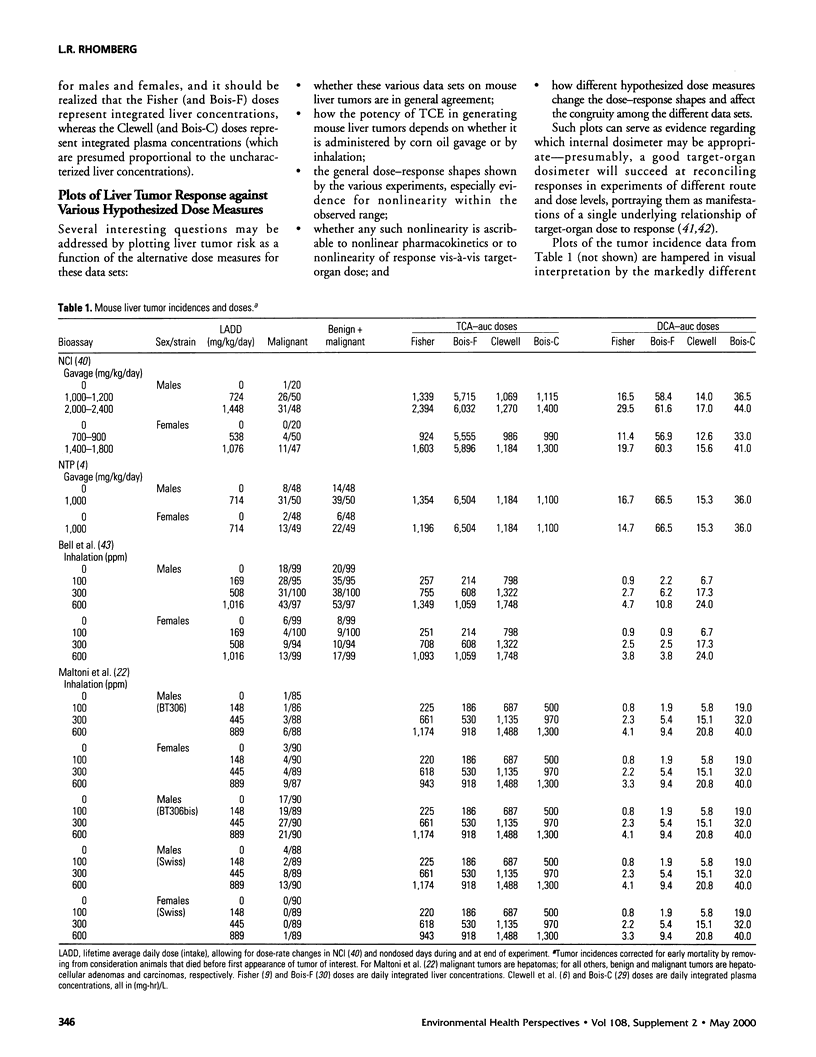
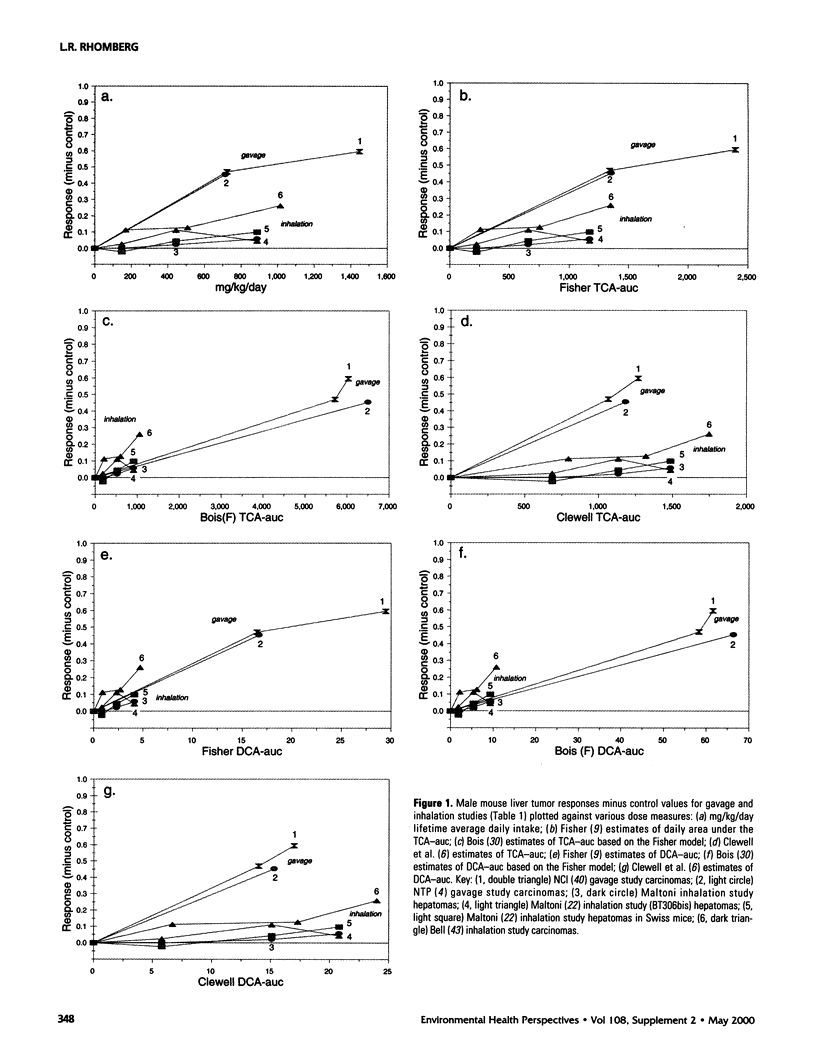
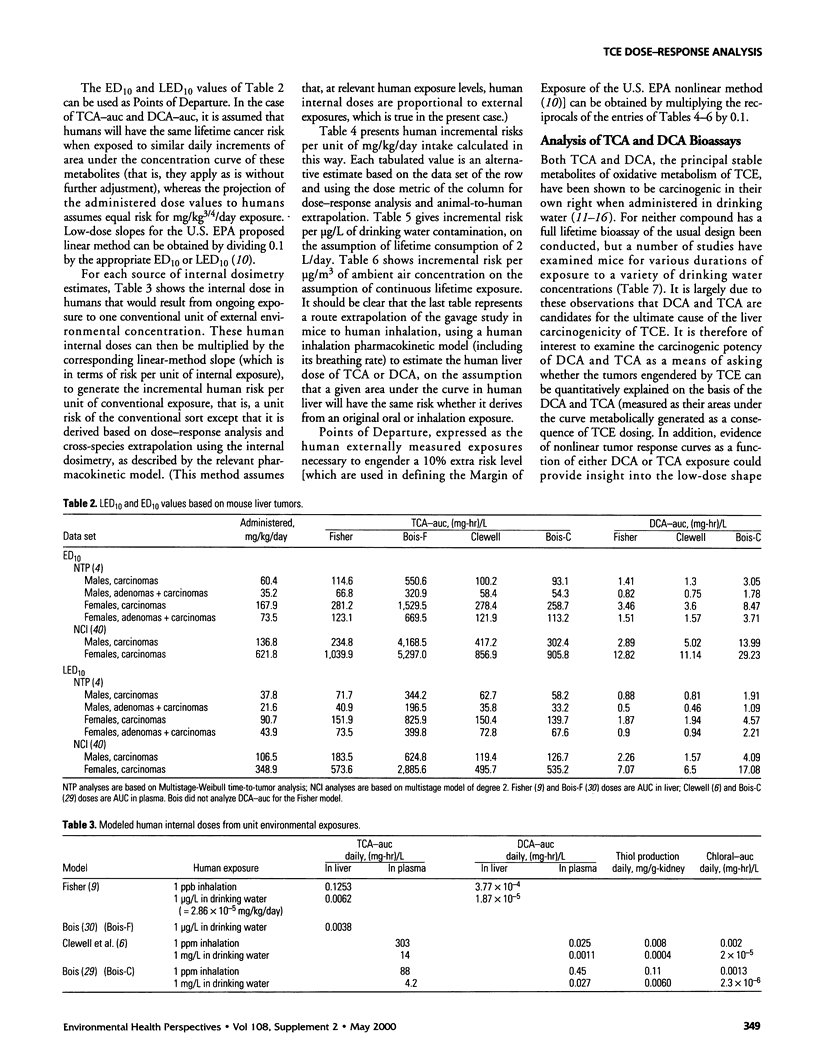
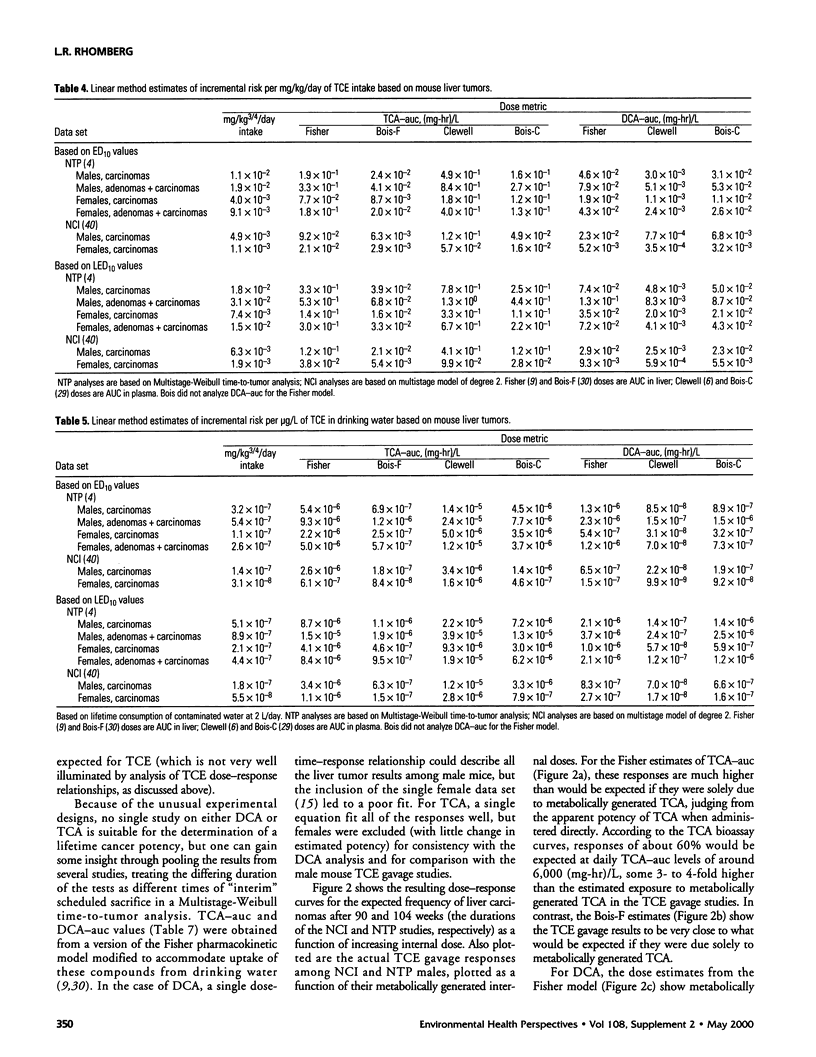
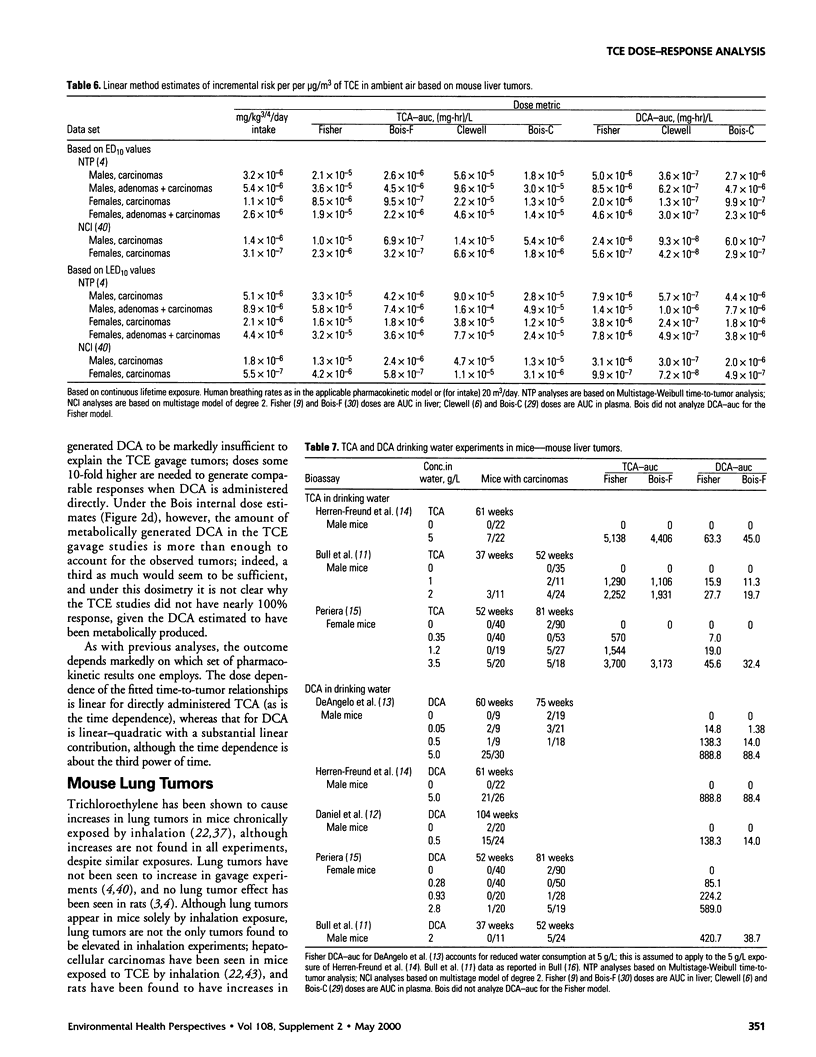
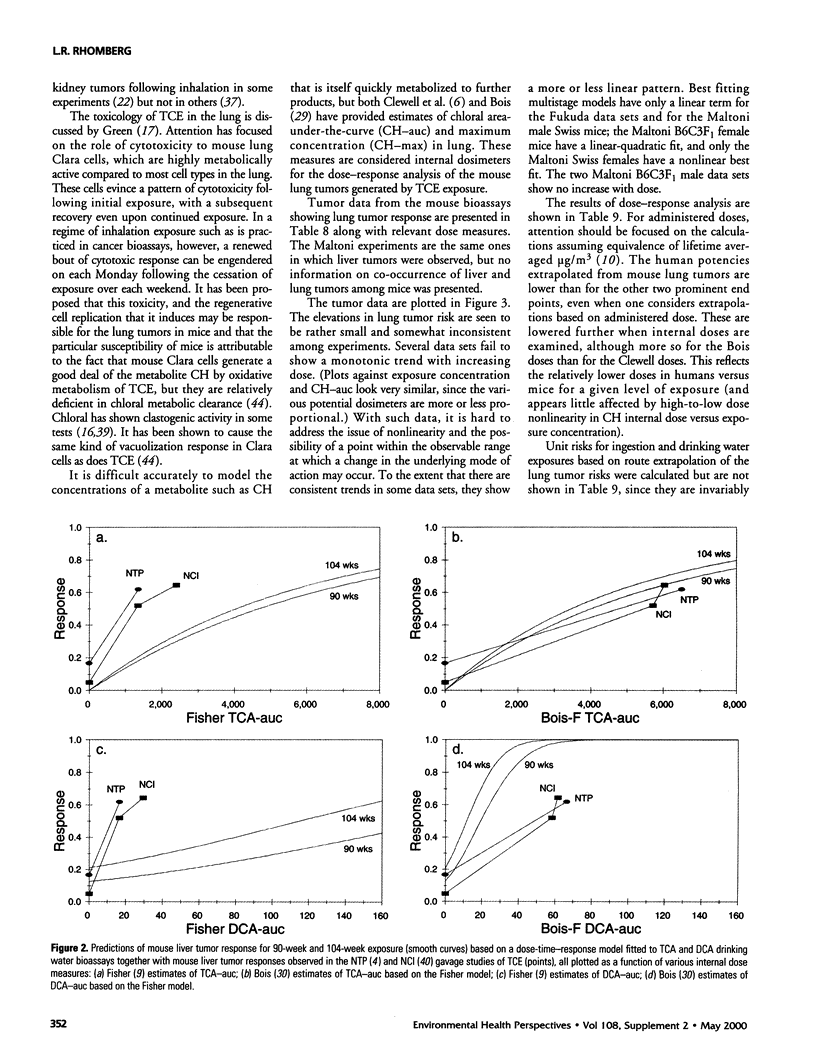
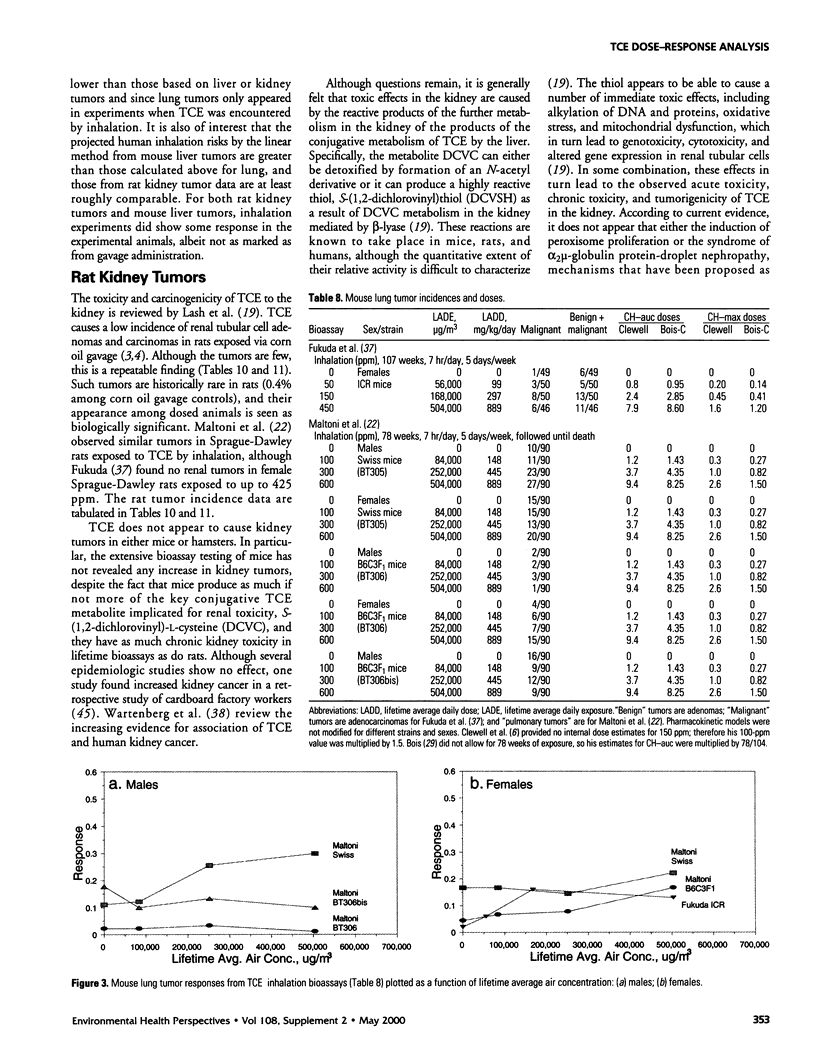
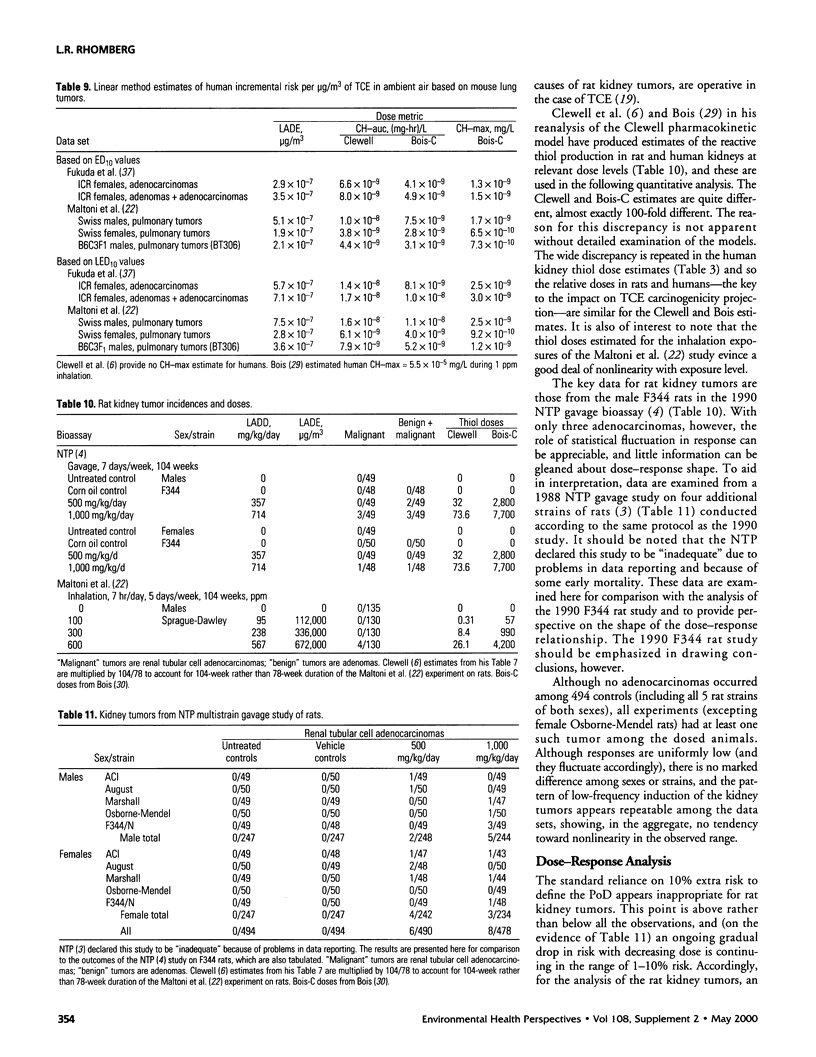
In lifetime bioassays, trichloroethylene (TCE, CAS No. 79-01-6) causes liver tumors in mice following gavage, liver and lung tumors in mice following inhalation, and kidney tumors in rats following gavage or inhalation. Recently developed pharmacokinetic models provide estimates of internal, target-organ doses of the TCE metabolites thought responsible for these tumor responses. Dose-response analyses following recently proposed methods for carcinogen risk assessment from the U.S. Environmental Protection Agency (U.S. EPA) are conducted on the animal tumor data using the pharmacokinetic dosimeters to derive a series of alternative projections of the potential carcinogenic potency of TCE in humans exposed to low environmental concentrations. Although mechanistic considerations suggest action of possibly nonlinear processes, dose-response shapes in the observable range of tumor incidence evince little sign of such patterns. Results depend on which of several alternative pharmacokinetic analyses are used to define target-organ doses. Human potency projections under the U.S. EPA linear method based on mouse liver tumors and internal dosimetry equal or somewhat exceed calculations based on administered dose, and projections based on mouse liver tumors exceed those from mouse lung or rat kidney tumors. Estimates of the carcinogenic potency of the two primary oxidative metabolites of TCE--trichloroacetic acid and dichloroacetic acid, which are mouse liver carcinogens in their own right--are also made, but it is not clear whether the carcinogenic potency of TCE can be quantitatively ascribed to metabolic generation of these metabolites.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen B. C., Fisher J. W. Pharmacokinetic modeling of trichloroethylene and trichloroacetic acid in humans. Risk Anal. 1993 Feb;13(1):71–86. doi: 10.1111/j.1539-6924.1993.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Andersen M. E., Clewell H. J., 3rd, Gargas M. L., Smith F. A., Reitz R. H. Physiologically based pharmacokinetics and the risk assessment process for methylene chloride. Toxicol Appl Pharmacol. 1987 Feb;87(2):185–205. doi: 10.1016/0041-008x(87)90281-x. [DOI] [PubMed] [Google Scholar]
- Andersen M. E., Clewell H., 3rd, Krishnan K. Tissue dosimetry, pharmacokinetic modeling, and interspecies scaling factors. Risk Anal. 1995 Aug;15(4):533–537. doi: 10.1111/j.1539-6924.1995.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Barton H. A., Clewell H. J., 3rd Evaluating noncancer effects of trichloroethylene: dosimetry, mode of action, and risk assessment. Environ Health Perspect. 2000 May;108 (Suppl 2):323–334. doi: 10.1289/ehp.00108s2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois F. Y. Statistical analysis of Clewell et al. PBPK model of trichloroethylene kinetics. Environ Health Perspect. 2000 May;108 (Suppl 2):307–316. doi: 10.1289/ehp.00108s2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bois F. Y. Statistical analysis of Fisher et al. PBPK model of trichloroethylene kinetics. Environ Health Perspect. 2000 May;108 (Suppl 2):275–282. doi: 10.1289/ehp.00108s2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R. J. Mode of action of liver tumor induction by trichloroethylene and its metabolites, trichloroacetate and dichloroacetate. Environ Health Perspect. 2000 May;108 (Suppl 2):241–259. doi: 10.1289/ehp.00108s2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull R. J., Sanchez I. M., Nelson M. A., Larson J. L., Lansing A. J. Liver tumor induction in B6C3F1 mice by dichloroacetate and trichloroacetate. Toxicology. 1990 Sep;63(3):341–359. doi: 10.1016/0300-483x(90)90195-m. [DOI] [PubMed] [Google Scholar]
- Clewell H. J., 3rd, Gentry P. R., Covington T. R., Gearhart J. M. Development of a physiologically based pharmacokinetic model of trichloroethylene and its metabolites for use in risk assessment. Environ Health Perspect. 2000 May;108 (Suppl 2):283–305. doi: 10.1289/ehp.00108s2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell H. J., Gentry P. R., Gearhart J. M., Allen B. C., Andersen M. E. Considering pharmacokinetic and mechanistic information in cancer risk assessments for environmental contaminants: examples with vinyl chloride and trichloroethylene. Chemosphere. 1995 Jul;31(1):2561–2578. doi: 10.1016/0045-6535(95)00124-q. [DOI] [PubMed] [Google Scholar]
- Daniel F. B., DeAngelo A. B., Stober J. A., Olson G. R., Page N. P. Hepatocarcinogenicity of chloral hydrate, 2-chloroacetaldehyde, and dichloroacetic acid in the male B6C3F1 mouse. Fundam Appl Toxicol. 1992 Aug;19(2):159–168. doi: 10.1016/0272-0590(92)90147-a. [DOI] [PubMed] [Google Scholar]
- DeAngelo A. B., Daniel F. B., Stober J. A., Olson G. R. The carcinogenicity of dichloroacetic acid in the male B6C3F1 mouse. Fundam Appl Toxicol. 1991 Feb;16(2):337–347. doi: 10.1016/0272-0590(91)90118-n. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Allen B. C. Evaluating the risk of liver cancer in humans exposed to trichloroethylene using physiological models. Risk Anal. 1993 Feb;13(1):87–95. doi: 10.1111/j.1539-6924.1993.tb00731.x. [DOI] [PubMed] [Google Scholar]
- Fisher J. W. Physiologically based pharmacokinetic models for trichloroethylene and its oxidative metabolites. Environ Health Perspect. 2000 May;108 (Suppl 2):265–273. doi: 10.1289/ehp.00108s2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K., Takemoto K., Tsuruta H. Inhalation carcinogenicity of trichloroethylene in mice and rats. Ind Health. 1983;21(4):243–254. doi: 10.2486/indhealth.21.243. [DOI] [PubMed] [Google Scholar]
- Green T. Pulmonary toxicity and carcinogenicity of trichloroethylene: species differences and modes of action. Environ Health Perspect. 2000 May;108 (Suppl 2):261–264. doi: 10.1289/ehp.00108s2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henschler D., Romen W., Elsässer H. M., Reichert D., Eder E., Radwan Z. Carcinogenicity study of trichloroethylene by longterm inhalation in three animal species. Arch Toxicol. 1980 Feb;43(4):237–248. doi: 10.1007/BF00366179. [DOI] [PubMed] [Google Scholar]
- Henschler D., Vamvakas S., Lammert M., Dekant W., Kraus B., Thomas B., Ulm K. Increased incidence of renal cell tumors in a cohort of cardboard workers exposed to trichloroethene. Arch Toxicol. 1995;69(5):291–299. doi: 10.1007/s002040050173. [DOI] [PubMed] [Google Scholar]
- Herren-Freund S. L., Pereira M. A., Khoury M. D., Olson G. The carcinogenicity of trichloroethylene and its metabolites, trichloroacetic acid and dichloroacetic acid, in mouse liver. Toxicol Appl Pharmacol. 1987 Sep 15;90(2):183–189. doi: 10.1016/0041-008x(87)90325-5. [DOI] [PubMed] [Google Scholar]
- Krewski D., Crump K. S., Farmer J., Gaylor D. W., Howe R., Portier C., Salsburg D., Sielken R. L., Van Ryzin J. A comparison of statistical methods for low dose extrapolation utilizing time-to-tumor data. Fundam Appl Toxicol. 1983 May-Jun;3(3):140–160. doi: 10.1016/s0272-0590(83)80075-x. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Fisher J. W., Lipscomb J. C., Parker J. C. Metabolism of trichloroethylene. Environ Health Perspect. 2000 May;108 (Suppl 2):177–200. doi: 10.1289/ehp.00108s2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L. H., Parker J. C., Scott C. S. Modes of action of trichloroethylene for kidney tumorigenesis. Environ Health Perspect. 2000 May;108 (Suppl 2):225–240. doi: 10.1289/ehp.00108s2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltoni C., Lefemine G., Cotti G., Perino G. Long-term carcinogenicity bioassays on trichloroethylene administered by inhalation to Sprague-Dawley rats and Swiss and B6C3F1 mice. Ann N Y Acad Sci. 1988;534:316–342. doi: 10.1111/j.1749-6632.1988.tb30120.x. [DOI] [PubMed] [Google Scholar]
- Mordenti J. Man versus beast: pharmacokinetic scaling in mammals. J Pharm Sci. 1986 Nov;75(11):1028–1040. doi: 10.1002/jps.2600751104. [DOI] [PubMed] [Google Scholar]
- Odum J., Foster J. R., Green T. A mechanism for the development of Clara cell lesions in the mouse lung after exposure to trichloroethylene. Chem Biol Interact. 1992 Aug 14;83(2):135–153. doi: 10.1016/0009-2797(92)90042-j. [DOI] [PubMed] [Google Scholar]
- Pereira M. A. Carcinogenic activity of dichloroacetic acid and trichloroacetic acid in the liver of female B6C3F1 mice. Fundam Appl Toxicol. 1996 Jun;31(2):192–199. doi: 10.1006/faat.1996.0091. [DOI] [PubMed] [Google Scholar]
- Rhomberg L. Use of quantitative modelling in methylene chloride risk assessment. Toxicology. 1995 Sep 1;102(1-2):95–114. doi: 10.1016/0300-483x(95)03039-i. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Gray G. M., Evans J. S. The ability of predicted internal dose measures to reconcile tumor bioassay data for chloroform. Regul Toxicol Pharmacol. 1995 Jun;21(3):339–351. doi: 10.1006/rtph.1995.1048. [DOI] [PubMed] [Google Scholar]
- Travis C. C., White R. K., Ward R. C. Interspecies extrapolation of pharmacokinetics. J Theor Biol. 1990 Feb 9;142(3):285–304. doi: 10.1016/s0022-5193(05)80554-5. [DOI] [PubMed] [Google Scholar]
- Wartenberg D., Reyner D., Scott C. S. Trichloroethylene and cancer: epidemiologic evidence. Environ Health Perspect. 2000 May;108 (Suppl 2):161–176. doi: 10.1289/ehp.00108s2161. [DOI] [PMC free article] [PubMed] [Google Scholar]


