Abstract
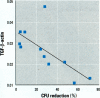
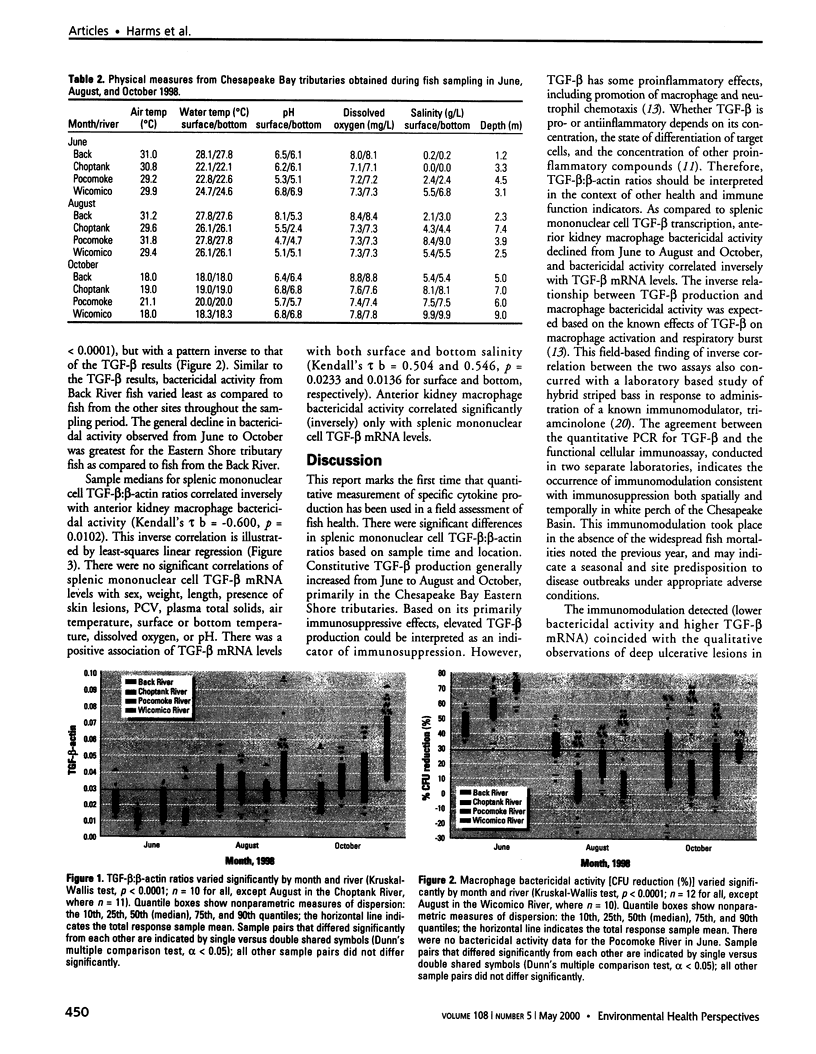
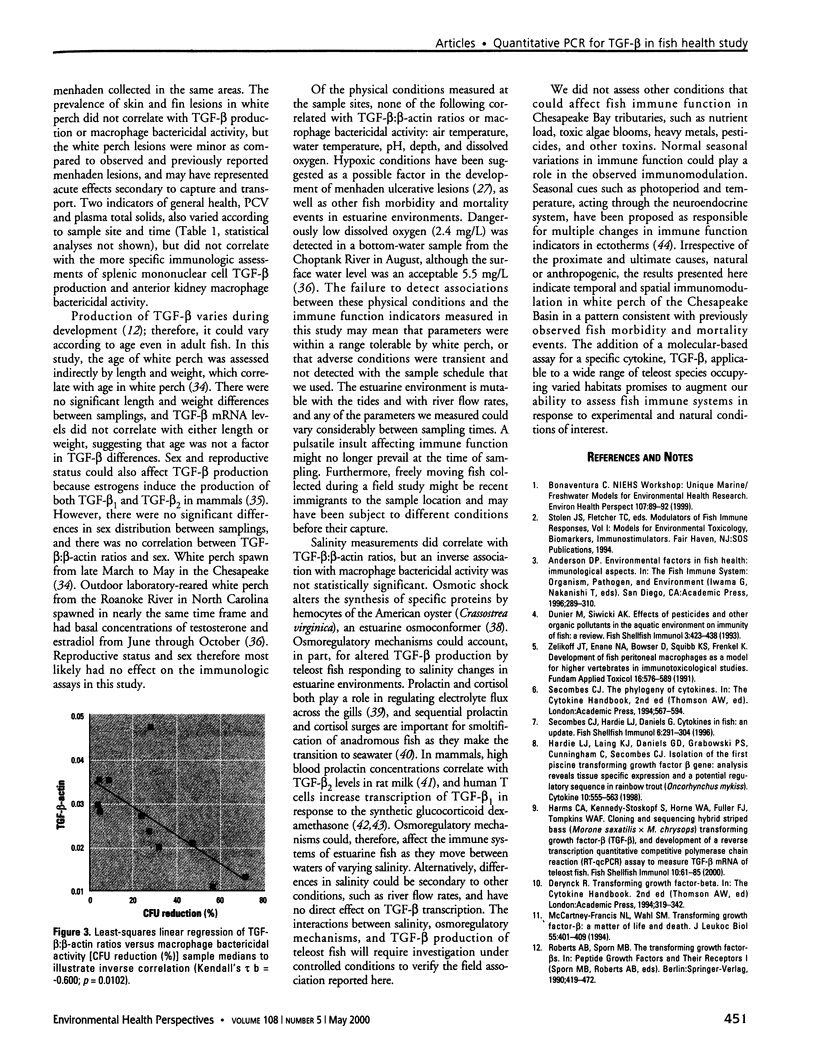
Fish morbidity and mortality events in Chesapeake Bay tributaries have aroused concern over the health of this important aquatic ecosystem. We applied a recently described method for quantifying mRNA of an immunosuppressive cytokine, transforming growth factor-beta (TGF-beta), by reverse transcription quantitative-competitive polymerase chain reaction to a field study of fish health in the Chesapeake Basin, and compared the results to those of a traditional cellular immunoassay macrophage bactericidal activity. We selected the white perch (Morone americana) as the sentinel fish species because of its abundance at all of the collection sites. White perch were sampled from Chesapeake Bay tributaries in June, August, and October 1998. Splenic mononuclear cell TGF-beta mRNA levels increased and anterior kidney macrophage bactericidal activity decreased, particularly in eastern shore tributaries, from June to August and October. The results of the two assays correlated inversely (Kendall's [Tau] b = -0.600; p = 0.0102). The results indicated both temporal and spatial modulation of white perch immune systems in the Chesapeake Basin, and demonstrated the utility of quantitative PCR for TGF-beta as a molecular biomarker for field assessment of teleost fish immune status.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AyanlarBatuman O., Ferrero A. P., Diaz A., Jimenez S. A. Regulation of transforming growth factor-beta 1 gene expression by glucocorticoids in normal human T lymphocytes. J Clin Invest. 1991 Nov;88(5):1574–1580. doi: 10.1172/JCI115469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batuman O. A., Ferrero A., Cupp C., Jimenez S. A., Khalili K. Differential regulation of transforming growth factor beta-1 gene expression by glucocorticoids in human T and glial cells. J Immunol. 1995 Nov 1;155(9):4397–4405. [PubMed] [Google Scholar]
- Bonaventura C. NIEHS workshop: unique marine/freshwater models for environmental health research. Environ Health Perspect. 1999 Jan;107(1):89–92. doi: 10.1289/ehp.9910789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkholder J. M., Noga E. J., Hobbs C. H., Glasgow H. B., Jr, Smith S. A. New 'phantom' dinoflagellate is the causative agent of major estuarine fish kills. Nature. 1992 Jul 30;358(6385):407–410. doi: 10.1038/358407a0. [DOI] [PubMed] [Google Scholar]
- Burt D. W., Law A. S. Evolution of the transforming growth factor-beta superfamily. Prog Growth Factor Res. 1994;5(1):99–118. doi: 10.1016/0955-2235(94)90020-5. [DOI] [PubMed] [Google Scholar]
- Daniels G. D., Secombes C. J. Genomic organisation of rainbow trout, Oncorhynchus mykiss TGF-beta. Dev Comp Immunol. 1999 Mar-Apr;23(2):139–147. doi: 10.1016/s0145-305x(98)00051-2. [DOI] [PubMed] [Google Scholar]
- Hardie L. J., Laing K. J., Daniels G. D., Grabowski P. S., Cunningham C., Secombes C. J. Isolation of the first piscine transforming growth factor beta gene: analysis reveals tissue specific expression and a potential regulatory sequence in rainbow trout (Oncorhynchus mykiss). Cytokine. 1998 Aug;10(8):555–563. doi: 10.1006/cyto.1997.0334. [DOI] [PubMed] [Google Scholar]
- Harms C. A., Kennedy-Stoskopf S., Horne W. A., Fuller F. J., Tompkins W. A. Cloning and sequencing hybrid striped bass (Morone saxatilis x M. chrysops) transforming growth factor-beta (TGF-beta), and development of a reverse transcription quantitative competitive polymerase chain reaction (RT-qcPCR) assay to measure TGF-beta mRNA of teleost fish. Fish Shellfish Immunol. 2000 Jan;10(1):61–85. doi: 10.1006/fsim.1999.0230. [DOI] [PubMed] [Google Scholar]
- Jang S. I., Hardie L. J., Secombes C. J. Effects of transforming growth factor beta 1 on rainbow trout Oncorhynchus mykiss macrophage respiratory burst activity. Dev Comp Immunol. 1994 Jul-Aug;18(4):315–323. doi: 10.1016/s0145-305x(94)90357-3. [DOI] [PubMed] [Google Scholar]
- Jang S. I., Hardie L. J., Secombes C. J. Elevation of rainbow trout Oncorhynchus mykiss macrophage respiratory burst activity with macrophage-derived supernatants. J Leukoc Biol. 1995 Jun;57(6):943–947. doi: 10.1002/jlb.57.6.943. [DOI] [PubMed] [Google Scholar]
- Macilwain C. Scientists close in on 'cell from hell' lurking in Chesapeake Bay. Nature. 1997 Sep 25;389(6649):317–318. doi: 10.1038/38551. [DOI] [PubMed] [Google Scholar]
- McCartney-Francis N. L., Wahl S. M. Transforming growth factor beta: a matter of life and death. J Leukoc Biol. 1994 Mar;55(3):401–409. doi: 10.1002/jlb.55.3.401. [DOI] [PubMed] [Google Scholar]
- Rottman J. B., Tompkins W. A., Tompkins M. B. A reverse transcription-quantitative competitive polymerase chain reaction (RT-qcPCR) technique to measure cytokine gene expression in domestic mammals. Vet Pathol. 1996 Mar;33(2):242–248. doi: 10.1177/030098589603300217. [DOI] [PubMed] [Google Scholar]
- Ruscetti F. W., Palladino M. A. Transforming growth factor-beta and the immune system. Prog Growth Factor Res. 1991;3(2):159–175. doi: 10.1016/s0955-2235(05)80006-7. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Pollard R. B., Schmitt D., Suzuki F. Transforming growth factor-beta in the regulation of the immune response. Clin Immunol Immunopathol. 1992 Oct;65(1):1–9. doi: 10.1016/0090-1229(92)90241-f. [DOI] [PubMed] [Google Scholar]
- Schneider S. L., Gollnick S. O., Grande C., Pazik J. E., Tomasi T. B. Differential regulation of TGF-beta 2 by hormones in rat uterus and mammary gland. J Reprod Immunol. 1996 Dec;32(2):125–144. doi: 10.1016/s0165-0378(96)00997-7. [DOI] [PubMed] [Google Scholar]
- Sharp G. J., Pike A. W., Secombes C. J. Leucocyte migration in rainbow trout (Oncorhynchus mykiss [Walbaum]): optimization of migration conditions and responses to host and pathogen (Diphyllobothrium dendriticum [Nitzsch]) derived chemoattractants. Dev Comp Immunol. 1991 Fall;15(4):295–305. doi: 10.1016/0145-305x(91)90022-q. [DOI] [PubMed] [Google Scholar]
- Zapata A. G., Varas A., Torroba M. Seasonal variations in the immune system of lower vertebrates. Immunol Today. 1992 Apr;13(4):142–147. doi: 10.1016/0167-5699(92)90112-K. [DOI] [PubMed] [Google Scholar]
- Zelikoff J. T., Enane N. A., Bowser D., Squibb K. S., Frenkel K. Development of fish peritoneal macrophages as a model for higher vertebrates in immunotoxicological studies. I. Characterization of trout macrophage morphological, functional, and biochemical properties. Fundam Appl Toxicol. 1991 Apr;16(3):576–589. doi: 10.1016/0272-0590(91)90097-n. [DOI] [PubMed] [Google Scholar]