Abstract
A major focus in the study of metabolism and disposition of trichloroethylene (TCE) is to identify metabolites that can be used reliably to assess flux through the various pathways of TCE metabolism and to identify those metabolites that are causally associated with toxic responses. Another important issue involves delineation of sex- and species-dependent differences in biotransformation pathways. Defining these differences can play an important role in the utility of laboratory animal data for understanding the pharmacokinetics and pharmacodynamics of TCE in humans. Sex-, species-, and strain-dependent differences in absorption and distribution of TCE may play some role in explaining differences in metabolism and susceptibility to toxicity from TCE exposure. The majority of differences in susceptibility, however, are likely due to sex-, species-, and strain-dependent differences in activities of the various enzymes that can metabolize TCE and its subsequent metabolites. An additional factor that plays a role in human health risk assessment for TCE is the high degree of variability in the activity of certain enzymes. TCE undergoes metabolism by two major pathways, cytochrome P450 (P450)-dependent oxidation and conjugation with glutathione (GSH). Key P450-derived metabolites of TCE that have been associated with specific target organs, such as the liver and lungs, include chloral hydrate, trichloroacetate, and dichloroacetate. Metabolites derived from the GSH conjugate of TCE, in contrast, have been associated with the kidney as a target organ. Specifically, metabolism of the cysteine conjugate of TCE by the cysteine conjugate ss-lyase generates a reactive metabolite that is nephrotoxic and may be nephrocarcinogenic. Although the P450 pathway is a higher activity and higher affinity pathway than the GSH conjugation pathway, one should not automatically conclude that the latter pathway is only important at very high doses. A synthesis of this information is then presented to assess how experimental data, from either animals or from (italic)in vitro (/italic)studies, can be extrapolated to humans for risk assessment. (italic)Key words(/italic): conjugate beta-lyase, cysteine glutathione, cytochrome P450, glutathione (italic)S(/italic)-transferases, metabolism, sex dependence, species dependence, tissue dependence, trichloroethylene.
Full text
PDF
















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
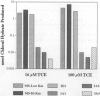
- Abbas R. R., Seckel C. S., Kidney J. K., Fisher J. W. Pharmacokinetic analysis of chloral hydrate and its metabolism in B6C3F1 mice. Drug Metab Dispos. 1996 Dec;24(12):1340–1346. [PubMed] [Google Scholar]
- Abbas R., Fisher J. W. A physiologically based pharmacokinetic model for trichloroethylene and its metabolites, chloral hydrate, trichloroacetate, dichloroacetate, trichloroethanol, and trichloroethanol glucuronide in B6C3F1 mice. Toxicol Appl Pharmacol. 1997 Nov;147(1):15–30. doi: 10.1006/taap.1997.8190. [DOI] [PubMed] [Google Scholar]
- Abraham D. G., Cooper A. J. Glutamine transaminase K and cysteine S-conjugate beta-lyase activity stains. Anal Biochem. 1991 Sep 2;197(2):421–427. doi: 10.1016/0003-2697(91)90414-o. [DOI] [PubMed] [Google Scholar]
- Abraham D. G., Patel P. P., Cooper A. J. Isolation from rat kidney of a cytosolic high molecular weight cysteine-S-conjugate beta-lyase with activity toward leukotriene E4. J Biol Chem. 1995 Jan 6;270(1):180–188. doi: 10.1074/jbc.270.1.180. [DOI] [PubMed] [Google Scholar]
- Abraham D. G., Thomas R. J., Cooper A. J. Glutamine transaminase K is not a major cysteine S-conjugate beta-lyase of rat kidney mitochondria: evidence that a high-molecular weight enzyme fulfills this role. Mol Pharmacol. 1995 Nov;48(5):855–860. [PubMed] [Google Scholar]
- Akerboom T. P., Narayanaswami V., Kunst M., Sies H. ATP-dependent S-(2,4-dinitrophenyl)glutathione transport in canalicular plasma membrane vesicles from rat liver. J Biol Chem. 1991 Jul 15;266(20):13147–13152. [PubMed] [Google Scholar]
- Alberati-Giani D., Malherbe P., Köhler C., Lang G., Kiefer V., Lahm H. W., Cesura A. M. Cloning and characterization of a soluble kynurenine aminotransferase from rat brain: identity with kidney cysteine conjugate beta-lyase. J Neurochem. 1995 Apr;64(4):1448–1455. doi: 10.1046/j.1471-4159.1995.64041448.x. [DOI] [PubMed] [Google Scholar]
- Allen B. C., Fisher J. W. Pharmacokinetic modeling of trichloroethylene and trichloroacetic acid in humans. Risk Anal. 1993 Feb;13(1):71–86. doi: 10.1111/j.1539-6924.1993.tb00730.x. [DOI] [PubMed] [Google Scholar]
- Anders M. W., Jakobson I. Biotransformation of halogenated solvents. Scand J Work Environ Health. 1985;11 (Suppl 1):23–32. [PubMed] [Google Scholar]
- Anderson P. M., Schultze M. O. Cleavage of S-(1,2-dichlorovinyl)-L-cysteine by an enzyme of bovine origin. Arch Biochem Biophys. 1965 Sep;111(3):593–602. doi: 10.1016/0003-9861(65)90240-7. [DOI] [PubMed] [Google Scholar]
- Badawy A. A., Aliyu S. U. Antagonism of acute alcohol intoxication by naloxone. Alcohol Alcohol. 1984;19(3):199–201. [PubMed] [Google Scholar]
- Barton H. A., Flemming C. D., Lipscomb J. C. Evaluating human variability in chemical risk assessment: hazard identification and dose-response assessment for noncancer oral toxicity of trichloroethylene. Toxicology. 1996 Jul 17;111(1-3):271–287. doi: 10.1016/0300-483x(96)03382-3. [DOI] [PubMed] [Google Scholar]
- Bernauer U., Birner G., Dekant W., Henschler D. Biotransformation of trichloroethene: dose-dependent excretion of 2,2,2-trichloro-metabolites and mercapturic acids in rats and humans after inhalation. Arch Toxicol. 1996;70(6):338–346. doi: 10.1007/s002040050283. [DOI] [PubMed] [Google Scholar]
- Birner G., Richling C., Henschler D., Anders M. W., Dekant W. Metabolism of tetrachloroethene in rats: identification of N epsilon-(dichloroacetyl)-L-lysine and N epsilon-(trichloroacetyl)-L-lysine as protein adducts. Chem Res Toxicol. 1994 Nov-Dec;7(6):724–732. doi: 10.1021/tx00042a003. [DOI] [PubMed] [Google Scholar]
- Birner G., Vamvakas S., Dekant W., Henschler D. Nephrotoxic and genotoxic N-acetyl-S-dichlorovinyl-L-cysteine is a urinary metabolite after occupational 1,1,2-trichloroethene exposure in humans: implications for the risk of trichloroethene exposure. Environ Health Perspect. 1993 Mar;99:281–284. doi: 10.1289/ehp.9399281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen K. T., Colston B. W., Jr, Machicao L. K. Dermal absorption of dilute aqueous chloroform, trichloroethylene, and tetrachloroethylene in hairless guinea pigs. Fundam Appl Toxicol. 1992 Jan;18(1):30–39. doi: 10.1016/0272-0590(92)90192-k. [DOI] [PubMed] [Google Scholar]
- Brüning T., Lammert M., Kempkes M., Thier R., Golka K., Bolt H. M. Influence of polymorphisms of GSTM1 and GSTT1 for risk of renal cell cancer in workers with long-term high occupational exposure to trichloroethene. Arch Toxicol. 1997;71(9):596–599. doi: 10.1007/s002040050432. [DOI] [PubMed] [Google Scholar]
- Brüning T., Vamvakas S., Makropoulos V., Birner G. Acute intoxication with trichloroethene: clinical symptoms, toxicokinetics, metabolism, and development of biochemical parameters for renal damage. Toxicol Sci. 1998 Feb;41(2):157–165. doi: 10.1006/toxs.1997.2401. [DOI] [PubMed] [Google Scholar]
- Bull R. J. Mode of action of liver tumor induction by trichloroethylene and its metabolites, trichloroacetate and dichloroacetate. Environ Health Perspect. 2000 May;108 (Suppl 2):241–259. doi: 10.1289/ehp.00108s2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlile D. J., Zomorodi K., Houston J. B. Scaling factors to relate drug metabolic clearance in hepatic microsomes, isolated hepatocytes, and the intact liver: studies with induced livers involving diazepam. Drug Metab Dispos. 1997 Aug;25(8):903–911. [PubMed] [Google Scholar]
- Clewell H. J., 3rd, Gentry P. R., Covington T. R., Gearhart J. M. Development of a physiologically based pharmacokinetic model of trichloroethylene and its metabolites for use in risk assessment. Environ Health Perspect. 2000 May;108 (Suppl 2):283–305. doi: 10.1289/ehp.00108s2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commandeur J. N., Vermeulen N. P. Identification of N-acetyl(2,2-dichlorovinyl)- and N-acetyl(1,2-dichlorovinyl)-L-cysteine as two regioisomeric mercapturic acids of trichloroethylene in the rat. Chem Res Toxicol. 1990 May-Jun;3(3):212–218. doi: 10.1021/tx00015a005. [DOI] [PubMed] [Google Scholar]
- D'Souza R. W., Bruckner J. V., Feldman S. Oral and intravenous trichloroethylene pharmacokinetics in the rat. J Toxicol Environ Health. 1985;15(5):587–601. doi: 10.1080/15287398509530688. [DOI] [PubMed] [Google Scholar]
- DANIEL J. W. THE METABOLISM OF 36C1-LABELLED TRICHLOROETHYLENE AND TETRACHLOROETHYLENE IN THE RAT. Biochem Pharmacol. 1963 Aug;12:795–802. doi: 10.1016/0006-2952(63)90109-6. [DOI] [PubMed] [Google Scholar]
- Dallas C. E., Gallo J. M., Ramanathan R., Muralidhara S., Bruckner J. V. Physiological pharmacokinetic modeling of inhaled trichloroethylene in rats. Toxicol Appl Pharmacol. 1991 Sep 1;110(2):303–314. doi: 10.1016/s0041-008x(05)80013-4. [DOI] [PubMed] [Google Scholar]
- Dantzler W. H., Evans K. K., Wright S. H. Kinetics of interactions of para-aminohippurate, probenecid, cysteine conjugates and N-acetyl cysteine conjugates with basolateral organic anion transporter in isolated rabbit proximal renal tubules. J Pharmacol Exp Ther. 1995 Feb;272(2):663–672. [PubMed] [Google Scholar]
- Davidson I. W., Beliles R. P. Consideration of the target organ toxicity of trichloroethylene in terms of metabolite toxicity and pharmacokinetics. Drug Metab Rev. 1991;23(5-6):493–599. doi: 10.3109/03602539109029772. [DOI] [PubMed] [Google Scholar]
- Dekant W., Berthold K., Vamvakas S., Henschler D., Anders M. W. Thioacylating intermediates as metabolites of S-(1,2-dichlorovinyl)-L-cysteine and S-(1,2,2-trichlorovinyl)-L-cysteine formed by cysteine conjugate beta-lyase. Chem Res Toxicol. 1988 May-Jun;1(3):175–178. doi: 10.1021/tx00003a008. [DOI] [PubMed] [Google Scholar]
- Dekant W., Koob M., Henschler D. Metabolism of trichloroethene--in vivo and in vitro evidence for activation by glutathione conjugation. Chem Biol Interact. 1990;73(1):89–101. doi: 10.1016/0009-2797(90)90110-9. [DOI] [PubMed] [Google Scholar]
- Dekant W., Lash L. H., Anders M. W. Bioactivation mechanism of the cytotoxic and nephrotoxic S-conjugate S-(2-chloro-1,1,2-trifluoroethyl)-L-cysteine. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7443–7447. doi: 10.1073/pnas.84.21.7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekant W., Metzler M., Henschler D. Identification of S-1,2-dichlorovinyl-N-acetyl-cysteine as a urinary metabolite of trichloroethylene: a possible explanation for its nephrocarcinogenicity in male rats. Biochem Pharmacol. 1986 Aug 1;35(15):2455–2458. doi: 10.1016/0006-2952(86)90039-0. [DOI] [PubMed] [Google Scholar]
- Dekant W., Metzler M., Henschler D. Novel metabolites of trichloroethylene through dechlorination reactions in rats, mice and humans. Biochem Pharmacol. 1984 Jul 1;33(13):2021–2027. doi: 10.1016/0006-2952(84)90568-9. [DOI] [PubMed] [Google Scholar]
- Dekant W., Schulz A., Metzler M., Henschler D. Absorption, elimination and metabolism of trichloroethylene: a quantitative comparison between rats and mice. Xenobiotica. 1986 Feb;16(2):143–152. doi: 10.3109/00498258609043517. [DOI] [PubMed] [Google Scholar]
- Dohn D. R., Anders M. W. Assay of cysteine conjugate beta-lyase activity with S-(2-benzothiazolyl)cysteine as the substrate. Anal Biochem. 1982 Mar 1;120(2):379–386. doi: 10.1016/0003-2697(82)90361-x. [DOI] [PubMed] [Google Scholar]
- Dowsley T. F., Ulreich J. B., Bolton J. L., Park S. S., Forkert P. G. CYP2E1-dependent bioactivation of 1,1-dichloroethylene in murine lung: formation of reactive intermediates and glutathione conjugates. Toxicol Appl Pharmacol. 1996 Jul;139(1):42–48. doi: 10.1006/taap.1996.0141. [DOI] [PubMed] [Google Scholar]
- Duffel M. W., Jakoby W. B. Cysteine S-conjugate N-acetyltransferase from rat kidney microsomes. Mol Pharmacol. 1982 Mar;21(2):444–448. [PubMed] [Google Scholar]
- Elfarra A. A., Anders M. W. Renal processing of glutathione conjugates. Role in nephrotoxicity. Biochem Pharmacol. 1984 Dec 1;33(23):3729–3732. doi: 10.1016/0006-2952(84)90032-7. [DOI] [PubMed] [Google Scholar]
- Elfarra A. A., Hwang I. Y. In vivo metabolites of S-(2-benzothiazolyl)-L-cysteine as markers of in vivo cysteine conjugate beta-lyase and thiol glucuronosyl transferase activities. Drug Metab Dispos. 1990 Nov-Dec;18(6):917–922. [PubMed] [Google Scholar]
- Elfarra A. A., Jakobson I., Anders M. W. Mechanism of S-(1,2-dichlorovinyl)glutathione-induced nephrotoxicity. Biochem Pharmacol. 1986 Jan 15;35(2):283–288. doi: 10.1016/0006-2952(86)90527-7. [DOI] [PubMed] [Google Scholar]
- Elfarra A. A., Krause R. J., Last A. R., Lash L. H., Parker J. C. Species- and sex-related differences in metabolism of trichloroethylene to yield chloral and trichloroethanol in mouse, rat, and human liver microsomes. Drug Metab Dispos. 1998 Aug;26(8):779–785. [PubMed] [Google Scholar]
- Elfarra A. A., Lash L. H., Anders M. W. Alpha-ketoacids stimulate rat renal cysteine conjugate beta-lyase activity and potentiate the cytotoxicity of S-(1,2-dichlorovinyl)-L-cysteine. Mol Pharmacol. 1987 Feb;31(2):208–212. [PubMed] [Google Scholar]
- Eyre R. J., Stevens D. K., Parker J. C., Bull R. J. Acid-labile adducts to protein can be used as indicators of the cysteine S-conjugate pathway of trichloroethene metabolism. J Toxicol Environ Health. 1995 Dec;46(4):443–464. doi: 10.1080/15287399509532048. [DOI] [PubMed] [Google Scholar]
- Eyre R. J., Stevens D. K., Parker J. C., Bull R. J. Renal activation of trichloroethene and S-(1,2-dichlorovinyl)-L-cysteine and cell proliferative responses in the kidneys of F344 rats and B6C3F1 mice. J Toxicol Environ Health. 1995 Dec;46(4):465–481. doi: 10.1080/15287399509532049. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Gargas M. L., Allen B. C., Andersen M. E. Physiologically based pharmacokinetic modeling with trichloroethylene and its metabolite, trichloroacetic acid, in the rat and mouse. Toxicol Appl Pharmacol. 1991 Jun 15;109(2):183–195. doi: 10.1016/0041-008x(91)90167-d. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Mahle D., Abbas R. A human physiologically based pharmacokinetic model for trichloroethylene and its metabolites, trichloroacetic acid and free trichloroethanol. Toxicol Appl Pharmacol. 1998 Oct;152(2):339–359. doi: 10.1006/taap.1998.8486. [DOI] [PubMed] [Google Scholar]
- Fisher J. W., Whittaker T. A., Taylor D. H., Clewell H. J., 3rd, Andersen M. E. Physiologically based pharmacokinetic modeling of the pregnant rat: a multiroute exposure model for trichloroethylene and its metabolite, trichloroacetic acid. Toxicol Appl Pharmacol. 1989 Jul;99(3):395–414. doi: 10.1016/0041-008x(89)90149-x. [DOI] [PubMed] [Google Scholar]
- Forkert P. G. 1,1-Dichloroethylene-induced Clara cell damage is associated with in situ formation of the reactive epoxide. Immunohistochemical detection of its glutathione conjugate. Am J Respir Cell Mol Biol. 1999 Jun;20(6):1310–1318. doi: 10.1165/ajrcmb.20.6.3525. [DOI] [PubMed] [Google Scholar]
- Forkert P. G., Birch D. W. Pulmonary toxicity of trichloroethylene in mice. Covalent binding and morphological manifestations. Drug Metab Dispos. 1989 Jan-Feb;17(1):106–113. [PubMed] [Google Scholar]
- Forkert P. G. In vivo formation and localization of 1,1-dichloroethylene epoxide in murine liver: identification of its glutathione conjugate 2-S-glutathionyl acetate. J Pharmacol Exp Ther. 1999 Sep;290(3):1299–1306. [PubMed] [Google Scholar]
- Forkert P. G., Malkinson A. M., Rice P., Moussa M. Diminished CYP2E1 expression and formation of 2-S-glutathionyl acetate, a glutathione conjugate derived from 1,1-dichloroethylene epoxide, in murine lung tumors. Drug Metab Dispos. 1999 Jan;27(1):68–73. [PubMed] [Google Scholar]
- Goeptar A. R., Commandeur J. N., van Ommen B., van Bladeren P. J., Vermeulen N. P. Metabolism and kinetics of trichloroethylene in relation to toxicity and carcinogenicity. Relevance of the mercapturic acid pathway. Chem Res Toxicol. 1995 Jan-Feb;8(1):3–21. doi: 10.1021/tx00043a001. [DOI] [PubMed] [Google Scholar]
- Green T., Dow J., Ellis M. K., Foster J. R., Odum J. The role of glutathione conjugation in the development of kidney tumours in rats exposed to trichloroethylene. Chem Biol Interact. 1997 Jul 11;105(2):99–117. doi: 10.1016/s0009-2797(97)00040-9. [DOI] [PubMed] [Google Scholar]
- Green T., Mainwaring G. W., Foster J. R. Trichloroethylene-induced mouse lung tumors: studies of the mode of action and comparisons between species. Fundam Appl Toxicol. 1997 Jun;37(2):125–130. doi: 10.1006/faat.1997.2312. [DOI] [PubMed] [Google Scholar]
- Green T., Odum J., Nash J. A., Foster J. R. Perchloroethylene-induced rat kidney tumors: an investigation of the mechanisms involved and their relevance to humans. Toxicol Appl Pharmacol. 1990 Mar 15;103(1):77–89. doi: 10.1016/0041-008x(90)90264-u. [DOI] [PubMed] [Google Scholar]
- Green T., Prout M. S. Species differences in response to trichloroethylene. II. Biotransformation in rats and mice. Toxicol Appl Pharmacol. 1985 Jul;79(3):401–411. doi: 10.1016/0041-008x(85)90138-3. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Translocation of intracellular glutathione to membrane-bound gamma-glutamyl transpeptidase as a discrete step in the gamma-glutamyl cycle: glutathionuria after inhibition of transpeptidase. Proc Natl Acad Sci U S A. 1979 Jan;76(1):268–272. doi: 10.1073/pnas.76.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich F. P., Kim D. H., Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991 Mar-Apr;4(2):168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- Guengerich F. P., Shimada T. Oxidation of toxic and carcinogenic chemicals by human cytochrome P-450 enzymes. Chem Res Toxicol. 1991 Jul-Aug;4(4):391–407. doi: 10.1021/tx00022a001. [DOI] [PubMed] [Google Scholar]
- Henderson G. N., Yan Z., James M. O., Davydova N., Stacpoole P. W. Kinetics and metabolism of chloral hydrate in children: identification of dichloroacetate as a metabolite. Biochem Biophys Res Commun. 1997 Jun 27;235(3):695–698. doi: 10.1006/bbrc.1997.6868. [DOI] [PubMed] [Google Scholar]
- Hinchman C. A., Ballatori N. Glutathione-degrading capacities of liver and kidney in different species. Biochem Pharmacol. 1990 Sep 1;40(5):1131–1135. doi: 10.1016/0006-2952(90)90503-d. [DOI] [PubMed] [Google Scholar]
- Inoue M., Akerboom T. P., Sies H., Kinne R., Thao T., Arias I. M. Biliary transport of glutathione S-conjugate by rat liver canalicular membrane vesicles. J Biol Chem. 1984 Apr 25;259(8):4998–5002. [PubMed] [Google Scholar]
- Inoue M., Morino Y. Direct evidence for the role of the membrane potential in glutathione transport by renal brush-border membranes. J Biol Chem. 1985 Jan 10;260(1):326–331. [PubMed] [Google Scholar]
- Iyer R. A., Frink E. J., Jr, Ebert T. J., Anders M. W. Cysteine conjugate beta-lyase-dependent metabolism of compound A (2-[fluoromethoxy]-1,1,3,3,3-pentafluoro-1-propene) in human subjects anesthetized with sevoflurane and in rats given compound A. Anesthesiology. 1998 Mar;88(3):611–618. doi: 10.1097/00000542-199803000-00009. [DOI] [PubMed] [Google Scholar]
- James M. O., Cornett R., Yan Z., Henderson G. N., Stacpoole P. W. Glutathione-dependent conversion to glyoxylate, a major pathway of dichloroacetate biotransformation in hepatic cytosol from humans and rats, is reduced in dichloroacetate-treated rats. Drug Metab Dispos. 1997 Nov;25(11):1223–1227. [PubMed] [Google Scholar]
- Jones T. W., Qin C., Schaeffer V. H., Stevens J. L. Immunohistochemical localization of glutamine transaminase K, a rat kidney cysteine conjugate beta-lyase, and the relationship to the segment specificity of cysteine conjugate nephrotoxicity. Mol Pharmacol. 1988 Nov;34(5):621–627. [PubMed] [Google Scholar]
- KLEINFELD M., TABERSHAW I. R. Trichloroethylene toxicity; report of five fatal cases. AMA Arch Ind Hyg Occup Med. 1954 Aug;10(2):134–141. [PubMed] [Google Scholar]
- Kawamoto T., Hobara T., Aoki T., Kobayashi H., Iwamoto S., Sakai T., Takano T., Miyazaki Y., Imamura A., Ogino K. Metabolism of chloral hydrate in the anoxic perfused liver. Toxicol Lett. 1988 Mar;40(3):225–231. doi: 10.1016/0378-4274(88)90045-8. [DOI] [PubMed] [Google Scholar]
- Kedderis G. L. Extrapolation of in vitro enzyme induction data to humans in vivo. Chem Biol Interact. 1997 Nov 6;107(1-2):109–121. doi: 10.1016/s0009-2797(97)00076-8. [DOI] [PubMed] [Google Scholar]
- Ketcha M. M., Stevens D. K., Warren D. A., Bishop C. T., Brashear W. T. Conversion of trichloroacetic acid to dichloroacetic acid in biological samples. J Anal Toxicol. 1996 Jul-Aug;20(4):236–241. doi: 10.1093/jat/20.4.236. [DOI] [PubMed] [Google Scholar]
- Kobayashi K., Sogame Y., Hara H., Hayashi K. Mechanism of glutathione S-conjugate transport in canalicular and basolateral rat liver plasma membranes. J Biol Chem. 1990 May 15;265(14):7737–7741. [PubMed] [Google Scholar]
- Koob M., Dekant W. Metabolism of hexafluoropropene. Evidence for bioactivation by glutathione conjugate formation in the kidney. Drug Metab Dispos. 1990 Nov-Dec;18(6):911–916. [PubMed] [Google Scholar]
- Koop D. R., Crump B. L., Nordblom G. D., Coon M. J. Immunochemical evidence for induction of the alcohol-oxidizing cytochrome P-450 of rabbit liver microsomes by diverse agents: ethanol, imidazole, trichloroethylene, acetone, pyrazole, and isoniazid. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4065–4069. doi: 10.1073/pnas.82.12.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen G. L., Stevens J. L. Cysteine conjugate beta-lyase in the gastrointestinal bacterium Eubacterium limosum. Mol Pharmacol. 1986 Jan;29(1):97–103. [PubMed] [Google Scholar]
- Larson J. L., Bull R. J. Effect of ethanol on the metabolism of trichloroethylene. J Toxicol Environ Health. 1989;28(4):395–406. doi: 10.1080/15287398909531359. [DOI] [PubMed] [Google Scholar]
- Larson J. L., Bull R. J. Metabolism and lipoperoxidative activity of trichloroacetate and dichloroacetate in rats and mice. Toxicol Appl Pharmacol. 1992 Aug;115(2):268–277. doi: 10.1016/0041-008x(92)90332-m. [DOI] [PubMed] [Google Scholar]
- Larson J. L., Bull R. J. Species differences in the metabolism of trichloroethylene to the carcinogenic metabolites trichloroacetate and dichloroacetate. Toxicol Appl Pharmacol. 1992 Aug;115(2):278–285. doi: 10.1016/0041-008x(92)90333-n. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Anders M. W. Cytotoxicity of S-(1,2-dichlorovinyl)glutathione and S-(1,2-dichlorovinyl)-L-cysteine in isolated rat kidney cells. J Biol Chem. 1986 Oct 5;261(28):13076–13081. [PubMed] [Google Scholar]
- Lash L. H., Anders M. W. Uptake of nephrotoxic S-conjugates by isolated rat renal proximal tubular cells. J Pharmacol Exp Ther. 1989 Feb;248(2):531–537. [PubMed] [Google Scholar]
- Lash L. H., Elfarra A. A., Anders M. W. Renal cysteine conjugate beta-lyase. Bioactivation of nephrotoxic cysteine S-conjugates in mitochondrial outer membrane. J Biol Chem. 1986 May 5;261(13):5930–5935. [PubMed] [Google Scholar]
- Lash L. H., Elfarra A. A., Rakiewicz-Nemeth D., Anders M. W. Bioactivation mechanism of cytotoxic homocysteine S-conjugates. Arch Biochem Biophys. 1990 Feb 1;276(2):322–330. doi: 10.1016/0003-9861(90)90727-g. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Renal glutathione transport. Characteristics of the sodium-dependent system in the basal-lateral membrane. J Biol Chem. 1984 Dec 10;259(23):14508–14514. [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Transport of glutathione by renal basal-lateral membrane vesicles. Biochem Biophys Res Commun. 1983 Apr 15;112(1):55–60. doi: 10.1016/0006-291x(83)91796-5. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Jones D. P. Uptake of the glutathione conjugate S-(1,2-dichlorovinyl)glutathione by renal basal-lateral membrane vesicles and isolated kidney cells. Mol Pharmacol. 1985 Sep;28(3):278–282. [PubMed] [Google Scholar]
- Lash L. H., Lipscomb J. C., Putt D. A., Parker J. C. Glutathione conjugation of trichloroethylene in human liver and kidney: kinetics and individual variation. Drug Metab Dispos. 1999 Mar;27(3):351–359. [PubMed] [Google Scholar]
- Lash L. H., Nelson R. M., Van Dyke R. A., Anders M. W. Purification and characterization of human kidney cytosolic cysteine conjugate beta-lyase activity. Drug Metab Dispos. 1990 Jan-Feb;18(1):50–54. [PubMed] [Google Scholar]
- Lash L. H., Parker J. C., Scott C. S. Modes of action of trichloroethylene for kidney tumorigenesis. Environ Health Perspect. 2000 May;108 (Suppl 2):225–240. doi: 10.1289/ehp.00108s2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lash L. H., Putt D. A., Brashear W. T., Abbas R., Parker J. C., Fisher J. W. Identification of S-(1,2-dichlorovinyl)glutathione in the blood of human volunteers exposed to trichloroethylene. J Toxicol Environ Health A. 1999 Jan 8;56(1):1–21. doi: 10.1080/009841099158204. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Putt D. A. Renal cellular transport of exogenous glutathione: heterogeneity at physiological and pharmacological concentrations. Biochem Pharmacol. 1999 Sep 1;58(5):897–907. doi: 10.1016/s0006-2952(99)00155-0. [DOI] [PubMed] [Google Scholar]
- Lash L. H., Qian W., Putt D. A., Jacobs K., Elfarra A. A., Krause R. J., Parker J. C. Glutathione conjugation of trichloroethylene in rats and mice: sex-, species-, and tissue-dependent differences. Drug Metab Dispos. 1998 Jan;26(1):12–19. [PubMed] [Google Scholar]
- Lash L. H., Sausen P. J., Duescher R. J., Cooley A. J., Elfarra A. A. Roles of cysteine conjugate beta-lyase and S-oxidase in nephrotoxicity: studies with S-(1,2-dichlorovinyl)-L-cysteine and S-(1,2-dichlorovinyl)-L-cysteine sulfoxide. J Pharmacol Exp Ther. 1994 Apr;269(1):374–383. [PubMed] [Google Scholar]
- Lash L. H., Xu Y., Elfarra A. A., Duescher R. J., Parker J. C. Glutathione-dependent metabolism of trichloroethylene in isolated liver and kidney cells of rats and its role in mitochondrial and cellular toxicity. Drug Metab Dispos. 1995 Aug;23(8):846–853. [PubMed] [Google Scholar]
- Lau S. S., Kleiner H. E., Monks T. J. Metabolism as a determinant of species susceptibility to 2,3,5-(triglutathion-S-yl)hydroquinone-mediated nephrotoxicity. The role of N-acetylation and N-deacetylation. Drug Metab Dispos. 1995 Oct;23(10):1136–1142. [PubMed] [Google Scholar]
- Lee K. M., Bruckner J. V., Muralidhara S., Gallo J. M. Characterization of presystemic elimination of trichloroethylene and its nonlinear kinetics in rats. Toxicol Appl Pharmacol. 1996 Aug;139(2):262–271. doi: 10.1006/taap.1996.0165. [DOI] [PubMed] [Google Scholar]
- Lindahl R., Evces S. Comparative subcellular distribution of aldehyde dehydrogenase in rat, mouse and rabbit liver. Biochem Pharmacol. 1984 Nov 1;33(21):3383–3389. doi: 10.1016/0006-2952(84)90109-6. [DOI] [PubMed] [Google Scholar]
- Lipscomb J. C., Fisher J. W., Confer P. D., Byczkowski J. Z. In vitro to in vivo extrapolation for trichloroethylene metabolism in humans. Toxicol Appl Pharmacol. 1998 Oct;152(2):376–387. doi: 10.1006/taap.1998.8485. [DOI] [PubMed] [Google Scholar]
- Lipscomb J. C., Garrett C. M., Snawder J. E. Cytochrome P450-dependent metabolism of trichloroethylene: interindividual differences in humans. Toxicol Appl Pharmacol. 1997 Feb;142(2):311–318. doi: 10.1006/taap.1996.8040. [DOI] [PubMed] [Google Scholar]
- Lipscomb J. C., Mahle D. A., Brashear W. T., Barton H. A. Dichloroacetic acid: metabolism in cytosol. Drug Metab Dispos. 1995 Nov;23(11):1202–1205. [PubMed] [Google Scholar]
- Lipscomb J. C., Mahle D. A., Brashear W. T., Garrett C. M. A species comparison of chloral hydrate metabolism in blood and liver. Biochem Biophys Res Commun. 1996 Oct 14;227(2):340–350. doi: 10.1006/bbrc.1996.1511. [DOI] [PubMed] [Google Scholar]
- MacFarlane M., Foster J. R., Gibson G. G., King L. J., Lock E. A. Cysteine conjugate beta-lyase of rat kidney cytosol: characterization, immunocytochemical localization, and correlation with hexachlorobutadiene nephrotoxicity. Toxicol Appl Pharmacol. 1989 Apr;98(2):185–197. doi: 10.1016/0041-008x(89)90224-x. [DOI] [PubMed] [Google Scholar]
- MacFarlane M., Schofield M., Parker N., Roelandt L., David M., Lock E. A., King L. J., Goldfarb P. S., Gibson G. G. Dose-dependent induction or depression of cysteine conjugate beta-lyase in rat kidney by N-acetyl-S-(1,2,3,4,4-pentachloro-1,3-butadienyl)-L-cysteine. Toxicology. 1993 Jan 29;77(1-2):133–144. doi: 10.1016/0300-483x(93)90144-h. [DOI] [PubMed] [Google Scholar]
- Maiorino R. M., Gandolfi A. J., Sipes I. G. Gas-chromatographic method for the halothane metabolites, trifluoroacetic acid and bromide, in biological fluids. J Anal Toxicol. 1980 Sep-Oct;4(5):250–254. doi: 10.1093/jat/4.5.250. [DOI] [PubMed] [Google Scholar]
- Malherbe P., Alberati-Giani D., Köhler C., Cesura A. M. Identification of a mitochondrial form of kynurenine aminotransferase/glutamine transaminase K from rat brain. FEBS Lett. 1995 Jun 26;367(2):141–144. doi: 10.1016/0014-5793(95)00546-l. [DOI] [PubMed] [Google Scholar]
- Miller R. E., Guengerich F. P. Metabolism of trichloroethylene in isolated hepatocytes, microsomes, and reconstituted enzyme systems containing cytochrome P-450. Cancer Res. 1983 Mar;43(3):1145–1152. [PubMed] [Google Scholar]
- Moghaddam A. P., Abbas R., Fisher J. W., Lipscomb J. C. The role of mouse intestinal microflora in the metabolism of trichloroethylene, an in vivo study. Hum Exp Toxicol. 1997 Nov;16(11):629–635. doi: 10.1177/096032719701601101. [DOI] [PubMed] [Google Scholar]
- Moghaddam A. P., Abbas R., Fisher J. W., Stavrou S., Lipscomb J. C. Formation of dichloroacetic acid by rat and mouse gut microflora, an in vitro study. Biochem Biophys Res Commun. 1996 Nov 12;228(2):639–645. doi: 10.1006/bbrc.1996.1709. [DOI] [PubMed] [Google Scholar]
- Müller G., Spassovski M., Henschler D. Trichloroethylene exposure and trichloroethylene metabolites in urine and blood. Arch Toxikol. 1972;29(4):335–340. doi: 10.1007/BF00326650. [DOI] [PubMed] [Google Scholar]
- Müller G., Spassowski M., Henschler D. Metabolism of trichloroethylene in man. III. Interaction of trichloroethylene and ethanol. Arch Toxicol. 1975;33(3):173–189. doi: 10.1007/BF00311271. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Okino T., Okuyama S., Kaneko T., Yonekura I., Sato A. Ethanol-induced enhancement of trichloroethylene metabolism and hepatotoxicity: difference from the effect of phenobarbital. Toxicol Appl Pharmacol. 1988 Jun 30;94(2):227–237. doi: 10.1016/0041-008x(88)90264-5. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Wang R. S., Elovaara E., Park S. S., Gelboin H. V., Vainio H. A comparative study on the contribution of cytochrome P450 isozymes to metabolism of benzene, toluene and trichloroethylene in rat liver. Biochem Pharmacol. 1992 Jan 22;43(2):251–257. doi: 10.1016/0006-2952(92)90285-q. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Wang R. S., Elovaara E., Park S. S., Gelboin H. V., Vainio H. Cytochrome P450-related differences between rats and mice in the metabolism of benzene, toluene and trichloroethylene in liver microsomes. Biochem Pharmacol. 1993 Mar 9;45(5):1079–1085. doi: 10.1016/0006-2952(93)90252-r. [DOI] [PubMed] [Google Scholar]
- Nakajima T., Wang R. S., Katakura Y., Kishi R., Elovaara E., Park S. S., Gelboin H. V., Vainio H. Sex-, age- and pregnancy-induced changes in the metabolism of toluene and trichloroethylene in rat liver in relation to the regulation of cytochrome P450IIE1 and P450IIC11 content. J Pharmacol Exp Ther. 1992 Jun;261(3):869–874. [PubMed] [Google Scholar]
- Nakajima T., Wang R. S., Murayama N., Sato A. Three forms of trichloroethylene-metabolizing enzymes in rat liver induced by ethanol, phenobarbital, and 3-methylcholanthrene. Toxicol Appl Pharmacol. 1990 Mar 1;102(3):546–552. doi: 10.1016/0041-008x(90)90049-z. [DOI] [PubMed] [Google Scholar]
- Ni Y. C., Wong T. Y., Lloyd R. V., Heinze T. M., Shelton S., Casciano D., Kadlubar F. F., Fu P. P. Mouse liver microsomal metabolism of chloral hydrate, trichloroacetic acid, and trichloroethanol leading to induction of lipid peroxidation via a free radical mechanism. Drug Metab Dispos. 1996 Jan;24(1):81–90. [PubMed] [Google Scholar]
- Niinuma K., Takenaka O., Horie T., Kobayashi K., Kato Y., Suzuki H., Sugiyama Y. Kinetic analysis of the primary active transport of conjugated metabolites across the bile canalicular membrane: comparative study of S-(2,4-dinitrophenyl)-glutathione and 6-hydroxy-5,7-dimethyl-2-methylamino-4-(3-pyridylmethyl)benzothiazole glucuronide. J Pharmacol Exp Ther. 1997 Aug;282(2):866–872. [PubMed] [Google Scholar]
- Odum J., Foster J. R., Green T. A mechanism for the development of Clara cell lesions in the mouse lung after exposure to trichloroethylene. Chem Biol Interact. 1992 Aug 14;83(2):135–153. doi: 10.1016/0009-2797(92)90042-j. [DOI] [PubMed] [Google Scholar]
- Olive C., Board P. Glutathione S-conjugate transport by cultured human cells. Biochim Biophys Acta. 1994 Nov 10;1224(2):264–268. doi: 10.1016/0167-4889(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Perry S. J., Schofield M. A., MacFarlane M., Lock E. A., King L. J., Gibson G. G., Goldfarb P. S. Isolation and expression of a cDNA coding for rat kidney cytosolic cysteine conjugate beta-lyase. Mol Pharmacol. 1993 May;43(5):660–665. [PubMed] [Google Scholar]
- Peter R., Böcker R., Beaune P. H., Iwasaki M., Guengerich F. P., Yang C. S. Hydroxylation of chlorzoxazone as a specific probe for human liver cytochrome P-450IIE1. Chem Res Toxicol. 1990 Nov-Dec;3(6):566–573. doi: 10.1021/tx00018a012. [DOI] [PubMed] [Google Scholar]
- Prout M. S., Provan W. M., Green T. Species differences in response to trichloroethylene. I. Pharmacokinetics in rats and mice. Toxicol Appl Pharmacol. 1985 Jul;79(3):389–400. doi: 10.1016/0041-008x(85)90137-1. [DOI] [PubMed] [Google Scholar]
- Reitz R. H., Gargas M. L., Mendrala A. L., Schumann A. M. In vivo and in vitro studies of perchloroethylene metabolism for physiologically based pharmacokinetic modeling in rats, mice, and humans. Toxicol Appl Pharmacol. 1996 Feb;136(2):289–306. doi: 10.1006/taap.1996.0036. [DOI] [PubMed] [Google Scholar]
- Ripp S. L., Overby L. H., Philpot R. M., Elfarra A. A. Oxidation of cysteine S-conjugates by rabbit liver microsomes and cDNA-expressed flavin-containing mono-oxygenases: studies with S-(1,2-dichlorovinyl)-L-cysteine, S-(1,2,2-trichlorovinyl)-L-cysteine, S-allyl-L-cysteine, and S-benzyl-L-cysteine. Mol Pharmacol. 1997 Mar;51(3):507–515. [PubMed] [Google Scholar]
- Sato A., Nakajima T. Differences following skin or inhalation exposure in the absorption and excretion kinetics of trichloroethylene and toluene. Br J Ind Med. 1978 Feb;35(1):43–49. doi: 10.1136/oem.35.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A., Nakajima T., Fujiwara Y., Murayama N. A pharmacokinetic model to study the excretion of trichloroethylene and its metabolites after an inhalation exposure. Br J Ind Med. 1977 Feb;34(1):56–63. doi: 10.1136/oem.34.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sausen P. J., Duescher R. J., Elfarra A. A. Further characterization and purification of the flavin-dependent S-benzyl-L-cysteine S-oxidase activities of rat liver and kidney microsomes. Mol Pharmacol. 1993 Mar;43(3):388–396. [PubMed] [Google Scholar]
- Sausen P. J., Elfarra A. A. Cysteine conjugate S-oxidase. Characterization of a novel enzymatic activity in rat hepatic and renal microsomes. J Biol Chem. 1990 Apr 15;265(11):6139–6145. [PubMed] [Google Scholar]
- Sausen P. J., Elfarra A. A. Reactivity of cysteine S-conjugate sulfoxides: formation of S-[1-chloro-2-(S-glutathionyl)vinyl]-L-cysteine sulfoxide by the reaction of S-(1,2-dichlorovinyl)-L-cysteine sulfoxide with glutathione. Chem Res Toxicol. 1991 Nov-Dec;4(6):655–660. doi: 10.1021/tx00024a010. [DOI] [PubMed] [Google Scholar]
- Schaeffer V. H., Stevens J. L. Mechanism of transport for toxic cysteine conjugates in rat kidney cortex membrane vesicles. Mol Pharmacol. 1987 Aug;32(1):293–298. [PubMed] [Google Scholar]
- Sellers E. M., Lang-Sellers M., Koch-Weser J. Comparative metabolism of chloral hydrate and triclofos. J Clin Pharmacol. 1978 Oct;18(10):457–461. doi: 10.1002/j.1552-4604.1978.tb01571.x. [DOI] [PubMed] [Google Scholar]
- Sellers E. M., Lang M., Koch-Weser J., LeBlanc E., Kalant H. Interaction of chloral hydrate and ethanol in man. I. Metabolism. Clin Pharmacol Ther. 1972 Jan-Feb;13(1):37–49. doi: 10.1002/cpt197213137. [DOI] [PubMed] [Google Scholar]
- Shimada T., Yamazaki H., Mimura M., Inui Y., Guengerich F. P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther. 1994 Jul;270(1):414–423. [PubMed] [Google Scholar]
- Shultz J., Weiner H. Alteration of the enzymology of chloral hydrate reduction in the presence of ethanol. Biochem Pharmacol. 1979 Dec 1;28(23):3379–3384. doi: 10.1016/0006-2952(79)90076-5. [DOI] [PubMed] [Google Scholar]
- Simmons T. W., Anders M. W., Ballatori N. L-cysteine and S-(1,2-dichlorovinyl)-L-cysteine transport in rat liver canalicular membrane vesicles: potential reabsorption mechanisms for biliary metabolites of glutathione and its S-conjugates. J Pharmacol Exp Ther. 1992 Sep;262(3):1182–1188. [PubMed] [Google Scholar]
- Stevens J. L., Ayoubi N., Robbins J. D. The role of mitochondrial matrix enzymes in the metabolism and toxicity of cysteine conjugates. J Biol Chem. 1988 Mar 5;263(7):3395–3401. [PubMed] [Google Scholar]
- Stevens J. L. Cysteine conjugate beta-lyase activities in rat kidney cortex: subcellular localization and relationship to the hepatic enzyme. Biochem Biophys Res Commun. 1985 Jun 14;129(2):499–504. doi: 10.1016/0006-291x(85)90179-2. [DOI] [PubMed] [Google Scholar]
- Stevens J. L., Hatzinger P. B., Hayden P. J. Quantitation of multiple pathways for the metabolism of nephrotoxic cysteine conjugates using selective inhibitors of L-alpha-hydroxy acid oxidase (L-amino acid oxidase) and cysteine conjugate beta-lyase. Drug Metab Dispos. 1989 May-Jun;17(3):297–303. [PubMed] [Google Scholar]
- Stevens J. L. Isolation and characterization of a rat liver enzyme with both cysteine conjugate beta-lyase and kynureninase activity. J Biol Chem. 1985 Jul 5;260(13):7945–7950. [PubMed] [Google Scholar]
- Stevens J. L., Robbins J. D., Byrd R. A. A purified cysteine conjugate beta-lyase from rat kidney cytosol. Requirement for an alpha-keto acid or an amino acid oxidase for activity and identity with soluble glutamine transaminase K. J Biol Chem. 1986 Nov 25;261(33):15529–15537. [PubMed] [Google Scholar]
- Stevens J., Jakoby W. B. Cysteine conjugate beta-lyase. Mol Pharmacol. 1983 May;23(3):761–765. [PubMed] [Google Scholar]
- Tateishi M., Suzuki S., Shimizu H. Cysteine conjugate beta-lyase in rat liver. A novel enzyme catalyzing formation of thiol-containing metabolites of drugs. J Biol Chem. 1978 Dec 25;253(24):8854–8859. [PubMed] [Google Scholar]
- Templin M. V., Parker J. C., Bull R. J. Relative formation of dichloroacetate and trichloroacetate from trichloroethylene in male B6C3F1 mice. Toxicol Appl Pharmacol. 1993 Nov;123(1):1–8. doi: 10.1006/taap.1993.1214. [DOI] [PubMed] [Google Scholar]
- Templin M. V., Stevens D. K., Stenner R. D., Bonate P. L., Tuman D., Bull R. J. Factors affecting species differences in the kinetics of metabolites of trichloroethylene. J Toxicol Environ Health. 1995 Apr;44(4):435–447. doi: 10.1080/15287399509531972. [DOI] [PubMed] [Google Scholar]
- Tomisawa H., Ichihara S., Fukazawa H., Ichimoto N., Tateishi M., Yamamoto I. Purification and characterization of human hepatic cysteine-conjugate beta-lyase. Biochem J. 1986 Apr 15;235(2):569–575. doi: 10.1042/bj2350569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomisawa H., Suzuki S., Ichihara S., Fukazawa H., Tateishi M. Purification and characterization of C-S lyase from Fusobacterium varium. A C-S cleavage enzyme of cysteine conjugates and some S-containing amino acids. J Biol Chem. 1984 Feb 25;259(4):2588–2593. [PubMed] [Google Scholar]
- Tong Z., Board P. G., Anders M. W. Glutathione transferase zeta catalyses the oxygenation of the carcinogen dichloroacetic acid to glyoxylic acid. Biochem J. 1998 Apr 15;331(Pt 2):371–374. doi: 10.1042/bj3310371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. Z., Takano T., Tomita K., Nakata K., Nakamura K. [Interaction of trichloroethylene and isopropyl alcohol in the perfused rat liver]. Nihon Eiseigaku Zasshi. 1993 Dec;48(5):1000–1005. doi: 10.1265/jjh.48.1000. [DOI] [PubMed] [Google Scholar]
- Werner M., Birner G., Dekant W. Sulfoxidation of mercapturic acids derived from tri- and tetrachloroethene by cytochromes P450 3A: a bioactivation reaction in addition to deacetylation and cysteine conjugate beta-lyase mediated cleavage. Chem Res Toxicol. 1996 Jan-Feb;9(1):41–49. doi: 10.1021/tx950075u. [DOI] [PubMed] [Google Scholar]
- Withey J. R., Collins B. T., Collins P. G. Effect of vehicle on the pharmacokinetics and uptake of four halogenated hydrocarbons from the gastrointestinal tract of the rat. J Appl Toxicol. 1983 Oct;3(5):249–253. doi: 10.1002/jat.2550030506. [DOI] [PubMed] [Google Scholar]
- Wolfgang G. H., Gandolfi A. J., Stevens J. L., Brendel K. N-acetyl S-(1,2-dichlorovinyl)-L-cysteine produces a similar toxicity to S-(1,2-dichlorovinyl)-L-cysteine in rabbit renal slices: differential transport and metabolism. Toxicol Appl Pharmacol. 1989 Nov;101(2):205–219. doi: 10.1016/0041-008x(89)90270-6. [DOI] [PubMed] [Google Scholar]
- Wright S. H., Wunz T. M., North J., Stevens J. L. Na-dependent transport of S-(1,2-dichlorovinyl)-L-cysteine by renal brush-border membrane vesicles. J Pharmacol Exp Ther. 1998 Apr;285(1):162–169. [PubMed] [Google Scholar]
- Wrighton S. A., Stevens J. C. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22(1):1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- Wu C., Schaum J. Exposure assessment of trichloroethylene. Environ Health Perspect. 2000 May;108 (Suppl 2):359–363. doi: 10.1289/ehp.00108s2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. H., Stevens J. L. Transport and activation of S-(1,2-dichlorovinyl)-L-cysteine and N-acetyl-S-(1,2-dichlorovinyl)-L-cysteine in rat kidney proximal tubules. Toxicol Appl Pharmacol. 1989 Aug;100(1):51–61. doi: 10.1016/0041-008x(89)90091-4. [DOI] [PubMed] [Google Scholar]