Abstract
Certain polyhalogenated aromatic hydrocarbons such as polychlorinated biphenyls (PCBs) and dibenzo-p-dioxins (dioxins, 2,3,7, 8-tetrachlorodibenzo-p-dioxin) have been shown to have neurotoxic effects and to alter thyroid function during critical periods of thyroid hormone-dependent brain development. This has led to the suggestion that some of the neurotoxic effects of these compounds could be mediated through the thyroid system. Thyroid hormones are essential for normal brain development during a critical period beginning in utero and extending through the first 2 years postpartum. They regulate neuronal proliferation, migration, and differentiation in discrete regions of the brain during definitive time periods. Even transient disruption of this normal pattern can impair brain development. Thyroid hormones are necessary for normal cytoskeletal assembly and stability and the cytoskeletal system is essential for migration and neuronal outgrowth. In addition, they regulate development of cholinergic and dopaminergic systems serving the cerebral cortex and hippocampus. Animals perinatally exposed to certain environmental organohalogens such as many of the PCBs and dioxins have abnormal thyroid function and neurologic impairment. Although there are both species and congener variabilities, most reports show exposure results in thyroid enlargement and reduced serum T(4) levels with normal T(3) levels. Initial research concentrated on studying the direct actions of xenobiotics on the thyroid; however, some of these compounds bear a structural resemblance to the natural thyroid hormones and have high affinity with thyroid hormone-binding proteins such as transthyretin. These compounds could act as agonists or antagonists for receptors of the thyroid/steroid/retinoic acid superfamily. These structurally similar organohalogens could act at multiple points to alter thyroid hormone action. The similarity of the neurologic impairment seen in thyroid disorders to that seen following PCB or dioxin exposure suggests that one mechanism of neurotoxicity of these compounds could involve interaction with the thyroid system.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. M., Stein S. A., Palnitkar M., Anthony A., Gerrity L., Shanklin D. R. Evaluation and characterization of the hypothyroid hyt/hyt mouse. I: Somatic and behavioral studies. Neuroendocrinology. 1989 Feb;49(2):138–143. doi: 10.1159/000125105. [DOI] [PubMed] [Google Scholar]
- Agrawal A. K., Tilson H. A., Bondy S. C. 3,4,3',4'-Tetrachlorobiphenyl given to mice prenatally produces long-term decreases in striatal dopamine and receptor binding sites in the caudate nucleus. Toxicol Lett. 1981 Mar;7(6):417–424. doi: 10.1016/0378-4274(81)90087-4. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M., Iglesias T., Rodríguez-Peña A., Bernal J., Muñoz A. Expression of neurotrophins and the trk family of neurotrophin receptors in normal and hypothyroid rat brain. Brain Res Mol Brain Res. 1994 Dec;27(2):249–257. doi: 10.1016/0169-328x(94)90007-8. [DOI] [PubMed] [Google Scholar]
- Bastomsky C. H. Enhanced thyroxine metabolism and high uptake goiters in rats after a single dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Endocrinology. 1977 Jul;101(1):292–296. doi: 10.1210/endo-101-1-292. [DOI] [PubMed] [Google Scholar]
- Bernal J., Rodriguez-Pena A., Iniguez M. A., Ibarrola N., Munoz A. Influence of thyroid hormone on brain gene expression. Acta Med Austriaca. 1992;19 (Suppl 1):32–35. [PubMed] [Google Scholar]
- Bradley D. J., Towle H. C., Young W. S., 3rd Alpha and beta thyroid hormone receptor (TR) gene expression during auditory neurogenesis: evidence for TR isoform-specific transcriptional regulation in vivo. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):439–443. doi: 10.1073/pnas.91.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer A., Ahlborg U. G., Van den Berg M., Birnbaum L. S., Boersma E. R., Bosveld B., Denison M. S., Gray L. E., Hagmar L., Holene E. Functional aspects of developmental toxicity of polyhalogenated aromatic hydrocarbons in experimental animals and human infants. Eur J Pharmacol. 1995 May 26;293(1):1–40. doi: 10.1016/0926-6917(95)90015-2. [DOI] [PubMed] [Google Scholar]
- Burrow G. N., Fisher D. A., Larsen P. R. Maternal and fetal thyroid function. N Engl J Med. 1994 Oct 20;331(16):1072–1078. doi: 10.1056/NEJM199410203311608. [DOI] [PubMed] [Google Scholar]
- Calvo R., Obregón M. J., Ruiz de Oña C., Escobar del Rey F., Morreale de Escobar G. Congenital hypothyroidism, as studied in rats. Crucial role of maternal thyroxine but not of 3,5,3'-triiodothyronine in the protection of the fetal brain. J Clin Invest. 1990 Sep;86(3):889–899. doi: 10.1172/JCI114790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X. Y., Jiang X. M., Dou Z. H., Rakeman M. A., Zhang M. L., O'Donnell K., Ma T., Amette K., DeLong N., DeLong G. R. Timing of vulnerability of the brain to iodine deficiency in endemic cretinism. N Engl J Med. 1994 Dec 29;331(26):1739–1744. doi: 10.1056/NEJM199412293312603. [DOI] [PubMed] [Google Scholar]
- Chantoux F., Blondeau J. P., Francon J. Characterization of the thyroid hormone transport system of cerebrocortical rat neurons in primary culture. J Neurochem. 1995 Dec;65(6):2549–2554. doi: 10.1046/j.1471-4159.1995.65062549.x. [DOI] [PubMed] [Google Scholar]
- Charrasse S., Jehan F., Confort C., Brachet P., Clos J. Thyroid hormone promotes transient nerve growth factor synthesis in rat cerebellar neuroblasts. Dev Neurosci. 1992;14(4):282–289. doi: 10.1159/000111673. [DOI] [PubMed] [Google Scholar]
- Cheek A. O., Kow K., Chen J., McLachlan J. A. Potential mechanisms of thyroid disruption in humans: interaction of organochlorine compounds with thyroid receptor, transthyretin, and thyroid-binding globulin. Environ Health Perspect. 1999 Apr;107(4):273–278. doi: 10.1289/ehp.99107273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Guo Y. L., Hsu C. C., Rogan W. J. Cognitive development of Yu-Cheng ("oil disease") children prenatally exposed to heat-degraded PCBs. JAMA. 1992 Dec 9;268(22):3213–3218. [PubMed] [Google Scholar]
- Di Liegro I., Savettieri G., Coppolino M., Scaturro M., Monte M., Nastasi T., Salemi G., Castiglia D., Cestelli A. Expression of synapsin I gene in primary cultures of differentiating rat cortical neurons. Neurochem Res. 1995 Feb;20(2):239–243. doi: 10.1007/BF00970550. [DOI] [PubMed] [Google Scholar]
- Donati L., Antonelli A., Bertoni F., Moscogiuri D., Andreani M., Venturi S., Filippi T., Gasperini L., Neri S., Baschieri L. Clinical picture of endemic cretinism in central Apennines (Montefeltro). Thyroid. 1992 Winter;2(4):283–290. doi: 10.1089/thy.1992.2.283. [DOI] [PubMed] [Google Scholar]
- Farwell A. P., Leonard J. L. Dissociation of actin polymerization and enzyme inactivation in the hormonal regulation of type II iodothyronine 5'-deiodinase activity in astrocytes. Endocrinology. 1992 Aug;131(2):721–728. doi: 10.1210/endo.131.2.1322280. [DOI] [PubMed] [Google Scholar]
- Farwell A. P., Tranter M. P., Leonard J. L. Thyroxine-dependent regulation of integrin-laminin interactions in astrocytes. Endocrinology. 1995 Sep;136(9):3909–3915. doi: 10.1210/endo.136.9.7649099. [DOI] [PubMed] [Google Scholar]
- Figueiredo B. C., Otten U., Strauss S., Volk B., Maysinger D. Effects of perinatal hypo- and hyperthyroidism on the levels of nerve growth factor and its low-affinity receptor in cerebellum. Brain Res Dev Brain Res. 1993 Apr 16;72(2):237–244. doi: 10.1016/0165-3806(93)90188-g. [DOI] [PubMed] [Google Scholar]
- Fisher D. A. Fetal thyroid function: diagnosis and management of fetal thyroid disorders. Clin Obstet Gynecol. 1997 Mar;40(1):16–31. doi: 10.1097/00003081-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Forrest D., Hanebuth E., Smeyne R. J., Everds N., Stewart C. L., Wehner J. M., Curran T. Recessive resistance to thyroid hormone in mice lacking thyroid hormone receptor beta: evidence for tissue-specific modulation of receptor function. EMBO J. 1996 Jun 17;15(12):3006–3015. [PMC free article] [PubMed] [Google Scholar]
- Fuse Y. Development of the hypothalamic-pituitary-thyroid axis in humans. Reprod Fertil Dev. 1996;8(1):1–21. doi: 10.1071/rd9960001. [DOI] [PubMed] [Google Scholar]
- Goldey E. S., Kehn L. S., Lau C., Rehnberg G. L., Crofton K. M. Developmental exposure to polychlorinated biphenyls (Aroclor 1254) reduces circulating thyroid hormone concentrations and causes hearing deficits in rats. Toxicol Appl Pharmacol. 1995 Nov;135(1):77–88. doi: 10.1006/taap.1995.1210. [DOI] [PubMed] [Google Scholar]
- Goldey E. S., Kehn L. S., Rehnberg G. L., Crofton K. M. Effects of developmental hypothyroidism on auditory and motor function in the rat. Toxicol Appl Pharmacol. 1995 Nov;135(1):67–76. doi: 10.1006/taap.1995.1209. [DOI] [PubMed] [Google Scholar]
- Haddow J. E., Palomaki G. E., Allan W. C., Williams J. R., Knight G. J., Gagnon J., O'Heir C. E., Mitchell M. L., Hermos R. J., Waisbren S. E. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999 Aug 19;341(8):549–555. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- Hamburgh M., Mendoza L. A., Burkart J. F., Weil F. The thyroid as a time clock in the developing nervous system. UCLA Forum Med Sci. 1971;14:321–328. [PubMed] [Google Scholar]
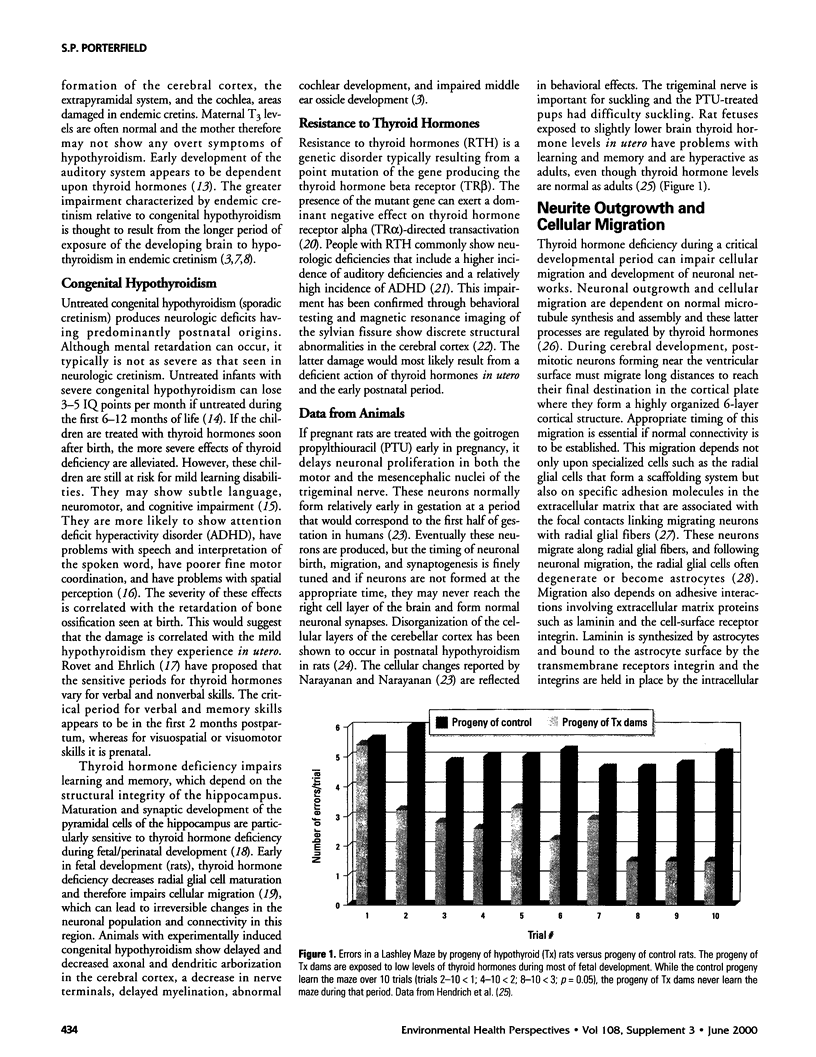
- Hendrich C. E., Jackson W. J., Porterfield S. P. Behavioral testing of progenies of Tx (hypothyroid) and growth hormone-treated Tx rats: an animal model for mental retardation. Neuroendocrinology. 1984 Jun;38(6):429–437. doi: 10.1159/000123931. [DOI] [PubMed] [Google Scholar]
- Hébert R., Langlois J. M., Dussault J. H. Permanent defects in rat peripheral auditory function following perinatal hypothyroidism: determination of a critical period. Brain Res. 1985 Dec;355(2):161–170. doi: 10.1016/0165-3806(85)90037-9. [DOI] [PubMed] [Google Scholar]
- Iglesias T., Caubín J., Zaballos A., Bernal J., Muñoz A. Identification of the mitochondrial NADH dehydrogenase subunit 3 (ND3) as a thyroid hormone regulated gene by whole genome PCR analysis. Biochem Biophys Res Commun. 1995 May 25;210(3):995–1000. doi: 10.1006/bbrc.1995.1755. [DOI] [PubMed] [Google Scholar]
- Jacobson J. L., Jacobson S. W. Intellectual impairment in children exposed to polychlorinated biphenyls in utero. N Engl J Med. 1996 Sep 12;335(11):783–789. doi: 10.1056/NEJM199609123351104. [DOI] [PubMed] [Google Scholar]
- Juárez de Ku L. M., Sharma-Stokkermans M., Meserve L. A. Thyroxine normalizes polychlorinated biphenyl (PCB) dose-related depression of choline acetyltransferase (ChAT) activity in hippocampus and basal forebrain of 15-day-old rats. Toxicology. 1994 Nov-Dec;94(1-3):19–30. doi: 10.1016/0300-483x(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Kastellakis A., Valcana T. Characterization of thyroid hormone transport in synaptosomes from rat brain. Mol Cell Endocrinol. 1989 Dec;67(2-3):231–241. doi: 10.1016/0303-7207(89)90213-x. [DOI] [PubMed] [Google Scholar]
- Klein A. H., Oddie T. H., Parslow M., Foley T. P., Jr, Fisher D. A. Developmental changes in pituitary-thyroid function in the human fetus and newborn. Early Hum Dev. 1982 Sep;6(4):321–330. doi: 10.1016/0378-3782(82)90070-6. [DOI] [PubMed] [Google Scholar]
- Kodavanti P. R., Shin D. S., Tilson H. A., Harry G. J. Comparative effects of two polychlorinated biphenyl congeners on calcium homeostasis in rat cerebellar granule cells. Toxicol Appl Pharmacol. 1993 Nov;123(1):97–106. doi: 10.1006/taap.1993.1226. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C., Morse D. C., Weisglas-Kuperus N., Lutkeschipholt I. J., Van der Paauw C. G., Tuinstra L. G., Brouwer A., Sauer P. J. Effects of dioxins and polychlorinated biphenyls on thyroid hormone status of pregnant women and their infants. Pediatr Res. 1994 Oct;36(4):468–473. doi: 10.1203/00006450-199410000-00009. [DOI] [PubMed] [Google Scholar]
- Lazar M. A. Thyroid hormone receptors: multiple forms, multiple possibilities. Endocr Rev. 1993 Apr;14(2):184–193. doi: 10.1210/edrv-14-2-184. [DOI] [PubMed] [Google Scholar]
- Leonard C. M., Martinez P., Weintraub B. D., Hauser P. Magnetic resonance imaging of cerebral anomalies in subjects with resistance to thyroid hormone. Am J Med Genet. 1995 Jun 19;60(3):238–243. doi: 10.1002/ajmg.1320600314. [DOI] [PubMed] [Google Scholar]
- Madeira M. D., Sousa N., Lima-Andrade M. T., Calheiros F., Cadete-Leite A., Paula-Barbosa M. M. Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. J Comp Neurol. 1992 Aug 22;322(4):501–518. doi: 10.1002/cne.903220405. [DOI] [PubMed] [Google Scholar]
- Maier W. E., Kodavanti P. R., Harry G. J., Tilson H. A. Sensitivity of adenosine triphosphatases in different brain regions to polychlorinated biphenyl congeners. J Appl Toxicol. 1994 May-Jun;14(3):225–229. doi: 10.1002/jat.2550140313. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Chae K., Oatley S. J., Blake C. C. Molecular interactions of toxic chlorinated dibenzo-p-dioxins and dibenzofurans with thyroxine binding prealbumin. J Med Chem. 1985 Mar;28(3):375–381. doi: 10.1021/jm00381a018. [DOI] [PubMed] [Google Scholar]
- McKinney J. D., Waller C. L. Polychlorinated biphenyls as hormonally active structural analogues. Environ Health Perspect. 1994 Mar;102(3):290–297. doi: 10.1289/ehp.94102290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellström B., Pipaón C., Naranjo J. R., Perez-Castillo A., Santos A. Differential effect of thyroid hormone on NGFI-A gene expression in developing rat brain. Endocrinology. 1994 Aug;135(2):583–588. doi: 10.1210/endo.135.2.8033806. [DOI] [PubMed] [Google Scholar]
- Mione M. C., Parnavelas J. G. How do developing cortical neurones know where to go? Trends Neurosci. 1994 Nov;17(11):443–445. doi: 10.1016/0166-2236(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Morse D. C., Groen D., Veerman M., van Amerongen C. J., Koëter H. B., Smits van Prooije A. E., Visser T. J., Koeman J. H., Brouwer A. Interference of polychlorinated biphenyls in hepatic and brain thyroid hormone metabolism in fetal and neonatal rats. Toxicol Appl Pharmacol. 1993 Sep;122(1):27–33. doi: 10.1006/taap.1993.1168. [DOI] [PubMed] [Google Scholar]
- Morse D. C., Wehler E. K., Wesseling W., Koeman J. H., Brouwer A. Alterations in rat brain thyroid hormone status following pre- and postnatal exposure to polychlorinated biphenyls (Aroclor 1254). Toxicol Appl Pharmacol. 1996 Feb;136(2):269–279. doi: 10.1006/taap.1996.0034. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Wrighton C., Seliger B., Bernal J., Beug H. Thyroid hormone receptor/c-erbA: control of commitment and differentiation in the neuronal/chromaffin progenitor line PC12. J Cell Biol. 1993 Apr;121(2):423–438. doi: 10.1083/jcb.121.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan C. H., Narayanan Y. Cell formation in the motor nucleus and mesencephalic nucleus of the trigeminal nerve of rats made hypothyroid by propylthiouracil. Exp Brain Res. 1985;59(2):257–266. doi: 10.1007/BF00230905. [DOI] [PubMed] [Google Scholar]
- Ness D. K., Schantz S. L., Moshtaghian J., Hansen L. G. Effects of perinatal exposure to specific PCB congeners on thyroid hormone concentrations and thyroid histology in the rat. Toxicol Lett. 1993 Jun;68(3):311–323. doi: 10.1016/0378-4274(93)90023-q. [DOI] [PubMed] [Google Scholar]
- Nunez J., Couchie D., Aniello F., Bridoux A. M. Regulation by thyroid hormone of microtubule assembly and neuronal differentiation. Neurochem Res. 1991 Sep;16(9):975–982. doi: 10.1007/BF00965840. [DOI] [PubMed] [Google Scholar]
- Oh J. D., Butcher L. L., Woolf N. J. Thyroid hormone modulates the development of cholinergic terminal fields in the rat forebrain: relation to nerve growth factor receptor. Brain Res Dev Brain Res. 1991 Apr 24;59(2):133–142. doi: 10.1016/0165-3806(91)90093-x. [DOI] [PubMed] [Google Scholar]
- Pharoah P., Connolly K., Hetzel B., Ekins R. Maternal thyroid function and motor competence in the child. Dev Med Child Neurol. 1981 Feb;23(1):76–82. doi: 10.1111/j.1469-8749.1981.tb08448.x. [DOI] [PubMed] [Google Scholar]
- Piosik P. A., van Groenigen M., Ponne N. J., Bolhuis P. A., Baas F. RC3/neurogranin structure and expression in the caprine brain in relation to congenital hypothyroidism. Brain Res Mol Brain Res. 1995 Mar;29(1):119–130. doi: 10.1016/0169-328x(94)00237-9. [DOI] [PubMed] [Google Scholar]
- Porterfield S. P., Hendrich C. E. The role of thyroid hormones in prenatal and neonatal neurological development--current perspectives. Endocr Rev. 1993 Feb;14(1):94–106. doi: 10.1210/edrv-14-1-94. [DOI] [PubMed] [Google Scholar]
- Porterfield S. P., Hendrich C. E. Tissue iodothyronine levels in fetuses of control and hypothyroid rats at 13 and 16 days gestation. Endocrinology. 1992 Jul;131(1):195–200. doi: 10.1210/endo.131.1.1611997. [DOI] [PubMed] [Google Scholar]
- Puymirat J., Barret A., Picart R., Vigny A., Loudes C., Faivre-Bauman A., Tixier-Vidal A. Triiodothyronine enhances the morphological maturation of dopaminergic neurons from fetal mouse hypothalamus cultured in serum-free medium. Neuroscience. 1983 Nov;10(3):801–810. doi: 10.1016/0306-4522(83)90217-8. [DOI] [PubMed] [Google Scholar]
- Puymirat J. Thyroid receptors in the rat brain. Prog Neurobiol. 1992 Sep;39(3):281–294. doi: 10.1016/0301-0082(92)90019-b. [DOI] [PubMed] [Google Scholar]
- Quirin-Stricker C., Nappey V., Simoni P., Toussaint J. L., Schmitt M. Trans-activation by thyroid hormone receptors of the 5' flanking region of the human ChAT gene. Brain Res Mol Brain Res. 1994 May;23(3):253–265. doi: 10.1016/0169-328x(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Rakic P. Principles of neural cell migration. Experientia. 1990 Sep 15;46(9):882–891. doi: 10.1007/BF01939380. [DOI] [PubMed] [Google Scholar]
- Rami A., Rabié A. Effect of thyroid deficiency on the development of glia in the hippocampal formation of the rat: an immunocytochemical study. Glia. 1988;1(5):337–345. doi: 10.1002/glia.440010506. [DOI] [PubMed] [Google Scholar]
- Rovet J. F., Ehrlich R. M. Long-term effects of L-thyroxine therapy for congenital hypothyroidism. J Pediatr. 1995 Mar;126(3):380–386. doi: 10.1016/s0022-3476(95)70452-3. [DOI] [PubMed] [Google Scholar]
- Sauer P. J., Huisman M., Koopman-Esseboom C., Morse D. C., Smits-van Prooije A. E., van de Berg K. J., Tuinstra L. G., van der Paauw C. G., Boersma E. R., Weisglas-Kuperus N. Effects of polychlorinated biphenyls (PCBs) and dioxins on growth and development. Hum Exp Toxicol. 1994 Dec;13(12):900–906. doi: 10.1177/096032719401301213. [DOI] [PubMed] [Google Scholar]
- Schwegler H., Crusio W. E. Correlations between radial-maze learning and structural variations of septum and hippocampus in rodents. Behav Brain Res. 1995 Feb;67(1):29–41. doi: 10.1016/0166-4328(95)91998-3. [DOI] [PubMed] [Google Scholar]
- Stanbury J. B. The pathogenesis of endemic cretinism. J Endocrinol Invest. 1984 Aug;7(4):409–419. doi: 10.1007/BF03351027. [DOI] [PubMed] [Google Scholar]
- Tilson H. A., Jacobson J. L., Rogan W. J. Polychlorinated biphenyls and the developing nervous system: cross-species comparisons. Neurotoxicol Teratol. 1990 May-Jun;12(3):239–248. doi: 10.1016/0892-0362(90)90095-t. [DOI] [PubMed] [Google Scholar]
- Vaccari A., Rossetti Z. L., de Montis G., Stefanini E., Martino E., Gessa G. L. Neonatal hypothyroidism induces striatal dopaminergic dysfunction. Neuroscience. 1990;35(3):699–706. doi: 10.1016/0306-4522(90)90340-a. [DOI] [PubMed] [Google Scholar]
- Vulsma T., Gons M. H., de Vijlder J. J. Maternal-fetal transfer of thyroxine in congenital hypothyroidism due to a total organification defect or thyroid agenesis. N Engl J Med. 1989 Jul 6;321(1):13–16. doi: 10.1056/NEJM198907063210103. [DOI] [PubMed] [Google Scholar]



