Abstract
Pharmacological studies have suggested that long-term potentiation (LTP) and long-term depression (LTD) and depotentiation, three forms of synaptic plasticity in the hippocampus, require the activity of the phosphatase calcineurin. At least two different isoforms of calcineurin are found in the central nervous system. To investigate whether all of these forms of synaptic plasticity require the same isoforms of calcineurin, we have examined LTD, depotentiation, and LTP in mice lacking the predominant calcineurin isoform in the central nervous system, Aα−/− mice. Depotentiation was abolished completely whereas neither LTD nor LTP were affected. These studies provide genetic evidence that the Aα isoform of calcineurin is important for the reversal of LTP in the hippocampus and indicate that depotentiation and LTD operate through somewhat different molecular mechanisms.
Calcineurin is a serine/threonine-protein phosphatase that is abundantly expressed in the central nervous system, including the hippocampus (1, 2). Because of its high affinity for the Ca2+/calmodulin complex, calcineurin is thought to play a critical role in synaptic plasticity (3–6). In particular, pharmacological studies using protein phosphatase inhibitors have suggested that both long-term depression (LTD) and depotentiation require the activation of protein phosphatases (7–9) and together with genetic studies also have suggested that these protein phosphatases antagonize the action of protein kinases required for long-term potentiation (LTP) (5, 10, 11).
Calcineurin also may have a direct role in LTPs because inhibitors of calcineurin affect the induction and expression of LTP (12–14). Because there are at least two calcineurin isoforms, these findings suggest that different isoforms may be involved in LTD and LTP or that pharmacological inhibitors produce calcineurin-independent effects that interfere with the induction of LTD or LTP (15). To address these issues, we examined LTD, depotentiation, and LTP in mice lacking the predominant calcineurin isoform in the central nervous system, Aα. We found that, in calcineurin Aα−/− mice, depotentiation was abolished completely whereas neither LTD nor LTP were affected.
MATERIALS AND METHODS
Immunohistochemistry.
To determine the localization of calcineurin A isoforms in the central nervous system, rabbit polyclonal antipeptide antisera to the calcineurin Aα isoform (peptide LSNSSNIQ; R2929) and to the calcineurin Aβ isoform (LMTEGEDEFDG; R2948) were used to stain paraffin sections of brain tissue from five wild-type and five mutant mice. To identify possible additional subtle abnormalities in the mutants, rabbit antibodies to glial fibrillary acidic protein (provided by L. F. Eng, Stanford University), synaptophysin (Zymed), and synapsin 1 (Biogenesis, Sandown, NH) also were used for immunohistochemical studies. Six-micrometer-thick paraffin sections were stained by using immunoperoxidase staining kits (Vector Laboratories) as described (30). In brief, after deparaffinization and hydration, the slides were washed in PBS (pH 7.3) and were incubated sequentially in 10% normal goat serum in PBS, primary antibody (diluted 1:100–1:200 in PBS), 0.03% H2O2 in PBS, biotinylated anti-rabbit Ig, and avidin biotin horseradish peroxidase complex. The sections were washed in PBS between each incubation step. Immunoperoxidase reaction product was visualized with 3-amino-9-ethyl carbazole (Aldrich) and were fixed in formol-acetate. The sections were counterstained with hematoxylin.
Electrophysiology.
Mice were on a Blackswiss/129 hybrid strain background. Most wild-type controls were littermates of mutants. Considering that hippocampal LTD is age-dependent, young mice (4 weeks old) were used in both LTD and depotentiation experiments. The protocol of electrical stimulation and recordings has been described (23, 24). All experiments used age-matched controls and were performed blind to genotype. Transverse slices of hippocampus were rapidly prepared and maintained in an interface chamber at 28°C, where they were subfused with ACSF consisting of 124 mM NaCl, 4.4 mM KCl, 2.0 mM CaCl2, 1.0 mM MgSO4, 25 mM NaHCO3, 1.0 mM Na2HPO4, and 10 mM glucose. Stimulus intensity was adjusted to produce a response of ≈1 mV amplitude, with an initial slope of ≈−0.5 mV/msec. Homosynaptic LTD was induced by a prolonged low frequency stimulation (1 Hz for 15 min). For LTP experiments, the field excitatory postsynaptic potential (EPSP) was tested every 50 sec. LTP was induced by tetanic stimulation (100 Hz twice for 1 sec with a 20-sec interval at testing intensity). For post-tetanic potentiation (PTP) experiments, the EPSP was tested every 10 sec before and after stimulation. Paired-pulse facilitation (PPF) of the response at various interpulse intervals (25–400 msec) was measured. For depotentiation experiments, the stimulus to produce LTP was 100 Hz for 1 sec, delivered twice with an interval of 20 sec. This was followed by a low frequency stimulus of 5 Hz for 3 min to produce depotentiation. In experiments with cyclosporin A (250 μM), slices were pretreated for 30 min before the induction of LTD.
RESULTS
Histological Analysis of Calcineurin Aα and Aβ.
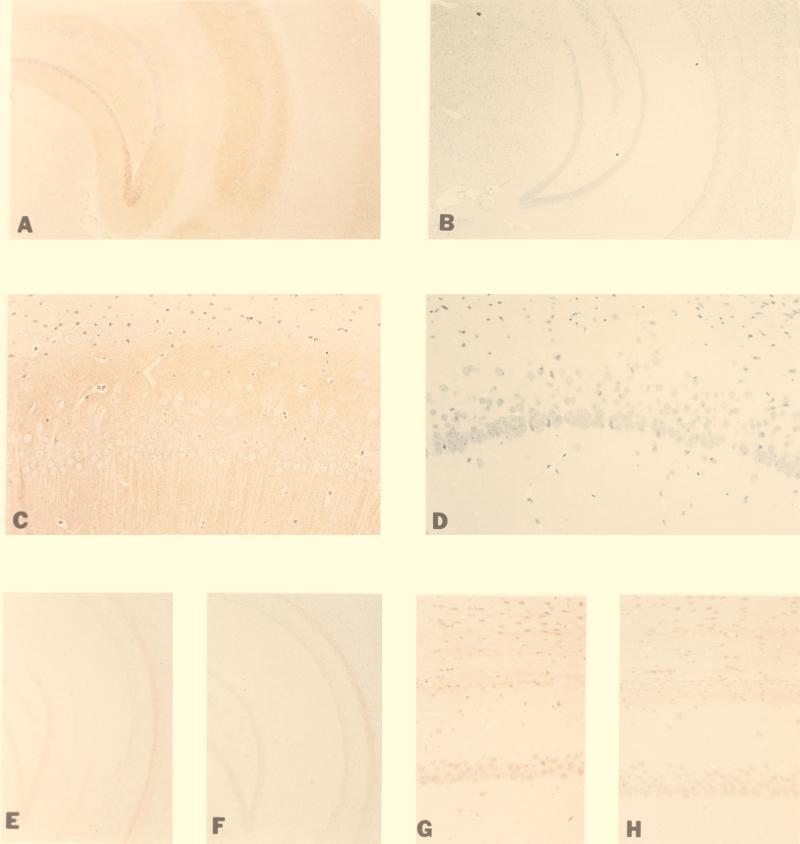
Calcineurin is a heterodimer consisting of a catalytic (A; 61 KD) and a regulatory (B; 19 KD) subunit. The catalytic subunit exists as two closely related isoforms, Aα and Aβ, both of which are expressed in the brain. Calcineurin Aα is the predominant isoform in the hippocampus, cerebral cortex, cerebellum, and striatum (1, 2, 16). Mice with a targeted mutation in the calcineurin Aα gene were generated by standard homologous recombination technique in embryonic stem cells (16). The distribution of calcineurin Aα and Aβ in the brain of wild-type and mutant mice was examined immunohistochemically by using calcineurin Aα- and calcineurin Aβ-specific peptide antibodies (Fig. 1). Consistent with previous reports (1, 2, 17), we found that in, wild-type mice, calcineurin Aα is highly expressed in the CA1 and CA3 regions of the hippocampus as well as in the dentate gyrus (Fig. 1 A and C). No calcineurin Aα protein was detected in the hippocampus of mutant mice (Fig. 1 B and D). Furthermore, no detectable difference was found in calcineurin Aβ expression between wild-type and mutant mice, indicating that calcineurin Aβ is not up-regulated during development in the mutant mice. These findings suggest that calcineurin Aα is not expressed in mutant mice and Aβ isoform does not compensate for the lack of Aα in the mutant mice. As expected, the activity of calcineurin (i.e., okadaic acid-resistant and EGTA-sensitive phosphatase activity) was reduced in the brain of the mutants by >85% (18).
Figure 1.
Immunohistochemical detection of calcineurin Aα and calcineurin Aβ in brains of wild-type and mutant calcineurin Aα knockout mice. A–D are stained with antiserum to calcineurin Aα peptide (R2929) in wild-type and mutant mice: Calcineurin Aα is expressed in the hippocampus of wild-type mice (A) and, in particular, in the CA1 region of the hippocampus (C) but not in the mutant mice (B and D). Calcineurin Aβ [stained with antiserum to calcineurin Aβ peptide (R2948)] is located in hippocampal neuron nuclei and in adjacent white matter tracts. The staining intensity is similar in wild-type [hippocampus (E) and CA1 region (G)] and mutant mice [hippocampus (F) and CA1 region (H)]. The amplification factor is 11 (A, B, E, and F) or 54 (C, D, G, and H).
LTD Is Normal in Calcineurin Aα-Deficient Mice.
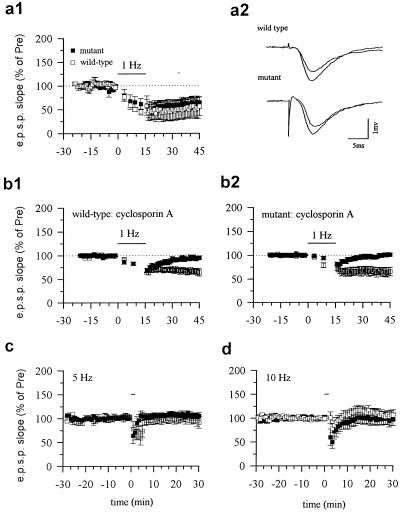
To determine the relative roles of calcineurin Aα and Aβ in hippocampal synaptic plasticity, we studied LTD, depotentiation, and LTP in the Schaffer collateral pathway in calcineurin Aα-deficient mice. We first evaluated LTD induced by low-frequency (1 Hz, 15 min) stimulation (19). Previous studies have shown that pharmacological inhibitors of calcineurin block the induction of LTD (ref. 8, but see ref. 20). To our surprise, LTD was normal in the calcineurin Aα mutant mice. One-hertz stimulation for 15 min produced an identical LTD of synaptic responses in mutant mice as that in wild-type mice (P > 0.05, not significantly different; Fig. 2a). Because it has been reported that Ca2+/calmodulin-dependent protein kinase II regulates the frequency-response function of hippocampal LTD (21), we wondered whether the deletion of calcineurin Aα might cause a frequency-shift in response to electrical stimulation. We therefore examined the effects of delivering the same number of pulses (900) at different frequencies (5 and 10 Hz). At both frequencies, wild-type and mutant mice were indistinguishable (Fig. 2 c and d). These results further support the evidence that calcineurin Aα is not critical for homosynaptic LTD.
Figure 2.
LTD is normal in calcineurin Aα mutant mice and is blocked by cyclosporin A. (a1) Low-frequency stimulation (1 Hz, 15 min) induced LTD in the CA1 region of the hippocampus from wild-type mice (open squares; 61.6 ± 9.4%, n = 14 slices/10 mice, t = 4.09, P < 0.01 comparing the potential 25 to 30 min after stimulation to the average of the potential before stimulation) or mutant mice (filled squares; 63.8 ± 12.0%, n = 9 slices from 5 mice, t = 3.02, P < 0.05). (a2) Representative recordings of the EPSP before and 30 min after low-frequency stimulation in wild-type (upper trace) and mutant (lower trace) mice. (b) LTD in wild-type and mutant mice was blocked by cyclosporin A. (b1) Pretreatment with cyclosporin A blocked LTD in wild-type mice (100.6 ± 4.0%, n = 6 slices/4 mice). LTD was not affected in vehicle-treated groups (open squares, 64.6 ± 0.5%, n = 6 slices/4 mice). (b2) Pretreatment with cyclosporin A blocked LTD in mutant mice (filled squares, 96.6 ± 5.1%, n = 6 slices/4 mice). LTD was not affected in vehicle-treated mutant mice (open squares, 64.5 ± 0.4%, n = 6 slices/5 mice). (c) Low-frequency stimulation at 5 Hz did not affect synaptic transmission. Neither LTP nor LTD was induced in wild-type (open squares, 94.5 ± 14.1%, n = 6 slices/6 mice) and mutant (filled squares, 103.9 ± 5.5%, n = 7 slices/7 mice) mice. (d) Stimulation at 10 Hz did not affect synaptic transmission. Ten-hertz stimulation produced neither potentiation nor depression in wild-type (open squares, 106.1 ± 9.7%, n = 11 slices/9 mice) or mutant (filled squares, 98.1 ± 13.0%, n = 8 slices/6 mice) mice.
Depotentiation Is Blocked in Calcineurin Aα-Deficient Mice.
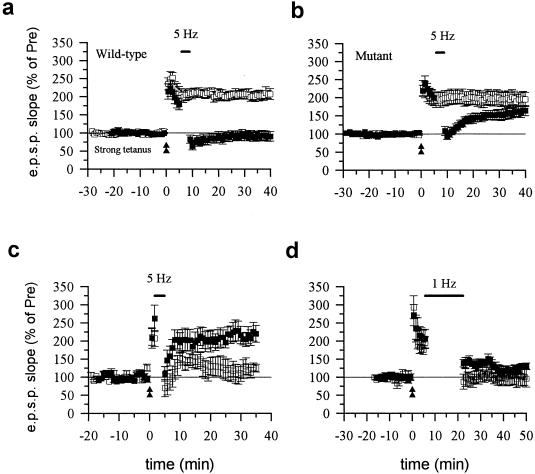
Does calcineurin Aα have a role in other forms of synaptic depression? Both in vivo and in vitro studies demonstrate that depotentiation is a form of synaptic depression that is different from LTD (22). We therefore examined depotentiation in calcineurin Aα wild-type and mutant mice. In wild-type mice, theta frequency stimulation (5 Hz for 3 min) applied 5 min after LTP induction produced long-lasting depression of the potentiated synaptic response (Fig. 3a). The same stimulation did not produce any significant effect on the response in slices that did not undergo LTP. Rather, depotentiation was reduced significantly in mutant mice. Theta frequency stimulation induced only a brief depression followed by a return toward the potentiated state (Fig. 3b). Theta frequency stimulation alone did not induce potentiation or depression (Fig. 2c). These results demonstrate that calcineurin Aα plays a role in homosynaptic depotentiation.
Figure 3.
Depotentiation is abolished in calcineurin Aα mutant mice. (a) Theta frequency stimulation induced depotentiation in wild-type mice. Strong tetanic stimulation (100 Hz, twice for 1 sec with a 20-sec interval) produced long-lasting enhancement of synaptic response (open squares). Five-hertz stimulation (3 min) induced long-lasting depression when delivered 5 min after the induction of LTP (filled squares, 91.4 ± 12.9%, n = 14 slices/10 mice, t = 5.57, P < 0.01 comparing the potential 25 to 30 min after tetanus with normal LTP without 5 Hz stimulation). (b) Theta frequency stimulation did not induce depotentiation in calcineurin Aα mutant mice. Strong tetanic stimulation produced similar long-lasting enhancement of synaptic responses (open squares) as in wild-type mice. However, 5-Hz stimulation only produced a brief depression, but the field EPSP returned to the potentiated level 30 min later (filled squares, 162.5 ± 14.1%, n = 13 slices/7 mice, not significantly different comparing with LTP without 5-Hz stimulation in slices of mutant mice). (c) Theta frequency stimulation delivered 1 min after LTP induction induced depotentiation in wild-type mice (open squares, 122.9 ± 15.1%, n = 5 slices/5 mice) but not mutant mice (filled squares, 213.6 ± 20.5%, n = 7 slices/5 mice, t = 3.28, P < 0.005 comparing the potential 25 to 30 min after theta frequency stimulation between wild-type and mutant mice). (d) Low-frequency stimulation (1 Hz, 15 min) induced similar LTD in mutant (filled squares, 130.3 ± 6.3%, n = 6 slices/6 mice) and wild-type (open squares, 103.5 ± 23.3%, n = 6 slices/5 mice, t = 1.45, not significantly different comparing the potential 25 to 30 min after low-frequency stimulation between wild-type and mutant mice) mice when delivered at 5 min after the induction of LTP.
What could explain the reduction in depotentiation in the mutant mice? One possibility is that the critical period for LTP reversal becomes shorter as a consequence of the mutation. If that were so, then delivering theta frequency stimulation closer to the end of the tetanus might be more successful in reversing LTP. To test this possibility, we delivered theta frequency stimulation 1 min after LTP induction. Theta frequency stimulation again failed to reverse LTP in mutant mice (n = 6; Fig. 3c) whereas it reversed LTP in wild-type mice (n = 5; Fig. 3c). We also tested the effect of 1-Hz stimulation (15 min) delivered 1 min after LTP induction. This stimulation induced similar LTD in mutant and wild-type mice even after LTP (Fig. 3d), indicating that the involvement of calcineurin Aα in depotentiation is frequency- but not time-dependent.
Pharmacological Inhibition of Calcineurin Blocks LTD.
If calcineurin Aα is critical for depotentiation but not LTD, does calcineurin Aβ play a role in LTD? To test this idea, we examined the effect of a calcineurin inhibitor, cyclosporin A, on LTD in calcineurin Aα mutant mice. Consistent with a previous report (8), pretreatment with cyclosporin A blocked LTD in wild-type mice (Fig. 2). Moreover, cyclosporin A also abolished LTD in mutant mice (Fig. 2b). One explanation of this result is that calcineurin Aβ, unlike Aα, might be selectively important for hippocampal LTD. However, the present work cannot completely exclude the possibility that pharmacological inhibitors (e.g., FK506 and cyclosporin A) affect LTD by acting on other target proteins unrelated to calcineurin (15, 25).
LTP and Paired Pulse Facilitation Are Normal in Mutant Mice.
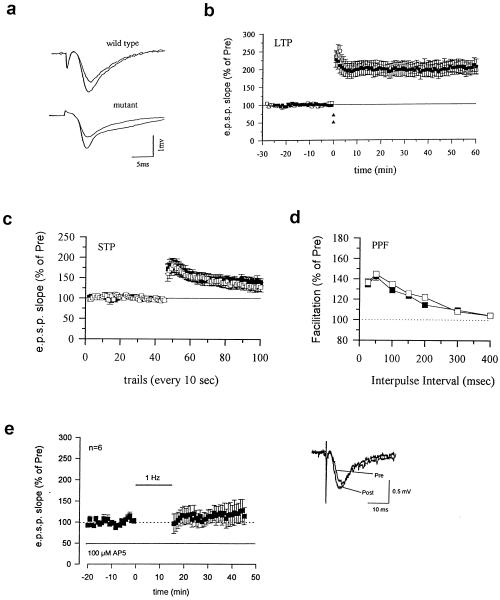
Pharmacological inhibitors of calcineurin can affect N-methyl-d-aspartate (NMDA) receptor-mediated currents and the induction of LTP (12, 13, 26, 27). To examine the involvement of calcineurin Aα in LTP, we induced LTP with a strong tetanic stimulation (two 100-Hz trains) in both wild-type and mutant mice. This stimulation induced a rapid and long-lasting enhancement of synaptic response that remained potentiated for at least 1 hour in both wild-type and mutant mice (P > 0.05) (Fig. 4a). We detected no obvious abnormality in other aspects of synaptic transmission or simple short-term form of synaptic plasticity. The NMDA-mediated EPSPs isolated pharmacologically by using CNQX were not significantly different in wild-type (n = 6) and mutant mice (n = 7) (P > 0.05, not significantly different comparing the two groups). Moreover, PTP, short-term potentiation, and paired-pulse facilitation (PPF) were similar in mutant and wild-type mice (P > 0.05; Fig. 4). Basal synaptic excitability also was not affected. The EPSP-volley measured in wild-type (n = 7) and mutant mice (n = 7) were similar (P > 0.05). Finally (Fig 4c), we found that LTD was blocked by the NMDA receptor antagonist AP-5 (100 μM), indicating that this form of LTD depended on NMDA receptor activation.
Figure 4.
LTP, PTP, and PPF were not affected in calcineurin Aα mutant mice. (a) Representative recordings of the EPSP before and 50–60 min after strong-tetanic stimulation in wild-type (upper trace) and mutant (lower trace) mice. (b) LTP is normal in calcineurin Aα mutant mice. Strong tetanus (100 Hz, two 1-sec trains delivered at 20-sec intervals) induced long-lasting enhancement in wild-type (open squares, 196.7 ± 12.4%, n = 9 slices/6 mice, t = 7.80, P < 0.001 comparing the potential 50 to 60 min after tetanus to the average of the potential before tetanus) is similar to that in mutant mice (filled squares, 203.4 ± 19.6%, n = 6 slices/5 mice, t = 5.28, P < 0.01). (c) PTP and STP induced a single train tetanic stimulus (100 Hz, 1 sec) and are similar in wild-type and mutant mice. PTP in wild-type (first measurement after tetanic stimulation; 162.1 ± 10.7%, n = 8 slices/4 mice) and mutant (filled squares, 172.5 ± 12.4%, n = 8 slices/5 mice) mice were identical. (d) PPF in wild-type mice (open squares) is similar to that in mutant mice (filled squares). At a 50-msec interval when PPF is at its maximum, PPF was 144.9 ± 2.8% (wild-type, n = 11 slices/7 mice) and 142.5 ± 3.9% (mutant, n = 17 slices/9 mice). (e) Low-frequency stimulation (1 Hz, 15 min) failed to induce LTD in the CA1 region of the hippocampus in the presence of 100 μM AP-5, a NMDA receptor antagonist (n = 6, 114.2 ± 20.4% of control, no significant difference comparing the potential 25 to 30 min after stimulation to the average of the potential before stimulation). (Inset) Representative recordings of the EPSP before and 30 min after low-frequency stimulation in the presence of AP-5.
Because metabotropic glutamate receptors have been suggested to contribute to depotentiation (28), we also studied the function of metabotropic glutamate receptors in mutant mice. We therefore measured the effect of the selective mGluR agonist tACPD on basal synaptic transmission in both mutant and wild-type mice. One effect of tACPD on synaptic transmission is through inhibition of voltage-gated calcium channels by a G protein-coupled process (29). We found that tACPD (200 μM) produced similar inhibitory effects on synaptic transmission in mutant mice (n = 7; 49.0 ± 7.7% of control) and wild-type mice (n = 4; 55.6 ± 7.3% of control) (P > 0.05; not significantly different comparing the two groups).
DISCUSSION
In the present study, we have combined genetic and pharmacological approaches and have provided strong evidence that calcineurin Aα is required for the generation of depotentiation in the CA1 region of the hippocampus. These findings support previous reports that the mechanism of depotentiation is distinct from that of LTD (9). Although depotentiation requires calcineurin Aα, our pharmacological data suggest that LTD might require calcineurin Aβ. However, we cannot exclude the potential effects of pharmacological inhibitors on other target proteins.
Recent studies show that two different forms of LTD exist in the CA1 region of the hippocampus: NMDA receptor-dependent LTD, which is sensitive to inhibition of protein phosphatases, and NMDA receptor-independent LTD, which is resistant to protein phosphatase inhibitors (32). If LTD studied in the present report is an NMDA receptor-independent form of LTD, it could be easy to explain why LTD is not affected in calcineruin Aα-deficient mice. However, we found that LTD is blocked in the presence of 100 μM AP-5 (Fig. 4e). Therefore, it is clear that we are here concerned with an NMDA receptor-mediated form of LTD.
Previous pharmacological studies using selective inhibitors of the cAMP-dependent protein kinase A (PKA) and genetic studies using PKA RIβ- and Cβ1-deficient mice (23, 24) demonstrated that cAMP-PKA signaling pathway plays an important role in both LTD and depotentiation but not in early phase LTP (23, 24). The present results provide direct evidence that calcineurin Aα is required selectively for depotentiation but not LTD. Thus, these several genetic studies indicate that LTD and depotentiation have both shared a distinctive molecular component. Future studies are needed to investigate whether LTD and depotentiation play distinctive roles in physiological functions of the central nervous system.
Acknowledgments
This work was supported in part by grants from the National Institute on Aging, the National Institute of Mental Health, and the Howard Hughes Medical Institute.
ABBREVIATIONS
- LTP
long-term potentiation
- LTD
long-term depression
- EPSP
excitatory postsynaptic potential
- NMDA
N-methyl-d-aspartate
- PTP
post-tetanic potentiation
- PPF
paired-pulse facilitation
- PKA
protein kinase A
Footnotes
To whom reprint requests should be addressed. e-mail: erk5@columbia.edu.
References
- 1.Kuno K, Mukai H, Ito A, Chang C-D, Kishima K, Saito N, Tanaka C. J Neurochem. 1992;58:1643–1651. doi: 10.1111/j.1471-4159.1992.tb10036.x. [DOI] [PubMed] [Google Scholar]
- 2.Takaishi T, Saito N, Kuno T, Tanaka C. Biochem Biophys Res Commun. 1991;174:393–398. doi: 10.1016/0006-291x(91)90533-d. [DOI] [PubMed] [Google Scholar]
- 3.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 4.Nicoll R A, Malenka R C. Nature (London) 1995;377:115–118. doi: 10.1038/377115a0. [DOI] [PubMed] [Google Scholar]
- 5.Lisman J. Trends Neurosci. 1994;17:406–412. doi: 10.1016/0166-2236(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 6.Chen C, Tonegawa S. Annu Rev Neurosci. 1997;20:157–184. doi: 10.1146/annurev.neuro.20.1.157. [DOI] [PubMed] [Google Scholar]
- 7.Mulkey R M, Herron C E, Malenka R C. Science. 1993;261:1051–1055. doi: 10.1126/science.8394601. [DOI] [PubMed] [Google Scholar]
- 8.Mulkey R M, Endo S, Shenolikar S, Malenka R C. Nature (London) 1994;369:486–488. doi: 10.1038/369486a0. [DOI] [PubMed] [Google Scholar]
- 9.O’Dell T J, Kandel E R. Learn Mem. 1994;1:129–139. [PubMed] [Google Scholar]
- 10.Blitzer R D, Wong T, Nouranifar R, Iyengar R, Landau E M. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- 11.Iyengar R. Science. 1996;271:461–463. doi: 10.1126/science.271.5248.461. [DOI] [PubMed] [Google Scholar]
- 12.Wang J H, Stelzer A. Neuroreport. 1994;5:2377–2380. doi: 10.1097/00001756-199411000-00041. [DOI] [PubMed] [Google Scholar]
- 13.Lu Y F, Hayashi Y, Moriwaki A, Tomizawa K, Matsui H. Neurosci Lett. 1996;205:103–106. doi: 10.1016/0304-3940(96)12384-3. [DOI] [PubMed] [Google Scholar]
- 14.Lu Y F, Tomizawa K, Moriwaki A, Hayashi Y, Tokuda M, Itano T, Hatase O, Matsui H. Brain Res. 1996;729:142–146. [PubMed] [Google Scholar]
- 15.Steiner J P, Connolly M A, Valentine H L, Hamilton G S, Dawson T M, Hester L, Snyder S H. Nat Med. 1997;3:412–428. doi: 10.1038/nm0497-421. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Zimmer G, Chen J, Ladd D, Li E, Alt F W, Wiederrecht G, Cryan J, O’Neill E A, Seidman C E, et al. J Exp Med. 1996;183:413–420. doi: 10.1084/jem.183.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Usuda N, Arai H, Sasaki H, Hanai T, Nagata T, Muranatsu T, Kincaid R L, Higuchi S. J Histochem Cytochem. 1996;44:13–18. doi: 10.1177/44.1.8543776. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Wei. Ph.D. thesis. Cambridge, MA: Harvard Univ.; 1995. [Google Scholar]
- 19.Dudek S M, Bear M F. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodgkiss J P, Kelly J S. Brain Res. 1995;705:241–246. doi: 10.1016/0006-8993(95)01168-4. [DOI] [PubMed] [Google Scholar]
- 21.Mayford M, Wang J, Kandel E R, O’Dell T J. Cell. 1995;81:891–904. doi: 10.1016/0092-8674(95)90009-8. (1995). [DOI] [PubMed] [Google Scholar]
- 22.Staubli U, Chun D. J Neurosci. 1996;16:853–860. doi: 10.1523/JNEUROSCI.16-02-00853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brandon E P, Zhuo M, Huang Y-Y, Qi M, Gerhold K A, Burton K A, Kandel E R, McKnight G S, Idzerda R L. Proc Natl Acad Sci USA. 1995;92:8851–8855. doi: 10.1073/pnas.92.19.8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qi M, Zhuo M, Skalhegg B S, Brandon E P, Kandel E R, McKnight G S, Idzerda R L. Proc Natl Acad Sci USA. 1996;93:1571–1576. doi: 10.1073/pnas.93.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shenolikar S. Annu Rev Cell Biol. 1994;10:55–86. doi: 10.1146/annurev.cb.10.110194.000415. [DOI] [PubMed] [Google Scholar]
- 26.Lieberman D N, Mody I. Nature (London) 1994;369:235–239. doi: 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- 27.Tang G, Shepherd D, Jahr C E. Science. 1995;267:1510–1512. doi: 10.1126/science.7878472. [DOI] [PubMed] [Google Scholar]
- 28.Bashir Z I, Collingridge G L. Exp Brain Res. 1994;100:437–443. doi: 10.1007/BF02738403. [DOI] [PubMed] [Google Scholar]
- 29.Wheeler D B, Randall A, Tsien R W. Science. 1994;264:107–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- 30.Sobel R A, Kuchroo V K. J Immunol. 1992;149:1444–1451. [PubMed] [Google Scholar]
- 31.Winder D G, Mansuy I M, Osman M, Moallem T M, Kandel E R. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 32.Oliet S H R, Malenka R C, Nicoll R A. Neuron. 1997;18:969–982. doi: 10.1016/s0896-6273(00)80336-0. [DOI] [PubMed] [Google Scholar]