Abstract
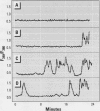
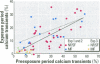
An effect on intracellular calcium continues to be proposed as a biochemical pathway for the mediation of biologic effects of electrical-power-frequency magnetic fields (MF). However, reproducible results among laboratories are difficult to attain and the characteristics of magnetic field effects on intracellular free calcium ([Ca(2+)](i)) are not well understood. We attempted to repeat the studies of Lindström et al. [Intracellular Calcium Oscillations in a T-Cell Line by a Weak 50 Hz Magnetic Field. J Cell Physiol 156:395-398 (1993)] by investigating the effect of a 1.5-G 50-Hz MF on [Ca(2+)](i) in the Jurkat lymphocyte T-cell line. Changes in [Ca(2+)](i) were determined using microscopic imaging of fura-2 loaded Jurkat cells on poly-l-lysine-coated glass coverslips. The MF was generated by a single coil constructed with bifilar wire and located in the same plane as the cells. Cells were randomly exposed for 8 min to MF, sham field (SF), or no field (NF) conditions. The exposure condition remained coded until data analysis was complete. Each exposure period was preceded by an 8-min data collection to establish a baseline for [Ca(2+)](i). After each exposure condition, cells were exposed to anti-CD3 antibody that induced a rapid increase in [Ca(2+)](i) in responsive cells; this provided a positive control. [Ca(2+)](i) was analyzed for individual cells as spatially-averaged background-corrected 340/380 nm ratios, and a [Ca(2+)](i) transient was considered significant for positive deviations from baseline of 3 [multiple] an estimate of noise in the baseline. Typically, 25-50 cells/field were viewed and approximately 50% had no [Ca(2+)](i) transients in the baseline period and also responded to positive control. Only cells responding to positive control and lacking changes in [Ca(2+)](i) during the baseline period were considered qualified for assessment during the exposure period. The incidences of [Ca(2+)](i) transients during the exposure period for two experiments (40 [multiple] objective) were 16.5, 14.6, and 14.2% for MF, SF, and NF, respectively, and were not statistically significantly different. Previous studies by Lindström et al. [Intracellular Calcium Oscillations in a T-Cell Line after Exposure to Extremely-Low-Frequency Magnetic Fields with Variable Frequencies and Flux Densities. Bioelectromagnetics 16:41-47 (1995)] showed a high response rate (92%) for exposure to 1. 5-G 50-Hz MF when individual cells were preselected for investigation. We found no such effect when examining many cells simultaneously in a random and blind fashion. These results do not preclude an effect of MF on [Ca(2+)](i), but suggest that responsive cells, if they exist, were not identified using the approaches that we used in this study.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellossi A. Lack of an effect of static magnetic field on calcium efflux from isolated chick brains. Bioelectromagnetics. 1986;7(4):381–386. doi: 10.1002/bem.2250070405. [DOI] [PubMed] [Google Scholar]
- Blackman C. F., Benane S. G., Elliott D. J., House D. E., Pollock M. M. Influence of electromagnetic fields on the efflux of calcium ions from brain tissue in vitro: a three-model analysis consistent with the frequency response up to 510 Hz. Bioelectromagnetics. 1988;9(3):215–227. doi: 10.1002/bem.2250090303. [DOI] [PubMed] [Google Scholar]
- Blackman C. F., Benane S. G., House D. E., Joines W. T. Effects of ELF (1-120 Hz) and modulated (50 Hz) RF fields on the efflux of calcium ions from brain tissue in vitro. Bioelectromagnetics. 1985;6(1):1–11. doi: 10.1002/bem.2250060102. [DOI] [PubMed] [Google Scholar]
- Blackman C. F., Benane S. G., House D. E. The influence of temperature during electric- and magnetic-field-induced alteration of calcium-ion release from in vitro brain tissue. Bioelectromagnetics. 1991;12(3):173–182. doi: 10.1002/bem.2250120305. [DOI] [PubMed] [Google Scholar]
- Blackman C. F., Benane S. G., Joines W. T., Hollis M. A., House D. E. Calcium-ion efflux from brain tissue: power-density versus internal field-intensity dependencies at 50-MHz RF radiation. Bioelectromagnetics. 1980;1(3):277–283. doi: 10.1002/bem.2250010304. [DOI] [PubMed] [Google Scholar]
- Blackman C. F., Kinney L. S., House D. E., Joines W. T. Multiple power-density windows and their possible origin. Bioelectromagnetics. 1989;10(2):115–128. doi: 10.1002/bem.2250100202. [DOI] [PubMed] [Google Scholar]
- Blanchard J. P., Blackman C. F. Clarification and application of an ion parametric resonance model for magnetic field interactions with biological systems. Bioelectromagnetics. 1994;15(3):217–238. doi: 10.1002/bem.2250150306. [DOI] [PubMed] [Google Scholar]
- Dutta S. K., Ghosh B., Blackman C. F. Radiofrequency radiation-induced calcium ion efflux enhancement from human and other neuroblastoma cells in culture. Bioelectromagnetics. 1989;10(2):197–202. doi: 10.1002/bem.2250100208. [DOI] [PubMed] [Google Scholar]
- Flipo D., Fournier M., Benquet C., Roux P., Le Boulaire C., Pinsky C., LaBella F. S., Krzystyniak K. Increased apoptosis, changes in intracellular Ca2+, and functional alterations in lymphocytes and macrophages after in vitro exposure to static magnetic field. J Toxicol Environ Health A. 1998 May 8;54(1):63–76. doi: 10.1080/009841098159033. [DOI] [PubMed] [Google Scholar]
- Galt S., Sandblom J., Hamnerius Y., Höjevik P., Saalman E., Nordén B. Experimental search for combined AC and DC magnetic field effects on ion channels. Bioelectromagnetics. 1993;14(4):315–327. doi: 10.1002/bem.2250140404. [DOI] [PubMed] [Google Scholar]
- Galvanovskis J., Sandblom J., Bergqvist B., Galt S., Hamnerius Y. The influence of 50-Hz magnetic fields on cytoplasmic Ca2+ oscillations in human leukemia T-cells. Sci Total Environ. 1996 Feb 2;180(1):19–33. doi: 10.1016/0048-9697(95)04916-9. [DOI] [PubMed] [Google Scholar]
- García-Sancho J., Montero M., Alvarez J., Fonteriz R. I., Sanchez A. Effects of extremely-low-frequency electromagnetic fields on ion transport in several mammalian cells. Bioelectromagnetics. 1994;15(6):579–588. doi: 10.1002/bem.2250150611. [DOI] [PubMed] [Google Scholar]
- Goodman E. M., Greenebaum B., Marron M. T. Effects of electromagnetic fields on molecules and cells. Int Rev Cytol. 1995;158:279–338. doi: 10.1016/s0074-7696(08)62489-4. [DOI] [PubMed] [Google Scholar]
- Höjevik P., Sandblom J., Galt S., Hamnerius Y. Ca2+ ion transport through patch-clamped cells exposed to magnetic fields. Bioelectromagnetics. 1995;16(1):33–40. doi: 10.1002/bem.2250160109. [DOI] [PubMed] [Google Scholar]
- Ihrig I., Heese C., Glaser R. Alterations of intracellular calcium concentration in mice neuroblastoma cells by electrical field and UVA. Bioelectromagnetics. 1997;18(8):595–597. [PubMed] [Google Scholar]
- Korzh-Sleptsova I. L., Lindström E., Mild K. H., Berglund A., Lundgren E. Low frequency MFs increased inositol 1,4,5-trisphosphate levels in the Jurkat cell line. FEBS Lett. 1995 Feb 13;359(2-3):151–154. doi: 10.1016/0014-5793(95)00031-4. [DOI] [PubMed] [Google Scholar]
- Liburdy R. P. Calcium signaling in lymphocytes and ELF fields. Evidence for an electric field metric and a site of interaction involving the calcium ion channel. FEBS Lett. 1992 Apr 13;301(1):53–59. doi: 10.1016/0014-5793(92)80209-y. [DOI] [PubMed] [Google Scholar]
- Liburdy R. P., Callahan D. E., Harland J., Dunham E., Sloma T. R., Yaswen P. Experimental evidence for 60 Hz magnetic fields operating through the signal transduction cascade. Effects on calcium influx and c-MYC mRNA induction. FEBS Lett. 1993 Nov 22;334(3):301–308. doi: 10.1016/0014-5793(93)80699-u. [DOI] [PubMed] [Google Scholar]
- Lindström E., Berglund A., Mild K. H., Lindström P., Lundgren E. CD45 phosphatase in Jurkat cells is necessary for response to applied ELF magnetic fields. FEBS Lett. 1995 Aug 14;370(1-2):118–122. doi: 10.1016/0014-5793(95)00807-l. [DOI] [PubMed] [Google Scholar]
- Lindström E., Lindström P., Berglund A., Lundgren E., Mild K. H. Intracellular calcium oscillations in a T-cell line after exposure to extremely-low-frequency magnetic fields with variable frequencies and flux densities. Bioelectromagnetics. 1995;16(1):41–47. doi: 10.1002/bem.2250160110. [DOI] [PubMed] [Google Scholar]
- Lindström E., Lindström P., Berglund A., Mild K. H., Lundgren E. Intracellular calcium oscillations induced in a T-cell line by a weak 50 Hz magnetic field. J Cell Physiol. 1993 Aug;156(2):395–398. doi: 10.1002/jcp.1041560223. [DOI] [PubMed] [Google Scholar]
- London S. J., Thomas D. C., Bowman J. D., Sobel E., Cheng T. C., Peters J. M. Exposure to residential electric and magnetic fields and risk of childhood leukemia. Am J Epidemiol. 1991 Nov 1;134(9):923–937. doi: 10.1093/oxfordjournals.aje.a116176. [DOI] [PubMed] [Google Scholar]
- Lyle D. B., Fuchs T. A., Casamento J. P., Davis C. C., Swicord M. L. Intracellular calcium signaling by Jurkat T-lymphocytes exposed to a 60 Hz magnetic field. Bioelectromagnetics. 1997;18(6):439–445. doi: 10.1002/(sici)1521-186x(1997)18:6<439::aid-bem6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Lyle D. B., Wang X. H., Ayotte R. D., Sheppard A. R., Adey W. R. Calcium uptake by leukemic and normal T-lymphocytes exposed to low frequency magnetic fields. Bioelectromagnetics. 1991;12(3):145–156. doi: 10.1002/bem.2250120303. [DOI] [PubMed] [Google Scholar]
- Parkinson W. C., Hanks C. T. Search for cyclotron resonance in cells in vitro. Bioelectromagnetics. 1989;10(2):129–145. doi: 10.1002/bem.2250100203. [DOI] [PubMed] [Google Scholar]
- Schieven G. L., Kirihara J. M., Gilliland L. K., Uckun F. M., Ledbetter J. A. Ultraviolet radiation rapidly induces tyrosine phosphorylation and calcium signaling in lymphocytes. Mol Biol Cell. 1993 May;4(5):523–530. doi: 10.1091/mbc.4.5.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walleczek J., Liburdy R. P. Nonthermal 60 Hz sinusoidal magnetic-field exposure enhances 45Ca2+ uptake in rat thymocytes: dependence on mitogen activation. FEBS Lett. 1990 Oct 1;271(1-2):157–160. doi: 10.1016/0014-5793(90)80396-z. [DOI] [PubMed] [Google Scholar]
- Wertheimer N., Leeper E. Electrical wiring configurations and childhood cancer. Am J Epidemiol. 1979 Mar;109(3):273–284. doi: 10.1093/oxfordjournals.aje.a112681. [DOI] [PubMed] [Google Scholar]
- Yost M. G., Liburdy R. P. Time-varying and static magnetic fields act in combination to alter calcium signal transduction in the lymphocyte. FEBS Lett. 1992 Jan 20;296(2):117–122. doi: 10.1016/0014-5793(92)80361-j. [DOI] [PubMed] [Google Scholar]