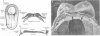Abstract
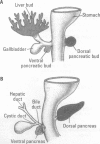
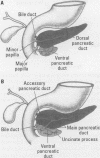
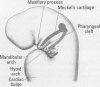
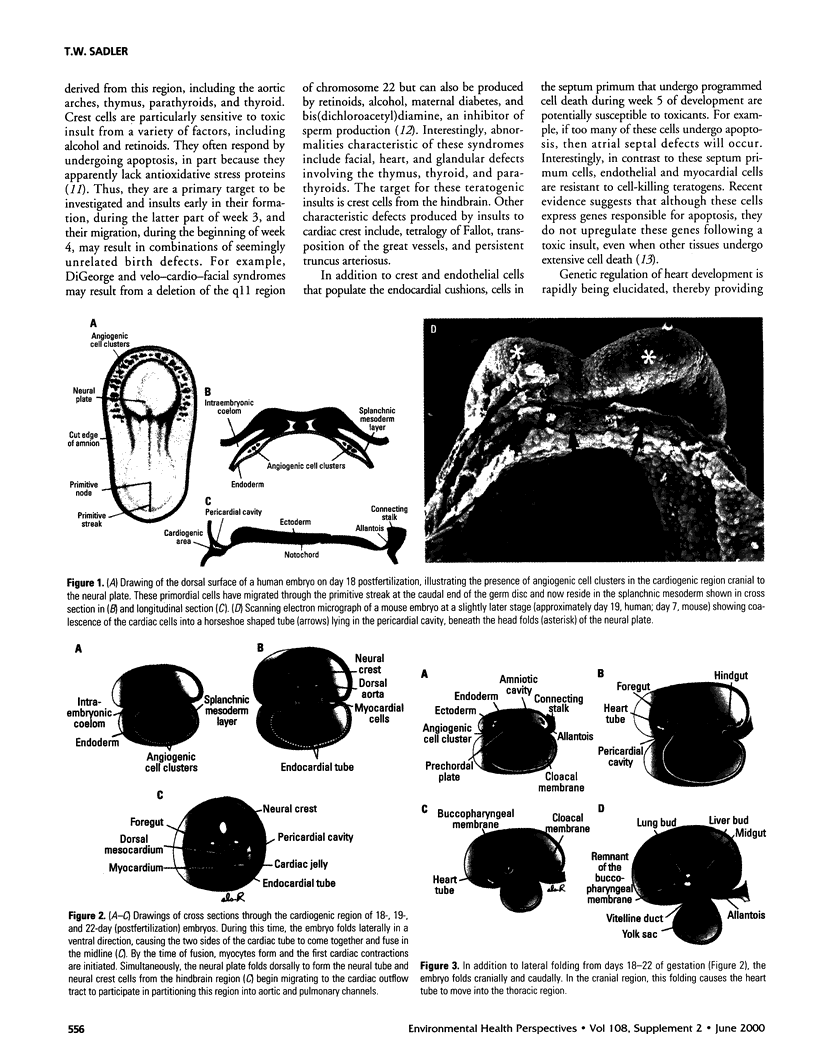
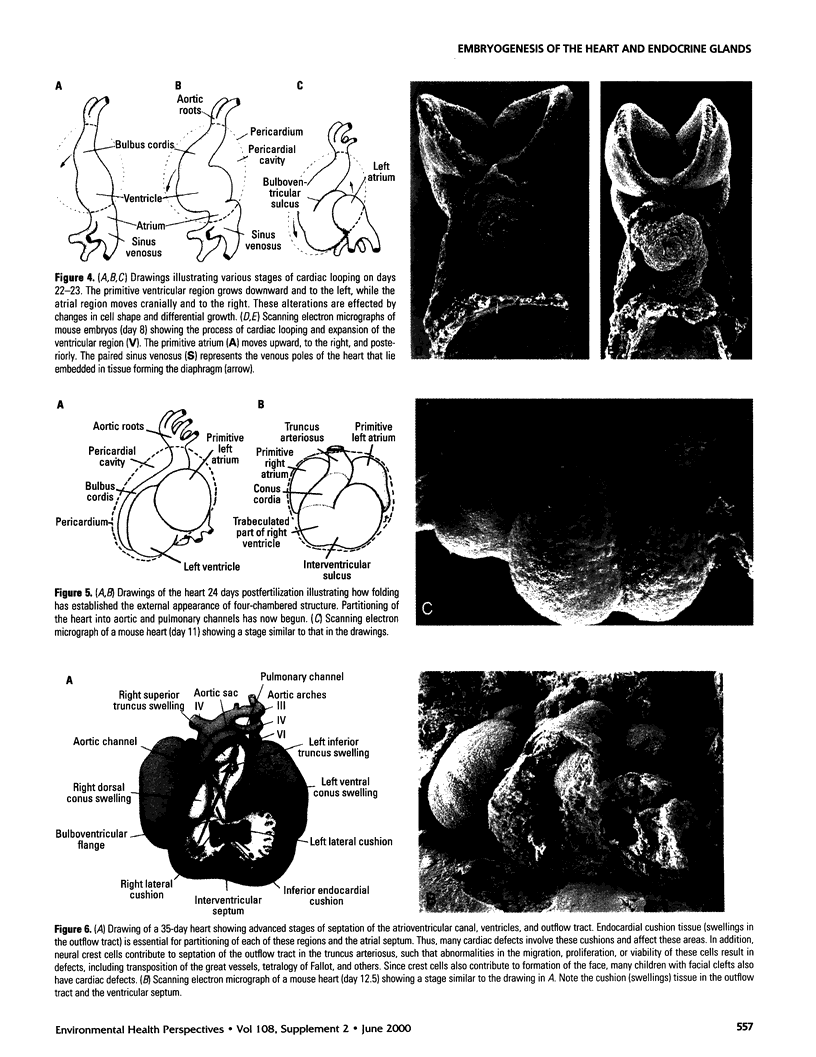
One of the original principles of teratology states that, "Susceptibility to teratogenesis varies with the developmental stage at the time of exposure to an adverse influence" [Wilson JG. Environment and Birth Defects. New York:Academic Press, 1973]. The time of greatest sensitivity encompasses the period of organ formation during weeks 3-8 following fertilization in human gestation. At this time, stem cell populations for each organ's morphogenesis are established and inductive events for the initiation of differentiation occur. Structural defects of the heart and endocrine system are no exception to this axiom and have their origins during this time frame. Although the function and maturation of these organs may be affected at later stages, structural defects and loss of cell types usually occur during these early phases of development. Thus, to determine critical windows for studying mechanisms of teratogenesis, it is essential to understand the developmental processes that establish these organs.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnhart K. M., Mellon P. L. The orphan nuclear receptor, steroidogenic factor-1, regulates the glycoprotein hormone alpha-subunit gene in pituitary gonadotropes. Mol Endocrinol. 1994 Jul;8(7):878–885. doi: 10.1210/mend.8.7.7527122. [DOI] [PubMed] [Google Scholar]
- Camper S. A., Saunders T. L., Katz R. W., Reeves R. H. The Pit-1 transcription factor gene is a candidate for the murine Snell dwarf mutation. Genomics. 1990 Nov;8(3):586–590. doi: 10.1016/0888-7543(90)90050-5. [DOI] [PubMed] [Google Scholar]
- Clemens J. W., Lala D. S., Parker K. L., Richards J. S. Steroidogenic factor-1 binding and transcriptional activity of the cholesterol side-chain cleavage promoter in rat granulosa cells. Endocrinology. 1994 Mar;134(3):1499–1508. doi: 10.1210/endo.134.3.8119192. [DOI] [PubMed] [Google Scholar]
- Couly G. F., Le Douarin N. M. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol. 1985 Aug;110(2):422–439. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Davis W. L., Crawford L. A., Cooper O. J., Farmer G. R., Thomas D. L., Freeman B. L. Ethanol induces the generation of reactive free radicals by neural crest cells in vitro. J Craniofac Genet Dev Biol. 1990;10(3):277–293. [PubMed] [Google Scholar]
- Easton H. S., Armstrong J. B., Smith S. C. Heart specification in the Mexican axolotl (Ambystoma mexicanum). Dev Dyn. 1994 Aug;200(4):313–320. doi: 10.1002/aja.1002000406. [DOI] [PubMed] [Google Scholar]
- Gage P. J., Brinkmeier M. L., Scarlett L. M., Knapp L. T., Camper S. A., Mahon K. A. The Ames dwarf gene, df, is required early in pituitary ontogeny for the extinction of Rpx transcription and initiation of lineage-specific cell proliferation. Mol Endocrinol. 1996 Dec;10(12):1570–1581. doi: 10.1210/mend.10.12.8961267. [DOI] [PubMed] [Google Scholar]
- Gage P. J., Roller M. L., Saunders T. L., Scarlett L. M., Camper S. A. Anterior pituitary cells defective in the cell-autonomous factor, df, undergo cell lineage specification but not expansion. Development. 1996 Jan;122(1):151–160. doi: 10.1242/dev.122.1.151. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez V., Schoenwolf G. C. Primitive-streak origin of the cardiovascular system in avian embryos. Dev Biol. 1993 Oct;159(2):706–719. doi: 10.1006/dbio.1993.1276. [DOI] [PubMed] [Google Scholar]
- George E. L., Georges-Labouesse E. N., Patel-King R. S., Rayburn H., Hynes R. O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993 Dec;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Halvorson L. M., Kaiser U. B., Chin W. W. Stimulation of luteinizing hormone beta gene promoter activity by the orphan nuclear receptor, steroidogenic factor-1. J Biol Chem. 1996 Mar 22;271(12):6645–6650. doi: 10.1074/jbc.271.12.6645. [DOI] [PubMed] [Google Scholar]
- Harvey R. P. NK-2 homeobox genes and heart development. Dev Biol. 1996 Sep 15;178(2):203–216. doi: 10.1006/dbio.1996.0212. [DOI] [PubMed] [Google Scholar]
- Honda S., Morohashi K., Nomura M., Takeya H., Kitajima M., Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993 Apr 5;268(10):7494–7502. [PubMed] [Google Scholar]
- Hunt P., Whiting J., Muchamore I., Marshall H., Krumlauf R. Homeobox genes and models for patterning the hindbrain and branchial arches. Dev Suppl. 1991;1:187–196. [PubMed] [Google Scholar]
- Ikeda H., Yoshimoto T. Developmental changes in proliferative activity of cells of the murine Rathke's pouch. Cell Tissue Res. 1991 Jan;263(1):41–47. doi: 10.1007/BF00318398. [DOI] [PubMed] [Google Scholar]
- Ito M., Yu R., Jameson J. L. DAX-1 inhibits SF-1-mediated transactivation via a carboxy-terminal domain that is deleted in adrenal hypoplasia congenita. Mol Cell Biol. 1997 Mar;17(3):1476–1483. doi: 10.1128/mcb.17.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri R. A., Nilson J. H. A steroidogenic factor-1 binding site is required for activity of the luteinizing hormone beta subunit promoter in gonadotropes of transgenic mice. J Biol Chem. 1996 May 3;271(18):10782–10785. doi: 10.1074/jbc.271.18.10782. [DOI] [PubMed] [Google Scholar]
- Kessel M. Respecification of vertebral identities by retinoic acid. Development. 1992 Jun;115(2):487–501. doi: 10.1242/dev.115.2.487. [DOI] [PubMed] [Google Scholar]
- Kim S. K., Hebrok M., Melton D. A. Pancreas development in the chick embryo. Cold Spring Harb Symp Quant Biol. 1997;62:377–383. [PubMed] [Google Scholar]
- Kim S. K., Melton D. A. Pancreas development is promoted by cyclopamine, a hedgehog signaling inhibitor. Proc Natl Acad Sci U S A. 1998 Oct 27;95(22):13036–13041. doi: 10.1073/pnas.95.22.13036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Hara Y., Pineau T., Fernandez-Salguero P., Fox C. H., Ward J. M., Gonzalez F. J. The T/ebp null mouse: thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes Dev. 1996 Jan 1;10(1):60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Krapp A., Knöfler M., Ledermann B., Bürki K., Berney C., Zoerkler N., Hagenbüchle O., Wellauer P. K. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998 Dec 1;12(23):3752–3763. doi: 10.1101/gad.12.23.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala D. S., Rice D. A., Parker K. L. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992 Aug;6(8):1249–1258. doi: 10.1210/mend.6.8.1406703. [DOI] [PubMed] [Google Scholar]
- Lammer E. J., Opitz J. M. The DiGeorge anomaly as a developmental field defect. Am J Med Genet Suppl. 1986;2:113–127. doi: 10.1002/ajmg.1320250615. [DOI] [PubMed] [Google Scholar]
- Le Bras S., Miralles F., Basmaciogullari A., Czernichow P., Scharfmann R. Fibroblast growth factor 2 promotes pancreatic epithelial cell proliferation via functional fibroblast growth factor receptors during embryonic life. Diabetes. 1998 Aug;47(8):1236–1242. [PubMed] [Google Scholar]
- Li Q. Y., Newbury-Ecob R. A., Terrett J. A., Wilson D. I., Curtis A. R., Yi C. H., Gebuhr T., Bullen P. J., Robson S. C., Strachan T. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997 Jan;15(1):21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- Li S., Crenshaw E. B., 3rd, Rawson E. J., Simmons D. M., Swanson L. W., Rosenfeld M. G. Dwarf locus mutants lacking three pituitary cell types result from mutations in the POU-domain gene pit-1. Nature. 1990 Oct 11;347(6293):528–533. doi: 10.1038/347528a0. [DOI] [PubMed] [Google Scholar]
- Linask K. K., Lash J. W. A role for fibronectin in the migration of avian precardiac cells. I. Dose-dependent effects of fibronectin antibody. Dev Biol. 1988 Oct;129(2):315–323. doi: 10.1016/0012-1606(88)90378-8. [DOI] [PubMed] [Google Scholar]
- Little C. D., Piquet D. M., Davis L. A., Walters L., Drake C. J. Distribution of laminin, collagen type IV, collagen type I, and fibronectin in chicken cardiac jelly/basement membrane. Anat Rec. 1989 Jul;224(3):417–425. doi: 10.1002/ar.1092240310. [DOI] [PubMed] [Google Scholar]
- Luo X., Ikeda Y., Parker K. L. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994 May 20;77(4):481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Lyons I., Parsons L. M., Hartley L., Li R., Andrews J. E., Robb L., Harvey R. P. Myogenic and morphogenetic defects in the heart tubes of murine embryos lacking the homeo box gene Nkx2-5. Genes Dev. 1995 Jul 1;9(13):1654–1666. doi: 10.1101/gad.9.13.1654. [DOI] [PubMed] [Google Scholar]
- Miralles F., Czernichow P., Scharfmann R. Follistatin regulates the relative proportions of endocrine versus exocrine tissue during pancreatic development. Development. 1998 Mar;125(6):1017–1024. doi: 10.1242/dev.125.6.1017. [DOI] [PubMed] [Google Scholar]
- Moore A. W., McInnes L., Kreidberg J., Hastie N. D., Schedl A. YAC complementation shows a requirement for Wt1 in the development of epicardium, adrenal gland and throughout nephrogenesis. Development. 1999 May;126(9):1845–1857. doi: 10.1242/dev.126.9.1845. [DOI] [PubMed] [Google Scholar]
- Moore A. W., Schedl A., McInnes L., Doyle M., Hecksher-Sorensen J., Hastie N. D. YAC transgenic analysis reveals Wilms' tumour 1 gene activity in the proliferating coelomic epithelium, developing diaphragm and limb. Mech Dev. 1998 Dec;79(1-2):169–184. doi: 10.1016/s0925-4773(98)00188-9. [DOI] [PubMed] [Google Scholar]
- Pepe G. J., Albrecht E. D. Regulation of the primate fetal adrenal cortex. Endocr Rev. 1990 Feb;11(1):151–176. doi: 10.1210/edrv-11-1-151. [DOI] [PubMed] [Google Scholar]
- Percival A. C., Slack J. M. Analysis of pancreatic development using a cell lineage label. Exp Cell Res. 1999 Feb 25;247(1):123–132. doi: 10.1006/excr.1998.4322. [DOI] [PubMed] [Google Scholar]
- Porterfield S. P. Vulnerability of the developing brain to thyroid abnormalities: environmental insults to the thyroid system. Environ Health Perspect. 1994 Jun;102 (Suppl 2):125–130. doi: 10.1289/ehp.94102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaee M., Isokawa K., Halligan N., Markwald R. R., Krug E. L. Identification of an extracellular 130-kDa protein involved in early cardiac morphogenesis. J Biol Chem. 1993 Jul 5;268(19):14404–14411. [PubMed] [Google Scholar]
- Sadovsky Y., Crawford P. A., Woodson K. G., Polish J. A., Clements M. A., Tourtellotte L. M., Simburger K., Milbrandt J. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):10939–10943. doi: 10.1073/pnas.92.24.10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott J. J., Benson D. W., Basson C. T., Pease W., Silberbach G. M., Moak J. P., Maron B. J., Seidman C. E., Seidman J. G. Congenital heart disease caused by mutations in the transcription factor NKX2-5. Science. 1998 Jul 3;281(5373):108–111. doi: 10.1126/science.281.5373.108. [DOI] [PubMed] [Google Scholar]
- Sheng H. Z., Moriyama K., Yamashita T., Li H., Potter S. S., Mahon K. A., Westphal H. Multistep control of pituitary organogenesis. Science. 1997 Dec 5;278(5344):1809–1812. doi: 10.1126/science.278.5344.1809. [DOI] [PubMed] [Google Scholar]
- Sornson M. W., Wu W., Dasen J. S., Flynn S. E., Norman D. J., O'Connell S. M., Gukovsky I., Carrière C., Ryan A. K., Miller A. P. Pituitary lineage determination by the Prophet of Pit-1 homeodomain factor defective in Ames dwarfism. Nature. 1996 Nov 28;384(6607):327–333. doi: 10.1038/384327a0. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda B., Chowdhury K., Torres M., Oliver G., Gruss P. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997 Mar 27;386(6623):399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- St-Onge L., Sosa-Pineda B., Chowdhury K., Mansouri A., Gruss P. Pax6 is required for differentiation of glucagon-producing alpha-cells in mouse pancreas. Nature. 1997 May 22;387(6631):406–409. doi: 10.1038/387406a0. [DOI] [PubMed] [Google Scholar]
- Tremblay J. J., Lanctôt C., Drouin J. The pan-pituitary activator of transcription, Ptx1 (pituitary homeobox 1), acts in synergy with SF-1 and Pit1 and is an upstream regulator of the Lim-homeodomain gene Lim3/Lhx3. Mol Endocrinol. 1998 Mar;12(3):428–441. doi: 10.1210/mend.12.3.0073. [DOI] [PubMed] [Google Scholar]
- Zanaria E., Muscatelli F., Bardoni B., Strom T. M., Guioli S., Guo W., Lalli E., Moser C., Walker A. P., McCabe E. R. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994 Dec 15;372(6507):635–641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]