Abstract
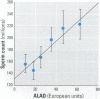
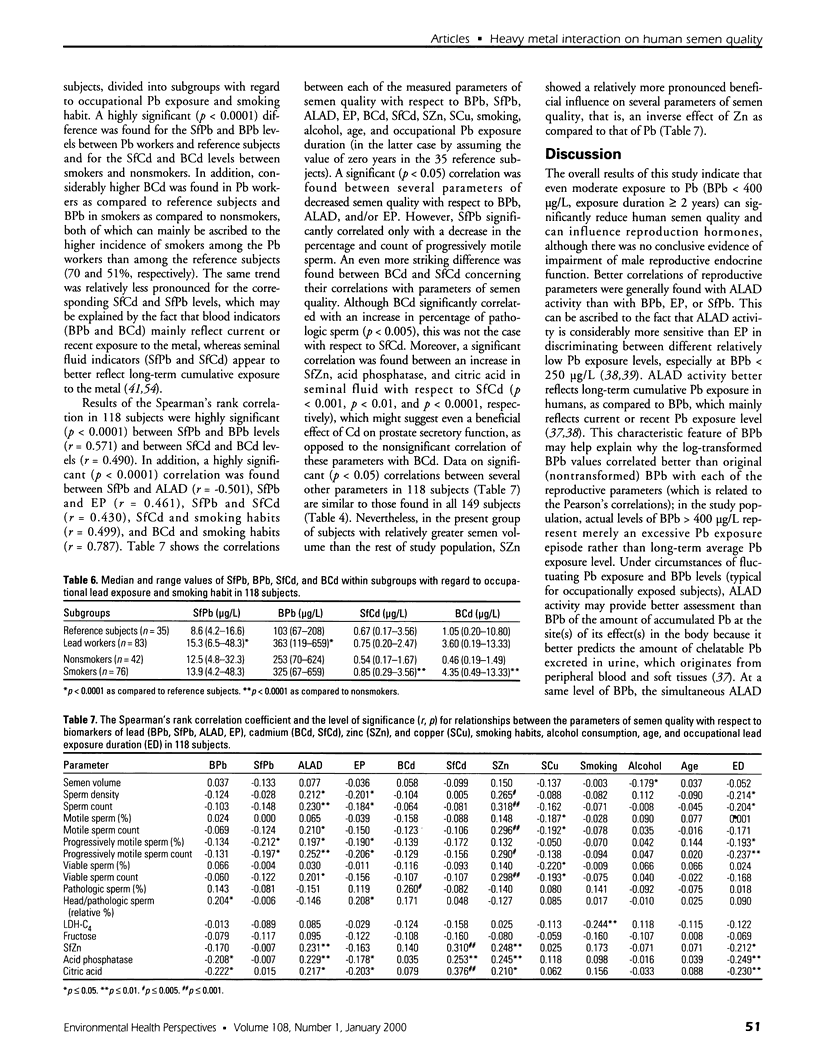
Blood lead (BPb), activity of delta-aminolevulinic acid dehydratase (ALAD), erythrocyte protoporphyrin (EP), blood cadmium (BCd), serum zinc (SZn), seminal fluid zinc (SfZn), serum copper (SCu), and parameters of semen quality and of reproductive endocrine function were measured in 149 healthy male industrial workers 20-43 years of age. The group contained 98 subjects with slight to moderate occupational exposure to Pb and 51 reference subjects. All of the subjects lived in Zagreb, Croatia. Significant (p < 0.05) correlations of BPb, ALAD, and/or EP with reproductive parameters indicated a Pb-related decrease in sperm density, in counts of total, motile, and viable sperm, in the percentage and count of progressively motile sperm, in parameters of prostate secretory function (SfZn, acid phosphatase, and citric acid in seminal fluid), and an increase in abnormal sperm head morphology, serum testosterone, and estradiol. These associations were confirmed by results of multiple regression, which also showed significant (p < 0. 05) influence of BCd, SZn, SCu, smoking habits, alcohol consumption, or age on certain reproductive parameters. These effects were mainly of lower rank and intensity as compared to Pb-related reproductive effects, whereas BCd contributed to a decrease in sperm motility and an increase in abnormal sperm morphology and serum testosterone. No significant Pb- or Cd-related influence was found on levels of the lactate dehydrogenase isoenzyme LDH-C(4) and fructose in seminal fluid or on follicle-stimulating hormone, luteinizing hormone, and prolactin in serum. The seminal fluid concentrations of Pb (SfPb) and Cd (SfCd) were measured in 118 of the 149 subjects, and a highly significant (p < 0.0001) correlation was found between BPb and SfPb levels (r = 0.571) and between BCd and SfCd levels (r = 0.490). The overall study results indicate that even moderate exposures to Pb (BPb < 400 microg/L) and Cd (BCd < 10 microg/L) can significantly reduce human semen quality without conclusive evidence of impairment of male reproductive endocrine function.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alessio L., Castoldi M. R., Odone P., Franchini I. Behaviour of indicators of exposure and effect after cessation of occupational exposure to lead. Br J Ind Med. 1981 Aug;38(3):262–267. doi: 10.1136/oem.38.3.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander B. H., Checkoway H., Costa-Mallen P., Faustman E. M., Woods J. S., Kelsey K. T., van Netten C., Costa L. G. Interaction of blood lead and delta-aminolevulinic acid dehydratase genotype on markers of heme synthesis and sperm production in lead smelter workers. Environ Health Perspect. 1998 Apr;106(4):213–216. doi: 10.1289/ehp.98106213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander B. H., Checkoway H., van Netten C., Muller C. H., Ewers T. G., Kaufman J. D., Mueller B. A., Vaughan T. L., Faustman E. M. Semen quality of men employed at a lead smelter. Occup Environ Med. 1996 Jun;53(6):411–416. doi: 10.1136/oem.53.6.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assennato G., Paci C., Baser M. E., Molinini R., Candela R. G., Altamura B. M., Giorgino R. Sperm count suppression without endocrine dysfunction in lead-exposed men. Arch Environ Health. 1986 Nov-Dec;41(6):387–390. doi: 10.1080/00039896.1986.9935784. [DOI] [PubMed] [Google Scholar]
- Berlin A., Schaller K. H. European standardized method for the determination of delta-aminolevulinic acid dehydratase activity in blood. Z Klin Chem Klin Biochem. 1974 Aug;12(8):389–390. [PubMed] [Google Scholar]
- Braunstein G. D., Dahlgren J., Loriaux D. L. Hypogonadism in chronically lead-poisoned men. Infertility. 1978;1(1):33–51. [PubMed] [Google Scholar]
- Chia S. E., Ong C. N., Lee S. T., Tsakok F. H. Blood concentrations of lead, cadmium, mercury, zinc, and copper and human semen parameters. Arch Androl. 1992 Sep-Oct;29(2):177–183. doi: 10.3109/01485019208987722. [DOI] [PubMed] [Google Scholar]
- Chia S. E., Xu B., Ong C. N., Tsakok F. M., Lee S. T. Effect of cadmium and cigarette smoking on human semen quality. Int J Fertil Menopausal Stud. 1994 Sep-Oct;39(5):292–298. [PubMed] [Google Scholar]
- Chisolm J., Jr, Brown D. H. Micro-scale photofluorometric determination of "free erythrocyte pophyrin" (protoporphyrin IX). Clin Chem. 1975 Oct;21(11):1669–1682. [PubMed] [Google Scholar]
- Clarkson T. W., Nordberg G. F., Sager P. R. Reproductive and developmental toxicity of metals. Scand J Work Environ Health. 1985 Jun;11(3 Spec No):145–154. doi: 10.5271/sjweh.2239. [DOI] [PubMed] [Google Scholar]
- Cullen M. R., Kayne R. D., Robins J. M. Endocrine and reproductive dysfunction in men associated with occupational inorganic lead intoxication. Arch Environ Health. 1984 Nov-Dec;39(6):431–440. doi: 10.1080/00039896.1984.10545877. [DOI] [PubMed] [Google Scholar]
- Fisher-Fischbein J., Fischbein A., Melnick H. D., Bardin C. W. Correlation between biochemical indicators of lead exposure and semen quality in a lead-poisoned firearms instructor. JAMA. 1987 Feb 13;257(6):803–805. [PubMed] [Google Scholar]
- Gavella M. A simple automated method for determination of citric acid levels in semen. Int J Androl. 1983 Dec;6(6):585–591. doi: 10.1111/j.1365-2605.1983.tb00349.x. [DOI] [PubMed] [Google Scholar]
- Gavella M., Cvitkovic P., Skrabalo Z. Seminal plasma isoenzyme LDH X in infertile men. Andrologia. 1982 Mar-Apr;14(2):104–109. doi: 10.1111/j.1439-0272.1982.tb03106.x. [DOI] [PubMed] [Google Scholar]
- Gavella M. Simple, rapid determination of zinc and acid phosphatase in seminal plasma with an ABA-100 bichromatic analyzer. Clin Chem. 1988 Aug;34(8):1605–1607. [PubMed] [Google Scholar]
- Gennart J. P., Bernard A., Lauwerys R. Assessment of thyroid, testes, kidney and autonomic nervous system function in lead-exposed workers. Int Arch Occup Environ Health. 1992;64(1):49–57. doi: 10.1007/BF00625951. [DOI] [PubMed] [Google Scholar]
- Gennart J. P., Buchet J. P., Roels H., Ghyselen P., Ceulemans E., Lauwerys R. Fertility of male workers exposed to cadmium, lead, or manganese. Am J Epidemiol. 1992 Jun 1;135(11):1208–1219. doi: 10.1093/oxfordjournals.aje.a116227. [DOI] [PubMed] [Google Scholar]
- Giwercman A., Carlsen E., Keiding N., Skakkebaek N. E. Evidence for increasing incidence of abnormalities of the human testis: a review. Environ Health Perspect. 1993 Jul;101 (Suppl 2):65–71. doi: 10.1289/ehp.93101s265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson A., Hedner P., Schütz A., Skerfving S. Occupational lead exposure and pituitary function. Int Arch Occup Environ Health. 1989;61(4):277–281. doi: 10.1007/BF00381426. [DOI] [PubMed] [Google Scholar]
- Keck C., Bramkamp G., Behre H. M., Müller C., Jockenhövel F., Nieschlag E. Lack of correlation between cadmium in seminal plasma and fertility status of nonexposed individuals and two cadmium-exposed patients. Reprod Toxicol. 1995 Jan-Feb;9(1):35–40. doi: 10.1016/0890-6238(94)00053-y. [DOI] [PubMed] [Google Scholar]
- Lancranjan I., Popescu H. I., GAvănescu O., Klepsch I., Serbănescu M. Reproductive ability of workmen occupationally exposed to lead. Arch Environ Health. 1975 Aug;30(8):396–401. doi: 10.1080/00039896.1975.10666733. [DOI] [PubMed] [Google Scholar]
- Lerda D. Study of sperm characteristics in persons occupationally exposed to lead. Am J Ind Med. 1992;22(4):567–571. doi: 10.1002/ajim.4700220411. [DOI] [PubMed] [Google Scholar]
- Lin S., Hwang S. A., Marshall E. G., Stone R., Chen J. Fertility rates among lead workers and professional bus drivers: a comparative study. Ann Epidemiol. 1996 May;6(3):201–208. doi: 10.1016/1047-2797(96)00010-5. [DOI] [PubMed] [Google Scholar]
- Lindbohm M. L., Sallmén M., Anttila A., Taskinen H., Hemminki K. Paternal occupational lead exposure and spontaneous abortion. Scand J Work Environ Health. 1991 Apr;17(2):95–103. doi: 10.5271/sjweh.1721. [DOI] [PubMed] [Google Scholar]
- Ng T. P., Goh H. H., Ng Y. L., Ong H. Y., Ong C. N., Chia K. S., Chia S. E., Jeyaratnam J. Male endocrine functions in workers with moderate exposure to lead. Br J Ind Med. 1991 Jul;48(7):485–491. doi: 10.1136/oem.48.7.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack-Füller G., De Beer C., Seibert H. Cadmium, lead, selenium, and zinc in semen of occupationally unexposed men. Andrologia. 1993 Jan-Feb;25(1):7–12. doi: 10.1111/j.1439-0272.1993.tb02674.x. [DOI] [PubMed] [Google Scholar]
- Oldereid N. B., Thomassen Y., Purvis K. Seminal plasma lead, cadmium and zinc in relation to tobacco consumption. Int J Androl. 1994 Feb;17(1):24–28. doi: 10.1111/j.1365-2605.1994.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Prpić-Majić D., Telisman S., Kezić S. In vitro study on lead and alcohol interaction and the inhibition of erythrocyte delta-aminolevulinic acid dehydratase in man. Scand J Work Environ Health. 1984 Aug;10(4):235–238. doi: 10.5271/sjweh.2336. [DOI] [PubMed] [Google Scholar]
- Rodamilans M., Osaba M. J., To-Figueras J., Rivera Fillat F., Marques J. M., Pérez P., Corbella J. Lead toxicity on endocrine testicular function in an occupationally exposed population. Hum Toxicol. 1988 Mar;7(2):125–128. doi: 10.1177/096032718800700203. [DOI] [PubMed] [Google Scholar]
- Saaranen M., Kantola M., Saarikoski S., Vanha-Perttula T. Human seminal plasma cadmium: comparison with fertility and smoking habits. Andrologia. 1989 Mar-Apr;21(2):140–145. [PubMed] [Google Scholar]
- Saaranen M., Suistomaa U., Kantola M., Saarikoski S., Vanha-Perttula T. Lead, magnesium, selenium and zinc in human seminal fluid: comparison with semen parameters and fertility. Hum Reprod. 1987 Aug;2(6):475–479. doi: 10.1093/oxfordjournals.humrep.a136573. [DOI] [PubMed] [Google Scholar]
- Safe S. H. Environmental and dietary estrogens and human health: is there a problem? Environ Health Perspect. 1995 Apr;103(4):346–351. doi: 10.1289/ehp.95103346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan S. H., Elkin E. P., Fenster L. Have sperm densities declined? A reanalysis of global trend data. Environ Health Perspect. 1997 Nov;105(11):1228–1232. doi: 10.1289/ehp.971051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tas S., Lauwerys R., Lison D. Occupational hazards for the male reproductive system. Crit Rev Toxicol. 1996 May;26(3):261–307. doi: 10.3109/10408449609012525. [DOI] [PubMed] [Google Scholar]
- Telisman S., Azarić J., Prpić-Majić D. Cadmium in blood as an indicator of integrated exposure to cadmium in the urban population. Bull Environ Contam Toxicol. 1986 Apr;36(4):491–495. doi: 10.1007/BF01623540. [DOI] [PubMed] [Google Scholar]
- Telisman S. Interactions of essential and/or toxic metals and metalloid regarding interindividual differences in susceptibility to various toxicants and chronic diseases in man. Arh Hig Rada Toksikol. 1995 Dec;46(4):459–476. [PubMed] [Google Scholar]
- Telisman S., Jurasović J., Pizent A., Cvitković P. Cadmium in the blood and seminal fluid of nonoccupationally exposed adult male subjects with regard to smoking habits. Int Arch Occup Environ Health. 1997;70(4):243–248. doi: 10.1007/s004200050214. [DOI] [PubMed] [Google Scholar]
- Telisman S., Kersanc A., Prpić-Majić D. The relevance of arguments for excluding ALAD from the recommended biological limit values in occupational exposure to inorganic lead (WHO 1980). Int Arch Occup Environ Health. 1982;50(4):397–412. doi: 10.1007/BF00377836. [DOI] [PubMed] [Google Scholar]
- Telisman S., Prpić-Majić D., Kersanc A. Relationships between blood lead and indicators of effect in cows environmentally exposed to lead. Toxicol Lett. 1990 Aug;52(3):347–356. doi: 10.1016/0378-4274(90)90045-n. [DOI] [PubMed] [Google Scholar]
- Telisman S., Prpić-Majić D., Kezić S. In vivo study on lead and alcohol interaction and the inhibition of erythrocyte delta-aminolevulinic acid dehydratase in man. Scand J Work Environ Health. 1984 Aug;10(4):239–244. doi: 10.5271/sjweh.2335. [DOI] [PubMed] [Google Scholar]
- Toppari J., Larsen J. C., Christiansen P., Giwercman A., Grandjean P., Guillette L. J., Jr, Jégou B., Jensen T. K., Jouannet P., Keiding N. Male reproductive health and environmental xenoestrogens. Environ Health Perspect. 1996 Aug;104 (Suppl 4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskum S., Rabjerg L., Jørgensen P. J., Grandjean P. Improvement in semen quality associated with decreasing occupational lead exposure. Am J Ind Med. 1999 Mar;35(3):257–263. doi: 10.1002/(sici)1097-0274(199903)35:3<257::aid-ajim5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Working P. K. Male reproductive toxicology: comparison of the human to animal models. Environ Health Perspect. 1988 Apr;77:37–44. doi: 10.1289/ehp.887737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu B., Chia S. E., Tsakok M., Ong C. N. Trace elements in blood and seminal plasma and their relationship to sperm quality. Reprod Toxicol. 1993 Nov-Dec;7(6):613–618. doi: 10.1016/0890-6238(93)90038-9. [DOI] [PubMed] [Google Scholar]
- Ziemsen B., Angerer J., Lehnert G., Benkmann H. G., Goedde H. W. Polymorphism of delta-aminolevulinic acid dehydratase in lead-exposed workers. Int Arch Occup Environ Health. 1986;58(3):245–247. doi: 10.1007/BF00432107. [DOI] [PubMed] [Google Scholar]