Abstract
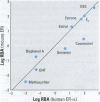
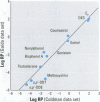
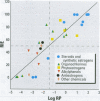
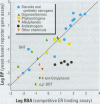
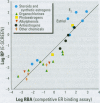
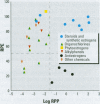
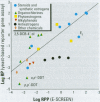
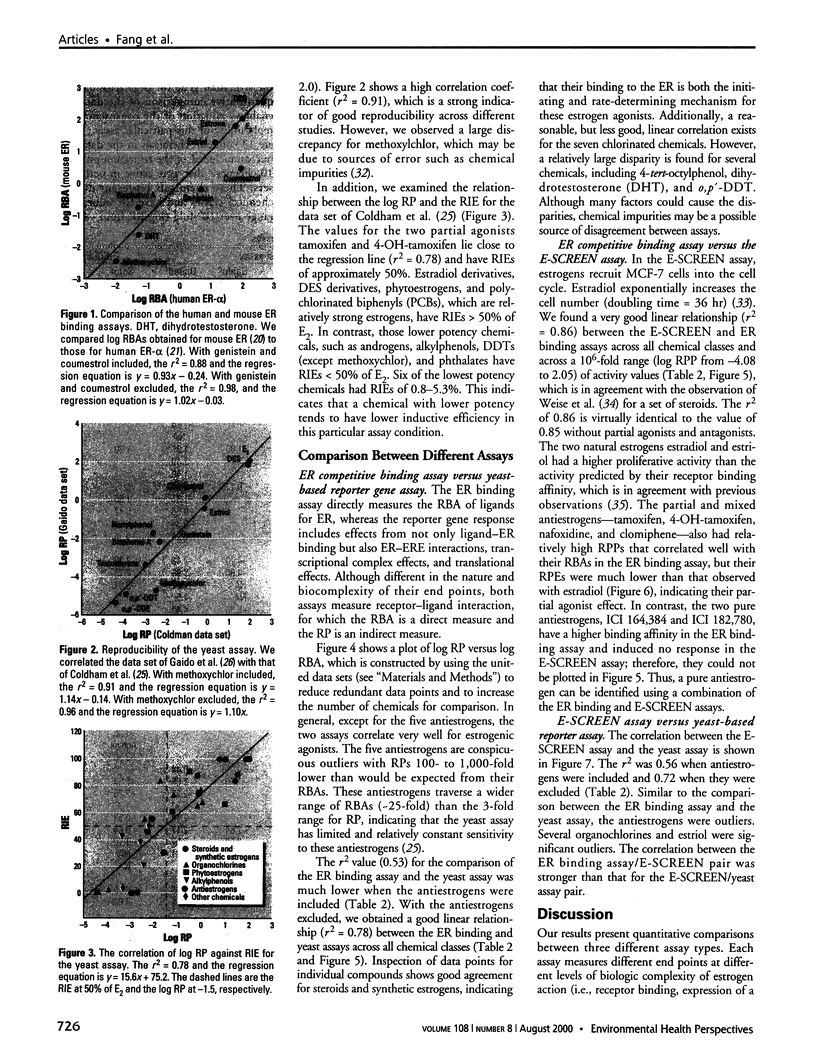
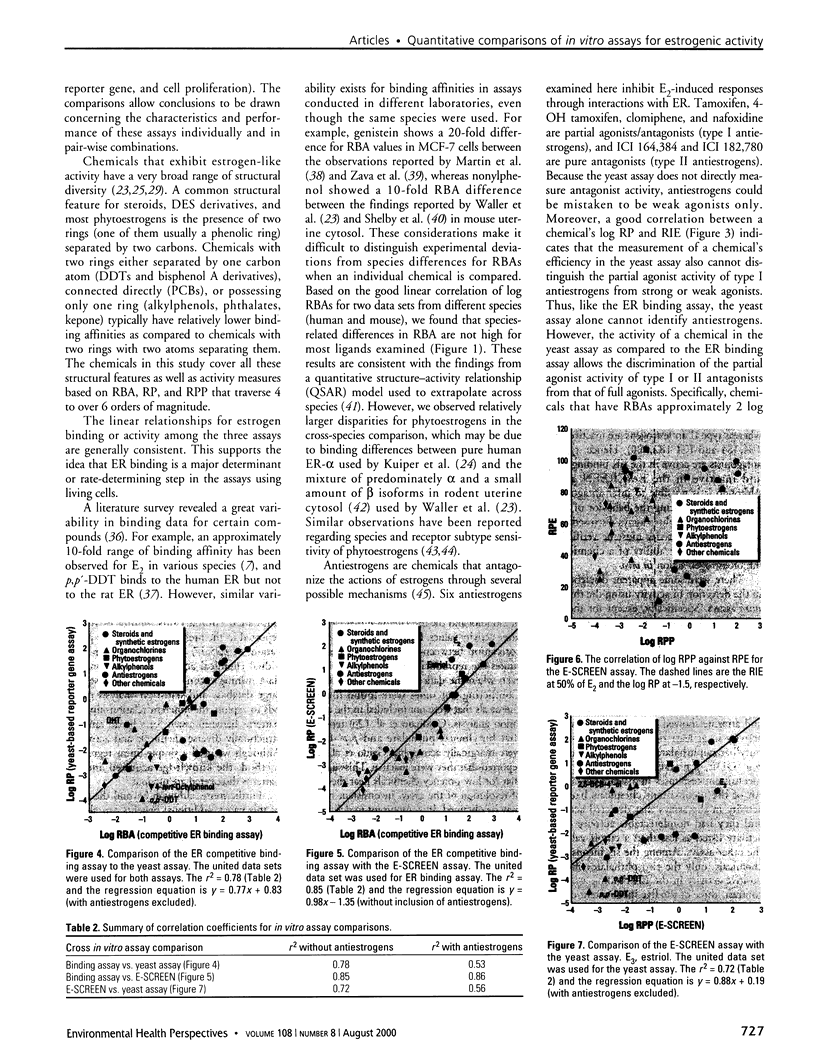
Substances that may act as estrogens show a broad chemical structural diversity. To thoroughly address the question of possible adverse estrogenic effects, reliable methods are needed to detect and identify the chemicals of these diverse structural classes. We compared three assays--in vitro estrogen receptor competitive binding assays (ER binding assays), yeast-based reporter gene assays (yeast assays), and the MCF-7 cell proliferation assay (E-SCREEN assay)--to determine their quantitative agreement in identifying structurally diverse estrogens. We examined assay performance for relative sensitivity, detection of active/inactive chemicals, and estrogen/antiestrogen activities. In this examination, we combined individual data sets in a specific, quantitative data mining exercise. Data sets for at least 29 chemicals from five laboratories were analyzed pair-wise by X-Y plots. The ER binding assay was a good predictor for the other two assay results when the antiestrogens were excluded (r(2) is 0.78 for the yeast assays and 0.85 for the E-SCREEN assays). Additionally, the examination strongly suggests that biologic information that is not apparent from any of the individual assays can be discovered by quantitative pair-wise comparisons among assays. Antiestrogens are identified as outliers in the ER binding/yeast assay, while complete antagonists are identified in the ER binding and E-SCREEN assays. Furthermore, the presence of outliers may be explained by different mechanisms that induce an endocrine response, different impurities in different batches of chemicals, different species sensitivity, or limitations of the assay techniques. Although these assays involve different levels of biologic complexity, the major conclusion is that they generally provided consistent information in quantitatively determining estrogenic activity for the five data sets examined. The results should provide guidance for expanded data mining examinations and the selection of appropriate assays to screen estrogenic endocrine disruptors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. R., Andersson A. M., Arnold S. F., Autrup H., Barfoed M., Beresford N. A., Bjerregaard P., Christiansen L. B., Gissel B., Hummel R. Comparison of short-term estrogenicity tests for identification of hormone-disrupting chemicals. Environ Health Perspect. 1999 Feb;107 (Suppl 1):89–108. doi: 10.1289/ehp.99107s189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anstead G. M., Carlson K. E., Katzenellenbogen J. A. The estradiol pharmacophore: ligand structure-estrogen receptor binding affinity relationships and a model for the receptor binding site. Steroids. 1997 Mar;62(3):268–303. doi: 10.1016/s0039-128x(96)00242-5. [DOI] [PubMed] [Google Scholar]
- Baker M. E., Medlock K. L., Sheehan D. M. Flavonoids inhibit estrogen binding to rat alpha-fetoprotein. Proc Soc Exp Biol Med. 1998 Mar;217(3):317–321. doi: 10.3181/00379727-217-44238. [DOI] [PubMed] [Google Scholar]
- Blair R. M., Fang H., Branham W. S., Hass B. S., Dial S. L., Moland C. L., Tong W., Shi L., Perkins R., Sheehan D. M. The estrogen receptor relative binding affinities of 188 natural and xenochemicals: structural diversity of ligands. Toxicol Sci. 2000 Mar;54(1):138–153. doi: 10.1093/toxsci/54.1.138. [DOI] [PubMed] [Google Scholar]
- Branham W. S., Zehr D. R., Sheehan D. M. Differential sensitivity of rat uterine growth and epithelium hypertrophy to estrogens and antiestrogens. Proc Soc Exp Biol Med. 1993 Jul;203(3):297–303. doi: 10.3181/00379727-203-43602. [DOI] [PubMed] [Google Scholar]
- Brazma A., Vilo J., Ukkonen E., Valtonen K. Data mining for regulatory elements in yeast genome. Proc Int Conf Intell Syst Mol Biol. 1997;5:65–74. [PubMed] [Google Scholar]
- Brossette S. E., Sprague A. P., Hardin J. M., Waites K. B., Jones W. T., Moser S. A. Association rules and data mining in hospital infection control and public health surveillance. J Am Med Inform Assoc. 1998 Jul-Aug;5(4):373–381. doi: 10.1136/jamia.1998.0050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger W. H., Muccitelli R. M., Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27(20):2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Bulger W. H., Muccitelli R. M., Kupfer D. Studies on the in vivo and in vitro estrogenic activities of methoxychlor and its metabolites. Role of hepatic mono-oxygenase in methoxychlor activation. Biochem Pharmacol. 1978;27(20):2417–2423. doi: 10.1016/0006-2952(78)90354-4. [DOI] [PubMed] [Google Scholar]
- Chen C. W., Hurd C., Vorojeikina D. P., Arnold S. F., Notides A. C. Transcriptional activation of the human estrogen receptor by DDT isomers and metabolites in yeast and MCF-7 cells. Biochem Pharmacol. 1997 Apr 25;53(8):1161–1172. doi: 10.1016/s0006-2952(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Coldham N. G., Dave M., Sivapathasundaram S., McDonnell D. P., Connor C., Sauer M. J. Evaluation of a recombinant yeast cell estrogen screening assay. Environ Health Perspect. 1997 Jul;105(7):734–742. doi: 10.1289/ehp.97105734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K., Ramamoorthy K., Moore M., Mustain M., Chen I., Safe S., Zacharewski T., Gillesby B., Joyeux A., Balaguer P. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: structure-activity relationships. Toxicol Appl Pharmacol. 1997 Jul;145(1):111–123. doi: 10.1006/taap.1997.8169. [DOI] [PubMed] [Google Scholar]
- Couse J. F., Lindzey J., Grandien K., Gustafsson J. A., Korach K. S. Tissue distribution and quantitative analysis of estrogen receptor-alpha (ERalpha) and estrogen receptor-beta (ERbeta) messenger ribonucleic acid in the wild-type and ERalpha-knockout mouse. Endocrinology. 1997 Nov;138(11):4613–4621. doi: 10.1210/endo.138.11.5496. [DOI] [PubMed] [Google Scholar]
- Gaido K. W., Leonard L. S., Lovell S., Gould J. C., Babaï D., Portier C. J., McDonnell D. P. Evaluation of chemicals with endocrine modulating activity in a yeast-based steroid hormone receptor gene transcription assay. Toxicol Appl Pharmacol. 1997 Mar;143(1):205–212. doi: 10.1006/taap.1996.8069. [DOI] [PubMed] [Google Scholar]
- Harris C. A., Henttu P., Parker M. G., Sumpter J. P. The estrogenic activity of phthalate esters in vitro. Environ Health Perspect. 1997 Aug;105(8):802–811. doi: 10.1289/ehp.97105802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M., Zacharewski T., Safe S. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds on the occupied nuclear estrogen receptor in MCF-7 human breast cancer cells. Cancer Res. 1990 Jun 15;50(12):3579–3584. [PubMed] [Google Scholar]
- Katzenellenbogen B. S., Montano M. M., Le Goff P., Schodin D. J., Kraus W. L., Bhardwaj B., Fujimoto N. Antiestrogens: mechanisms and actions in target cells. J Steroid Biochem Mol Biol. 1995 Jun;53(1-6):387–393. doi: 10.1016/0960-0760(95)00084-d. [DOI] [PubMed] [Google Scholar]
- Katzenellenbogen J. A. The structural pervasiveness of estrogenic activity. Environ Health Perspect. 1995 Oct;103 (Suppl 7):99–101. doi: 10.1289/ehp.95103s799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavlock R. J., Daston G. P., DeRosa C., Fenner-Crisp P., Gray L. E., Kaattari S., Lucier G., Luster M., Mac M. J., Maczka C. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ Health Perspect. 1996 Aug;104 (Suppl 4):715–740. doi: 10.1289/ehp.96104s4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper G. G., Carlsson B., Grandien K., Enmark E., Häggblad J., Nilsson S., Gustafsson J. A. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology. 1997 Mar;138(3):863–870. doi: 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Martin P. M., Horwitz K. B., Ryan D. S., McGuire W. L. Phytoestrogen interaction with estrogen receptors in human breast cancer cells. Endocrinology. 1978 Nov;103(5):1860–1867. doi: 10.1210/endo-103-5-1860. [DOI] [PubMed] [Google Scholar]
- McDonald J. M., Brossette S., Moser S. A. Pathology information systems: data mining leads to knowledge discovery. Arch Pathol Lab Med. 1998 May;122(5):409–411. [PubMed] [Google Scholar]
- Nesaretnam K., Corcoran D., Dils R. R., Darbre P. 3,4,3',4'-Tetrachlorobiphenyl acts as an estrogen in vitro and in vivo. Mol Endocrinol. 1996 Aug;10(8):923–936. doi: 10.1210/mend.10.8.8843409. [DOI] [PubMed] [Google Scholar]
- Norris J. D., Fan D., Kerner S. A., McDonnell D. P. Identification of a third autonomous activation domain within the human estrogen receptor. Mol Endocrinol. 1997 Jun;11(6):747–754. doi: 10.1210/mend.11.6.0008. [DOI] [PubMed] [Google Scholar]
- Odum J., Lefevre P. A., Tittensor S., Paton D., Routledge E. J., Beresford N. A., Sumpter J. P., Ashby J. The rodent uterotrophic assay: critical protocol features, studies with nonyl phenols, and comparison with a yeast estrogenicity assay. Regul Toxicol Pharmacol. 1997 Apr;25(2):176–188. doi: 10.1006/rtph.1997.1100. [DOI] [PubMed] [Google Scholar]
- Pappas T. C., Gametchu B., Watson C. S. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995 Mar;9(5):404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- Petersen D. N., Tkalcevic G. T., Koza-Taylor P. H., Turi T. G., Brown T. A. Identification of estrogen receptor beta2, a functional variant of estrogen receptor beta expressed in normal rat tissues. Endocrinology. 1998 Mar;139(3):1082–1092. doi: 10.1210/endo.139.3.5840. [DOI] [PubMed] [Google Scholar]
- Reel J. R., Lamb IV J. C., Neal B. H. Survey and assessment of mammalian estrogen biological assays for hazard characterization. Fundam Appl Toxicol. 1996 Dec;34(2):288–305. doi: 10.1006/faat.1996.0198. [DOI] [PubMed] [Google Scholar]
- Safe S., Krishnan V. Chlorinated hydrocarbons: estrogens and antiestrogens. Toxicol Lett. 1995 Dec;82-83:731–736. doi: 10.1016/0378-4274(95)03591-5. [DOI] [PubMed] [Google Scholar]
- Sheehan D. M., Young M. Diethylstilbestrol and estradiol binding to serum albumin and pregnancy plasma of rat and human. Endocrinology. 1979 May;104(5):1442–1446. doi: 10.1210/endo-104-5-1442. [DOI] [PubMed] [Google Scholar]
- Shelby M. D., Newbold R. R., Tully D. B., Chae K., Davis V. L. Assessing environmental chemicals for estrogenicity using a combination of in vitro and in vivo assays. Environ Health Perspect. 1996 Dec;104(12):1296–1300. doi: 10.1289/ehp.961041296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnenschein C., Soto A. M., Michaelson C. L. Human serum albumin shares the properties of estrocolyone-I, the inhibitor of the proliferation of estrogen-target cells. J Steroid Biochem Mol Biol. 1996 Oct;59(2):147–154. doi: 10.1016/s0960-0760(96)00112-4. [DOI] [PubMed] [Google Scholar]
- Soto A. M., Murai J. T., Siiteri P. K., Sonnenschein C. Control of cell proliferation: evidence for negative control on estrogen-sensitive T47D human breast cancer cells. Cancer Res. 1986 May;46(5):2271–2275. [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C., Chung K. L., Fernandez M. F., Olea N., Serrano F. O. The E-SCREEN assay as a tool to identify estrogens: an update on estrogenic environmental pollutants. Environ Health Perspect. 1995 Oct;103 (Suppl 7):113–122. doi: 10.1289/ehp.95103s7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A. M., Sonnenschein C. The role of estrogens on the proliferation of human breast tumor cells (MCF-7). J Steroid Biochem. 1985 Jul;23(1):87–94. doi: 10.1016/0022-4731(85)90265-1. [DOI] [PubMed] [Google Scholar]
- Tong W., Perkins R., Strelitz R., Collantes E. R., Keenan S., Welsh W. J., Branham W. S., Sheehan D. M. Quantitative structure-activity relationships (QSARs) for estrogen binding to the estrogen receptor: predictions across species. Environ Health Perspect. 1997 Oct;105(10):1116–1124. doi: 10.1289/ehp.971051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong W., Perkins R., Xing L., Welsh W. J., Sheehan D. M. QSAR models for binding of estrogenic compounds to estrogen receptor alpha and beta subtypes. Endocrinology. 1997 Sep;138(9):4022–4025. doi: 10.1210/endo.138.9.5487. [DOI] [PubMed] [Google Scholar]
- Waller C. L., Oprea T. I., Chae K., Park H. K., Korach K. S., Laws S. C., Wiese T. E., Kelce W. R., Gray L. E., Jr Ligand-based identification of environmental estrogens. Chem Res Toxicol. 1996 Dec;9(8):1240–1248. doi: 10.1021/tx960054f. [DOI] [PubMed] [Google Scholar]
- Watson C. S., Pappas T. C., Gametchu B. The other estrogen receptor in the plasma membrane: implications for the actions of environmental estrogens. Environ Health Perspect. 1995 Oct;103 (Suppl 7):41–50. doi: 10.1289/ehp.95103s741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese T. E., Polin L. A., Palomino E., Brooks S. C. Induction of the estrogen specific mitogenic response of MCF-7 cells by selected analogues of estradiol-17 beta: a 3D QSAR study. J Med Chem. 1997 Oct 24;40(22):3659–3669. doi: 10.1021/jm9703294. [DOI] [PubMed] [Google Scholar]
- Zacharewski T., Harris M., Safe S. Evidence for the mechanism of action of the 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated decrease of nuclear estrogen receptor levels in wild-type and mutant mouse Hepa 1c1c7 cells. Biochem Pharmacol. 1991 Jun 15;41(12):1931–1939. doi: 10.1016/0006-2952(91)90133-p. [DOI] [PubMed] [Google Scholar]
- Zacharewski T. Identification and assessment of endocrine disruptors: limitations of in vivo and in vitro assays. Environ Health Perspect. 1998 Apr;106 (Suppl 2):577–582. doi: 10.1289/ehp.98106577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zava D. T., Blen M., Duwe G. Estrogenic activity of natural and synthetic estrogens in human breast cancer cells in culture. Environ Health Perspect. 1997 Apr;105 (Suppl 3):637–645. doi: 10.1289/ehp.97105s3637. [DOI] [PMC free article] [PubMed] [Google Scholar]