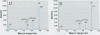Abstract
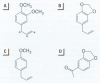
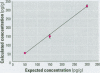
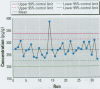
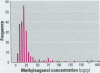
We developed a sensitive and accurate analytical method for quantifying methyleugenol (ME) in human serum. Our method uses a simple solid-phase extraction followed by a highly specific analysis using isotope dilution gas chromatography-high resolution mass spectrometry. Our method is very accurate; its limit of detection is 3.1 pg/g and its average coefficient of variation is 14% over a 200-pg/g range. We applied this method to measure serum ME concentrations in adults in the general U.S. population. ME was detected in 98% of our samples, with a mean ME concentration of 24 pg/g (range < 3.1-390 pg/g). Lipid adjustment of the data did not alter the distribution. Bivariate and multivariate analyses using selected demographic variables showed only marginal relationships between race/ethnicity and sex/fasting status with serum ME concentrations. Although no demographic variable was a good predictor of ME exposure or dose, our data indicate prevalent exposure of U.S. adults to ME. Detailed pharmacokinetic studies are required to determine the relationship between ME intake and human serum ME concentrations.
Full text
PDF

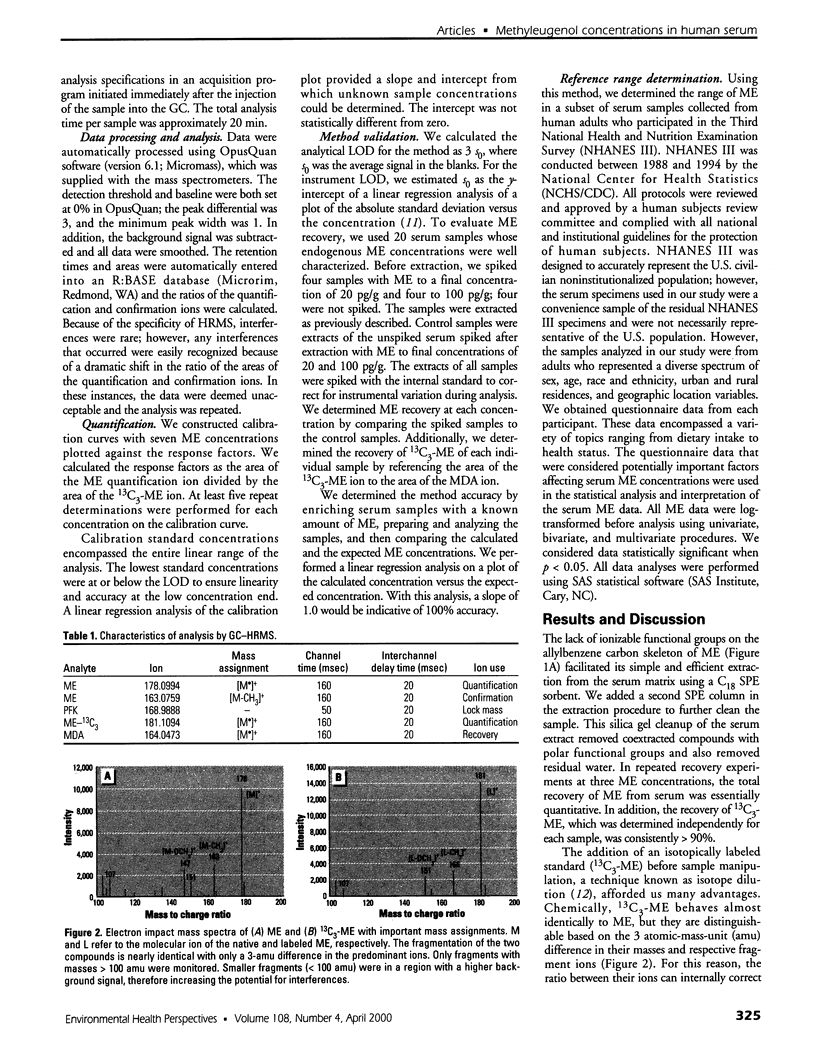
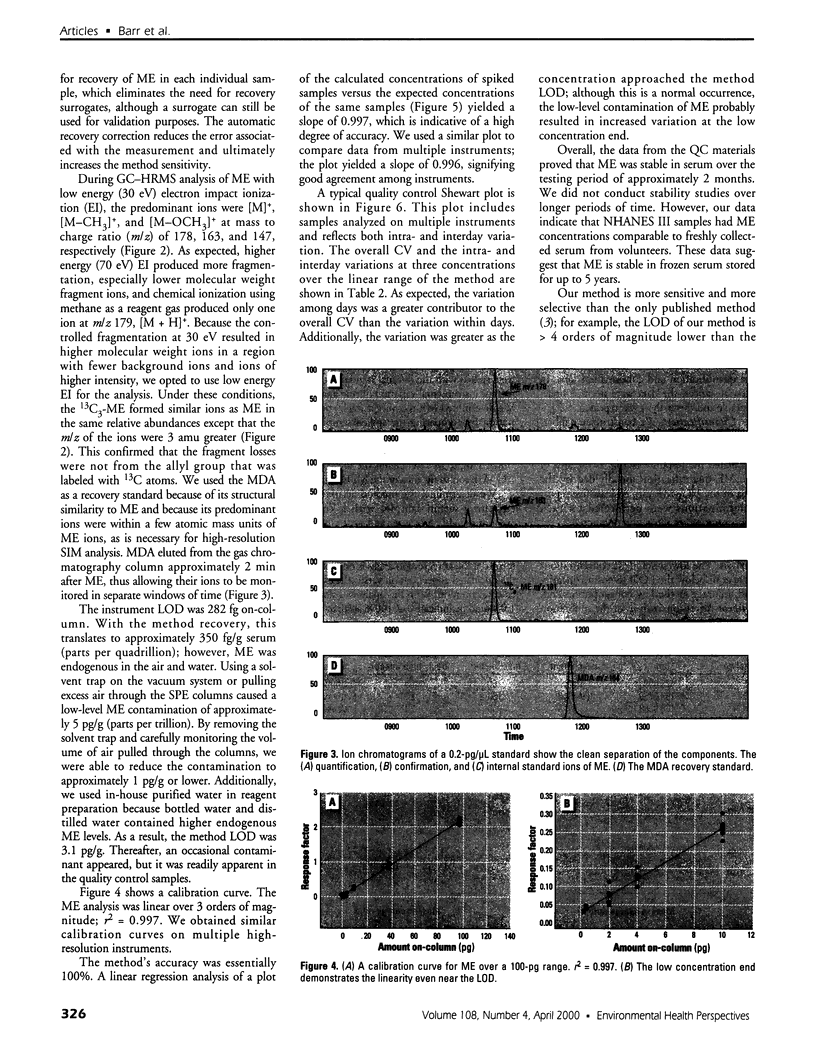
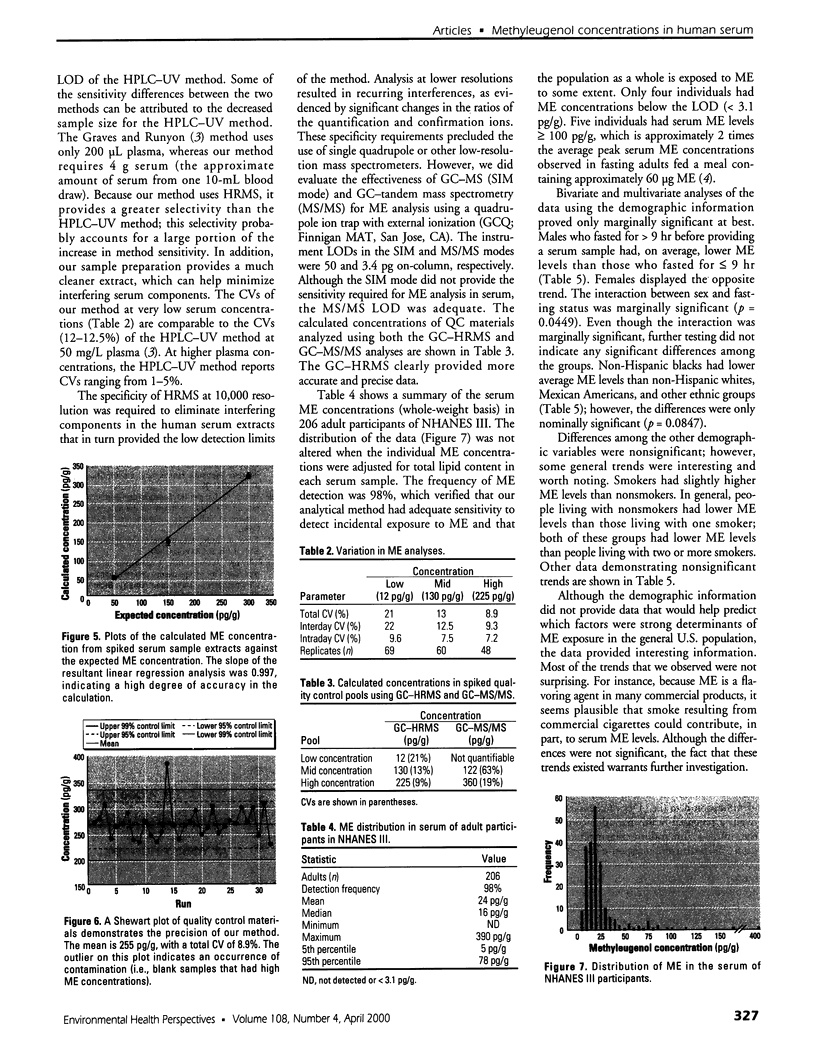
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Colby B. N., McCaman M. W. A comparison of calculation procedures for isotope dilution determinations using gas chromatography mass spectrometry. Biomed Mass Spectrom. 1979 Jun;6(6):225–230. doi: 10.1002/bms.1200060602. [DOI] [PubMed] [Google Scholar]
- Fischer I. U., Dengler H. J. Sensitive high-performance liquid chromatographic assay for the determination of eugenol in body fluids. J Chromatogr. 1990 Feb 23;525(2):369–377. doi: 10.1016/s0378-4347(00)83413-1. [DOI] [PubMed] [Google Scholar]
- Gardner I., Bergin P., Stening P., Kenna J. G., Caldwell J. Immunochemical detection of covalently modified protein adducts in livers of rats treated with methyleugenol. Chem Res Toxicol. 1996 Jun;9(4):713–721. doi: 10.1021/tx950211v. [DOI] [PubMed] [Google Scholar]
- Graves S. W., Runyon S. Determination of methyleugenol in rodent plasma by high-performance liquid chromatography. J Chromatogr B Biomed Appl. 1995 Jan 20;663(2):255–262. doi: 10.1016/0378-4347(94)00452-b. [DOI] [PubMed] [Google Scholar]
- Lucier G. W., Schecter A. Human exposure assessment and the National Toxicology Program. Environ Health Perspect. 1998 Oct;106(10):623–627. doi: 10.1289/ehp.106-1533173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller E. C., Swanson A. B., Phillips D. H., Fletcher T. L., Liem A., Miller J. A. Structure-activity studies of the carcinogenicities in the mouse and rat of some naturally occurring and synthetic alkenylbenzene derivatives related to safrole and estragole. Cancer Res. 1983 Mar;43(3):1124–1134. [PubMed] [Google Scholar]
- Needham L. L., Patterson D. G., Jr, Burse V. W., Paschal D. C., Turner W. E., Hill R. H., Jr Reference range data for assessing exposure to selected environmental toxicants. Toxicol Ind Health. 1996 May-Aug;12(3-4):507–513. doi: 10.1177/074823379601200322. [DOI] [PubMed] [Google Scholar]