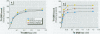Abstract
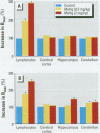
Methylmercury (MeHg) affects several parameters of cholinergic function. These alterations are thought to play a role in MeHg neurotoxicity. In vitro experiments have indicated that MeHg acts as a strong competitive inhibitor of radioligand binding to muscarinic cholinergic receptors (mAChRs) in rat brain. Furthermore, rat brain mAChRs share several pharmacologic characteristics of similar receptors present on lymphocytes. Using the muscarinic antagonist [(3)H]quinuclidinyl benzilate (QNB) to label receptors, we investigated the in vivo interactions of MeHg with rat brain mAChRs. We also investigated whether MeHg-induced central mAChR changes are reflected by similar alterations in splenic lymphocytes. Exposure to low doses of MeHg--0.5 or 2 mg/kg/day in drinking water--for 16 days significantly increased (20-44% of control) mAChRs density (B(max)) in the hippocampus and cerebellum without affecting receptor affinity (K(d)). The effect of MeHg did not occur immediately; it was not apparent until 2 weeks after the termination of treatment. No significant changes in [(3)H]QNB binding were observed in the cerebral cortex. In splenic lymphocytes, mAChR density was remarkably increased (95-198% of control) by day 14 of MeHg exposure and remained enhanced 14 days after the cessation of treatment. These results suggest up-regulation of mAChRs in selected brain regions (hippocampus and cerebellum) after prolonged low-level ingestion of MeHg in rats. These cerebral effects are delayed in onset and are preceded by a marked increase in density of mAChRs on lymphocytes. In chronic MeHg exposure, peripheral lymphocytes may represent a sensitive target for the interaction of MeHg with mAChRs and, therefore, may be predictive indicators of later adaptive response involving cerebral mAChRs. Additionally, the effect of MeHg on lymphocyte mAChRs in vivo indicates that this receptor system should be investigated further as a possible target for MeHg immunotoxicity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERLIN M., ULLBERG S. Accumulation and retention of mercury in the mouse. I. An autoradiographic study after a single intravenous injection of mercuric chloride. Arch Environ Health. 1963 May;6:589–601. doi: 10.1080/00039896.1963.10663447. [DOI] [PubMed] [Google Scholar]
- BERLIN M., ULLBERG S. Accumulation and retention of mercury in the mouse. II. An autoradiographic comparison of phenylmercuric acetate with inorganic mercury. Arch Environ Health. 1963 May;6:602–609. doi: 10.1080/00039896.1963.10663448. [DOI] [PubMed] [Google Scholar]
- BERLIN M., ULLBERG S. Accumulation and retention of mercury in the mouse. III. An autoradiographic comparison of methylmercuric dicyandiamide with inorganic mercury. Arch Environ Health. 1963 May;6:610–616. doi: 10.1080/00039896.1963.10663449. [DOI] [PubMed] [Google Scholar]
- Balduini W., Murphy S. D., Costa L. G. Developmental changes in muscarinic receptor-stimulated phosphoinositide metabolism in rat brain. J Pharmacol Exp Ther. 1987 May;241(2):421–427. [PubMed] [Google Scholar]
- Boischio A. A., Henshel D. S. Risk assessment of mercury exposure through fish consumption by the riverside people in the Madeira Basin, Amazon, 1991. Neurotoxicology. 1996 Spring;17(1):169–175. [PubMed] [Google Scholar]
- Bronzetti E., Adani O., Amenta F., Felici L., Mannino F., Ricci A. Muscarinic cholinergic receptor subtypes in human peripheral blood lymphocytes. Neurosci Lett. 1996 Apr 26;208(3):211–215. doi: 10.1016/0304-3940(96)12567-2. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Candura S. M., D'Agostino G., Castoldi A. F., Messori E., Liuzzi M., Manzo L., Tonini M. Effects of mercuric chloride and methyl mercury on cholinergic neuromuscular transmission in the guinea-pig ileum. Pharmacol Toxicol. 1997 May;80(5):218–224. doi: 10.1111/j.1600-0773.1997.tb01963.x. [DOI] [PubMed] [Google Scholar]
- Castoldi A. F., Candura S. M., Costa P., Manzo L., Costa L. G. Interaction of mercury compounds with muscarinic receptor subtypes in the rat brain. Neurotoxicology. 1996 Fall-Winter;17(3-4):735–741. [PubMed] [Google Scholar]
- Costa L. G., Kaylor G., Murphy S. D. Carbachol- and norepinephrine-stimulated phosphoinositide metabolism in rat brain: effect of chronic cholinesterase inhibition. J Pharmacol Exp Ther. 1986 Oct;239(1):32–37. [PubMed] [Google Scholar]
- Costa L. G., Kaylor G., Murphy S. D. In vitro and in vivo modulation of cholinergic muscarinic receptors in rat lymphocytes and brain by cholinergic agents. Int J Immunopharmacol. 1990;12(1):67–75. doi: 10.1016/0192-0561(90)90069-y. [DOI] [PubMed] [Google Scholar]
- Costa L. G., Kaylor G., Murphy S. D. Muscarinic cholinergic binding sites on rat lymphocytes. Immunopharmacology. 1988 Nov-Dec;16(3):139–149. doi: 10.1016/0162-3109(88)90002-1. [DOI] [PubMed] [Google Scholar]
- Costa P., Traver D. J., Auger C. B., Costa L. G. Expression of cholinergic muscarinic receptor subtypes mRNA in rat blood mononuclear cells. Immunopharmacology. 1994 Sep-Oct;28(2):113–123. doi: 10.1016/0162-3109(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Davis L. E., Kornfeld M., Mooney H. S., Fiedler K. J., Haaland K. Y., Orrison W. W., Cernichiari E., Clarkson T. W. Methylmercury poisoning: long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann Neurol. 1994 Jun;35(6):680–688. doi: 10.1002/ana.410350608. [DOI] [PubMed] [Google Scholar]
- Duman R. S., Heninger G. R., Nestler E. J. Molecular psychiatry. Adaptations of receptor-coupled signal transduction pathways underlying stress- and drug-induced neural plasticity. J Nerv Ment Dis. 1994 Dec;182(12):692–700. doi: 10.1097/00005053-199412000-00003. [DOI] [PubMed] [Google Scholar]
- Eldefrawi M. E., Mansour N. A., Eldefrawi A. T. Interactions of acetylcholine receptors with organic mercury compounds. Adv Exp Med Biol. 1977;84:449–463. doi: 10.1007/978-1-4684-3279-4_20. [DOI] [PubMed] [Google Scholar]
- Eva C., Ferrero P., Rocca P., Funaro A., Bergamasco B., Ravizza L., Genazzani E. [3H]N-methylscopolamine binding to muscarinic receptors in human peripheral blood lymphocytes: characterization, localization on T-lymphocyte subsets and age-dependent changes. Neuropharmacology. 1989 Jul;28(7):719–726. doi: 10.1016/0028-3908(89)90157-3. [DOI] [PubMed] [Google Scholar]
- Fitzgerald B. B., Costa L. G. Modulation of muscarinic receptors and acetylcholinesterase activity in lymphocytes and in brain areas following repeated organophosphate exposure in rats. Fundam Appl Toxicol. 1993 Feb;20(2):210–216. doi: 10.1006/faat.1993.1028. [DOI] [PubMed] [Google Scholar]
- Grandjean P., Weihe P., White R. F., Debes F., Araki S., Yokoyama K., Murata K., Sørensen N., Dahl R., Jørgensen P. J. Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury. Neurotoxicol Teratol. 1997 Nov-Dec;19(6):417–428. doi: 10.1016/s0892-0362(97)00097-4. [DOI] [PubMed] [Google Scholar]
- Hellström-Lindahl E., Nordberg A. Muscarinic receptor subtypes in subpopulations of human blood mononuclear cells as analyzed by RT-PCR technique. J Neuroimmunol. 1996 Aug;68(1-2):139–144. doi: 10.1016/0165-5728(96)00079-3. [DOI] [PubMed] [Google Scholar]
- Hrdina P. D., Peters D. A., Singhal R. L. Effects of chronic exposure to cadmium, lead and mercury of brain biogenic amines in the rat. Res Commun Chem Pathol Pharmacol. 1976 Nov;15(3):483–493. [PubMed] [Google Scholar]
- Hulme E. C., Birdsall N. J., Buckley N. J. Muscarinic receptor subtypes. Annu Rev Pharmacol Toxicol. 1990;30:633–673. doi: 10.1146/annurev.pa.30.040190.003221. [DOI] [PubMed] [Google Scholar]
- Hultman P., Hansson-Georgiadis H. Methyl mercury-induced autoimmunity in mice. Toxicol Appl Pharmacol. 1999 Feb 1;154(3):203–211. doi: 10.1006/taap.1998.8576. [DOI] [PubMed] [Google Scholar]
- Kobayashi H., Yuyama A., Matsusaka N., Takeno K., Yanagiya I. Effect of methylmercury on brain acetylcholine concentration and turnover in mice. Toxicol Appl Pharmacol. 1980 Jun 15;54(1):1–8. doi: 10.1016/0041-008x(80)90002-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee W., Nicklaus K. J., Manning D. R., Wolfe B. B. Ontogeny of cortical muscarinic receptor subtypes and muscarinic receptor-mediated responses in rat. J Pharmacol Exp Ther. 1990 Feb;252(2):482–490. [PubMed] [Google Scholar]
- Magos L., Butler W. H. The kinetics of methylmercury administered repeatedly to rats. Arch Toxicol. 1976 Jan 30;35(1):25–39. doi: 10.1007/BF00333983. [DOI] [PubMed] [Google Scholar]
- Manzo L., Artigas F., Martínez E., Mutti A., Bergamaschi E., Nicotera P., Tonini M., Candura S. M., Ray D. E., Costa L. G. Biochemical markers of neurotoxicity. A review of mechanistic studies and applications. Hum Exp Toxicol. 1996 Mar;15 (Suppl 1):S20–S35. [PubMed] [Google Scholar]
- Mash D. C., Flynn D. D., Potter L. T. Loss of M2 muscarine receptors in the cerebral cortex in Alzheimer's disease and experimental cholinergic denervation. Science. 1985 May 31;228(4703):1115–1117. doi: 10.1126/science.3992249. [DOI] [PubMed] [Google Scholar]
- Maslinski W., Kullberg M., Nordström O., Bartfai T. Muscarinic receptors and receptor-mediated actions on rat thymocytes. J Neuroimmunol. 1988 Mar;17(4):265–274. doi: 10.1016/0165-5728(88)90118-x. [DOI] [PubMed] [Google Scholar]
- Masuyama K., Uno K., Minoda R., Eura M., Samejima Y., Ishikawa T. Muscarinic acetylcholine receptors on human lymphocytes in patients with Menière's disease. Acta Otolaryngol. 1996 May;116(3):369–373. doi: 10.3109/00016489609137859. [DOI] [PubMed] [Google Scholar]
- Myers G. J., Davidson P. W., Cox C., Shamlaye C. F., Tanner M. A., Marsh D. O., Cernichiari E., Lapham L. W., Berlin M., Clarkson T. W. Summary of the Seychelles child development study on the relationship of fetal methylmercury exposure to neurodevelopment. Neurotoxicology. 1995 Winter;16(4):711–716. [PubMed] [Google Scholar]
- Nagashima K. A review of experimental methylmercury toxicity in rats: neuropathology and evidence for apoptosis. Toxicol Pathol. 1997 Nov-Dec;25(6):624–631. doi: 10.1177/019262339702500613. [DOI] [PubMed] [Google Scholar]
- Ortega H. G., Lopez M., Salvaggio J. E., Reimers R., Hsiao-Lin C., Bollinger J. E., George W. Lymphocyte proliferative response and tissue distribution of methylmercury sulfide and chloride in exposed rats. J Toxicol Environ Health. 1997 Apr 25;50(6):605–616. doi: 10.1080/15287399709532058. [DOI] [PubMed] [Google Scholar]
- Rabey J. M., Lewis A., Graff E., Korczyn A. D. Decreased (3H) quinuclidinyl benzilate binding to lymphocytes in Gilles de la Tourette syndrome. Biol Psychiatry. 1992 May 1;31(9):889–895. doi: 10.1016/0006-3223(92)90115-g. [DOI] [PubMed] [Google Scholar]
- Rinner I., Felsner P., Falus A., Skreiner E., Kukulansky T., Globerson A., Hirokawa K., Schauenstein K. Cholinergic signals to and from the immune system. Immunol Lett. 1995 Jan;44(2-3):217–220. doi: 10.1016/0165-2478(94)00220-l. [DOI] [PubMed] [Google Scholar]
- Schlumpf M., Palacios J. M., Cortes R., Lichtensteiger W. Regional development of muscarinic cholinergic binding sites in the prenatal rat brain. Neuroscience. 1991;45(2):347–357. doi: 10.1016/0306-4522(91)90232-d. [DOI] [PubMed] [Google Scholar]
- Shenker B. J., Guo T. L., Shapiro I. M. Low-level methylmercury exposure causes human T-cells to undergo apoptosis: evidence of mitochondrial dysfunction. Environ Res. 1998 May;77(2):149–159. doi: 10.1006/enrs.1997.3816. [DOI] [PubMed] [Google Scholar]
- Strom T. B., Sytkowski A. J., Carpenter C. B., Merrill J. P. Cholinergic augmentation of lymphocyte-mediated cytotoxicity. A study of the cholinergic receptor of cytotoxic T lymphocytes. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1330–1333. doi: 10.1073/pnas.71.4.1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. J., Fisher H. L., Sumler M. R., Marcus A. H., Mushak P., Hall L. L. Sexual differences in the distribution and retention of organic and inorganic mercury in methyl mercury-treated rats. Environ Res. 1986 Oct;41(1):219–234. doi: 10.1016/s0013-9351(86)80184-0. [DOI] [PubMed] [Google Scholar]
- Tsuzuki Y. Effect of chronic methylmercury exposure on activities of neurotransmitter enzymes in rat cerebellum. Toxicol Appl Pharmacol. 1981 Sep 15;60(2):379–381. doi: 10.1016/0041-008x(91)90241-6. [DOI] [PubMed] [Google Scholar]
- Von Burg R., Northington F. K., Shamoo A. Methylmercury inhibition of rat brain muscarinic receptors. Toxicol Appl Pharmacol. 1980 Apr;53(2):285–292. doi: 10.1016/0041-008x(80)90428-7. [DOI] [PubMed] [Google Scholar]
- Watanabe C., Satoh H. Evolution of our understanding of methylmercury as a health threat. Environ Health Perspect. 1996 Apr;104 (Suppl 2):367–379. doi: 10.1289/ehp.96104s2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler M. Measuring mercury. Environ Health Perspect. 1996 Aug;104(8):826–830. doi: 10.1289/ehp.96104826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y., Atchison W. D. Disruption by methylmercury of membrane excitability and synaptic transmission of CA1 neurons in hippocampal slices of the rat. Toxicol Appl Pharmacol. 1993 Jun;120(2):203–215. doi: 10.1006/taap.1993.1104. [DOI] [PubMed] [Google Scholar]
- Zanoli P., Truzzi C., Veneri C., Braghiroli D., Baraldi M. Methyl mercury during late gestation affects temporarily the development of cortical muscarinic receptors in rat offspring. Pharmacol Toxicol. 1994 Nov;75(5):261–264. doi: 10.1111/j.1600-0773.1994.tb00358.x. [DOI] [PubMed] [Google Scholar]