Abstract
We previously have reported corpus callosum defects in transgenic mice expressing the β-amyloid precursor protein (βAPP) with a deletion of exon 2 and at only 5% of normal levels. This finding indicates a possible involvement of βAPP in the regulation or guidance of axon growth during neural development. To determine to what degree the βAPP mutation interacts with genetic background alleles that predispose for forebrain commissure defects in some mouse lines, we have assessed the size of the forebrain commissures in a sample of 298 mice. Lines with mixed genetic background were compared with congenic lines obtained by backcrossing to the parental strains C57BL/6 and 129/SvEv. Mice bearing a null mutation of the βAPP gene also were included in the analysis. We show that, independently of genetic background, both lack and underexpression of βAPP are associated with reduced brain weight and reduced size of forebrain commissures, especially of the ventral hippocampal commissure. In addition, both mutations drastically increase the frequency and severity of callosal agenesis and hippocampal commissure defects in mouse lines with 129/SvEv or 129/Ola background.
Deficiency of the corpus callosum, ranging from a mere size reduction to total agenesis combined with defects of the ventral hippocampal commissure, occurs with varying frequencies in several mouse strains (1, 2). Cortical axons that fail to cross the midline gather in ectopic, longitudinal Probst bundles (3) and may connect to ipsilateral cortical targets (4). In the strains I/LnJ and RI-1 (5) all individuals are acallosal. Other strains show incomplete penetrance (6, 7). Depending on the substrain (8), 17–55% of BALB/c mice have a deficient corpus callosum and 2–21% have total agenesis. In 129/J and 129/ReJ mice, the relative frequencies are 71% and 77% for deficiency or 30% and 2% for total agenesis, respectively (6). In inbred 129/SvEv mice (unpublished data), we found 50% individuals with a deficient corpus callosum and 6% with total agenesis. In the strain ddN, only 8% have a defective corpus callosum. Crosses between different strains bearing callosal defects suggest recessive, autosomal multifactorial inheritance through two or three loci (1, 9–11). Environmental factors can modulate the penetrance of the genetic defects by a factor of two or more (8, 12). For example, penetrance can increase by a factor of 2.5 if BALB/c mothers are nursing a previous litter during pregnancy (13).
We recently have generated mice expressing βAPP in a mutated form (βAPPΔ) with a deletion of exon 2 (amino acids 20–75) and at only 5% of normal levels. These mice are viable and fertile, but show reduced open-field exploration and impaired acquisition of the Morris water-maze task (14). Their postnatal maturation is retarded with behavioral and neurological deficits emerging mainly in the second and third week (15). During initial anatomical characterization, we observed agenesis of the corpus callosum and formation of Probst bundles in seven of nine homozygous mutant individuals (βAPPΔ/Δ), compared with only one of eight wild-type controls (14). Later, another group found normal corpora callosa in mice homozygous for a null mutation of the βAPP gene (16). This finding raised the possibility that corpus callosum defects in βAPPΔ/Δ mice resulted from a toxic effect of the mutant protein product, rather than from the underexpression of βAPP. Furthermore, it was unclear to what degree environmental factors and genetic background had contributed to the phenotype of βAPPΔ/Δ mice. The necessity to consider possible influences of the genetic background on the phenotype of transgenic mice has been intensely discussed during the last few years (17–20).
Here, we present a systematic analysis of the forebrain commissures in a sample of 298 animals including βAPPΔ/Δ mutant mice, as well as βAPP0/0 mice bearing a complete deletion (βAPP0) of the βAPP gene (21) and respective littermate controls. Lines with mixed genetic background were compared with congenic lines obtained by backcrossing to the parental strains C57BL/6 and 129/SvEv.
MATERIALS AND METHODS
Animals.
The production of βAPPΔ/Δ (14) and βAPP0/0 (21) mutant mice has been described in detail. βAPPΔ/Δ mice were generated by using two different lines (D3 and GS) of 129/SvEv-derived embryonic stem (ES) cells. Because D3- and GS-derived mice are indistinguishable with respect to the variables discussed in this study, data obtained in the two lines were pooled. βAPP0/0 mice were produced by using E14.1 ES cells derived from the substrain 129/Ola. In all lines, ES cells with one engineered βAPP allele had been injected in C57BL/6 blastocysts, which resulted in transmitting chimeric males.
Five lines were analyzed in this study, three bearing the βAPPΔ mutation and two with the βAPP0 mutation. The mating of βAPPΔ/+ chimeras with 129/SvEv females resulted in a congenic line with 129/SvEv background (βAPPΔ/Δ Sv), whereas mating of 129/Ola-derived βAPP0/+ chimeras with 129/SvEv females gave rise to a line whose genetic background is a mixture of two 129 substrains (βAPP0/0 Sv-Ola). Lines with segregating mixed background (βAPPΔ/Δ B6-Sv and βAPP0/0 B6-Ola) were initiated by mating the respective chimeras with C57BL/6 females. The lines then were maintained by random mating of heterozygous offspring with a minimum of five breeding pairs per generation and avoiding littermate pairings wherever possible. Genotyping was carried out by PCR or Southern blot analysis as described (21). To prevent the formation of new recombinant inbred lines, mixed background lines were reinitiated after a maximum of four generations of interbreeding by mating mutant animals with 129 background to inbred C57BL/6 mice. A fifth line was obtained by backcrossing the βAPPΔ mutation into the C57BL/6 strain. After nine generations of backcrossing (≈ 99.9% C57BL/6 alleles), heterozygous offspring were interbred to obtain the sample designated here as βAPPΔ/Δ B6. In all lines, wild-type littermates (βAPP+/+) served as controls for the respective homozygous mutant mice (βAPPΔ/Δ and βAPP0/0). Heterozygous animals were not used because they had not shown mutation effects in earlier studies. We noted no gender effects in the variables reported here.
Inbred mice were from BRL Biological Research Laboratories, Füllinsdorf, Switzerland. Experimental animals were bred and housed in standard cages according to federal and local regulations under a light cycle of 12 hr with standard dry food pellets and water available ad libitum. In all lines breeding pairs usually were kept constantly together. Therefore, most litters were from mothers suckling a previous litter while pregnant.
Brain Weight and Frequency of Callosal Agenesis.
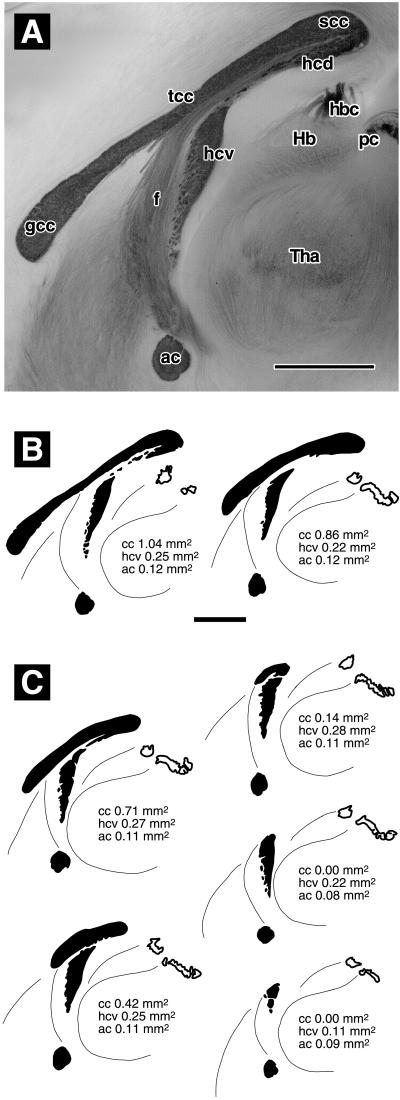
A total of 298 brains have been inspected over the past 4 years. Exact sample sizes are given in Table 1. Animals were killed by i.p. Nembutal or by exposure to carbon dioxide. Most had reached an age of 12–18 months, except for the βAPPΔ/Δ B6 mice that were about 18 weeks old. Dissected brains were split in the sagittal plane and fixed in 4% buffered paraformaldehyde. Part of the mice had been perfused transcardially with the same fixative. As in a previous report (14), the corpus callosum was examined by suspending brain halves from a cover glass in a drop of water and inspecting the sagittal cutting face by using a magnifying lens (12). Corpora callosa were compared with brains known to have a normal corpus callosum (Fig. 1 A and B) by a person who was blind to the genotype of the experimental animals. Based on their size and length, corpora callosa were assigned to one of three classes (Fig. 1C): normal, dyscallosal (shortened, reduced size), or acallosal (absent corpus callosum). Twenty-four brains were destined for stereology on coronal sections and could not be split sagittally. Their corpora callosa were classified by examination of a complete series of Giemsa-stained coronal sections. Examples of such sections are shown in Fig. 2. Brain weights (measured after fixation) were available from a total of 278 mice.
Table 1.
Effect of underexpression of βAPP on brain weight, corpus callosum defect frequencies, and forebrain commissure sizes in all five lines of transgenic mice
| Line | βAPPΔ/ΔB6 | βAPPΔ/ΔB6-Sv | βAPPΔ/ΔSv | βAPPΔ/ΔB6-Ola | βAPPΔ/ΔSv-Ola |
|---|---|---|---|---|---|
| Brain weight (g)178 | P < 0.0005 (n = 40) | P < 0.0030 (n = 68) | P < 0.0005 (n = 59) | P < 0.0030 (n = 37) | P < 0.0085 (n = 38) |
| Mutant (mean ± SE) | 0.415 ± 0.006 | 0.482 ± 0.006 | 0.436 ± 0.005 | 0.490 ± 0.007 | 0.478 ± 0.007 |
| Wild type (mean ± SE) | 0.455 ± 0.004 | 0.521 ± 0.009 | 0.490 ± 0.006 | 0.552 ± 0.016 | 0.519 ± 0.008 |
| Wild type - mutant† | 0.040 (9%) | 0.039 (8%) | 0.054 (12%) | 0.062 (12%) | 0.041 (8%) |
| Corpus callosum score‡ | ns(n = 40) | ns(n = 75) | P < 0.0005 (n = 108) | ns(n = 37) | P < 0.0015 (n = 37) |
| Mutant | n = 19 | n = 45 | n = 66 | n = 24 | n = 17 |
| Normal | 19 (100%) | 43 (95.6%) | 1 (1.5%) | 23 (95.8%) | 0 (0%) |
| Dyscallosal | 0 (0%) | 1 (2.2%) | 4 (6.1%) | 1 (4.2%) | 2 (11.1%) |
| Acallosal | 0 (0%) | 1 (2.2%) | 61 (92.4%) | 0 (0%) | 16 (88.9%) |
| Wild type | n = 21 | n = 30 | n = 42 | n = 13 | n = 20 |
| Normal | 21 (100%) | 30 (100%) | 28 (66.7%) | 13 (100%) | 6 (30.0%) |
| Dyscallosal | 0 (0%) | 0 (0%) | 8 (19%) | 0 (0%) | 9 (45.0%) |
| Acallosal | 0 (0%) | 0 (0%) | 6 (14.3%) | 0 (0%) | 5 (25.0%) |
| Corpus callosum, mm2178 | P < 0.0148 (n = 39) | P < 0.0472 (n = 58) | P < 0.0004 (n = 50) | (n = 10) | P < 0.0004 (n = 38) |
| Mutant (mean ± SE) | 1.090 ± 0.018 | 1.151 ± 0.034 | 0.000 ± 0.000 | 1.030 ± 0.104 | 0.043 ± 0.040 |
| Wild type (mean ± SE) | 1.171 ± 0.020 | 1.310 ± 0.045 | 0.678 ± 0.119 | — | 0.438 ± 0.930 |
| Wild type - mutant† | 0.081 (7%) | 0.159 (13%) | 0.678 (200%) | — | 0.395 (164%) |
| Adjusted to b.w. 0.476g:§ | |||||
| Mutant (mean ± SE) | 1.141 ± 0.018 | 1.144 ± 0.036 | 0.000 ± 0.000 | 1.034 ± 0.102 | 0.043 ± 0.036 |
| Wild type (mean ± SE) | 1.188 ± 0.020 | 1.270 ± 0.045 | 0.665 ± 0.117 | — | 0.413 ± 0.092 |
| Wild type - mutant† | 0.047 (4%) | 0.126 (10%) | 0.665 (200%) | — | 0.370 (162%) |
| Hipp. commissure, mm2178 | P < 0.0004 (n = 39) | P < 0.0064 (n = 58) | P < 0.0004 (n = 50) | (n = 10) | P < 0.0004 (n = 38) |
| Mutant (mean ± SE) | 0.259 ± 0.006 | 0.296 ± 0.007 | 0.121 ± 0.010 | 0.296 ± 0.015 | 0.133 ± 0.018 |
| Wild type (mean ± SE) | 0.304 ± 0.007 | 0.337 ± 0.008 | 0.291 ± 0.016 | — | 0.279 ± 0.016 |
| Wild type - mutant† | 0.045 (16%) | 0.041 (13%) | 0.170 (83%) | — | 0.146 (71%) |
| Adjusted to b.w. 0.476g:§ | |||||
| Mutant (mean ± SE) | 0.287 ± 0.004 | 0.292 ± 0.008 | 0.171 ± 0.010 | 0.297 ± 0.014 | 0.133 ± 0.018 |
| Wild type (mean ± SE) | 0.314 ± 0.006 | 0.317 ± 0.008 | 0.284 ± 0.015 | — | 0.257 ± 0.015 |
| Wild type - mutant† | 0.027 (9%) | 0.025 (8%) | 0.113 (50%) | — | 0.124 (64%) |
| Ant. commissure, mm2178 | P < 0.0080 (n = 39) | ns(n = 57) | P < 0.0020 (n = 50) | (n = 10) | P < 0.0008 (n = 38) |
| Mutant (mean ± SE) | 0.118 ± 0.003 | 0.134 ± 0.004 | 0.096 ± 0.002 | 0.123 ± 0.007 | 0.100 ± 0.003 |
| Wild type (mean ± SE) | 0.132 ± 0.003 | 0.144 ± 0.004 | 0.119 ± 0.005 | — | 0.116 ± 0.003 |
| Wild type - mutant† | 0.014 (11%) | 0.010 (7%) | 0.023 (21%) | — | 0.016 (15%) |
| Adjusted to b.w. 0.476g:§ | |||||
| Mutant (mean ± SE) | 0.126 ± 0.002 | 0.134 ± 0.004 | 0.100 ± 0.002 | 0.124 ± 0.006 | 0.099 ± 0.003 |
| Wild type (mean ± SE) | 0.134 ± 0.003 | 0.139 ± 0.004 | 0.119 ± 0.005 | — | 0.112 ± 0.003 |
| Wild type - mutant† | 0.008 (6%) | 0.005 (4%) | 0.019 (17%) | — | 0.013 (12%) |
Mann–Whitney U with Bonferroni correction for multiple comparisons.
Percentual differences are shown in parentheses and were computed by using the formula 200 (Xwild−Xmut)/(Xwild+Xmut).
χ2 with Bonferroni correction for multiple comparisons.
Commissure sizes were adjusted to an average brain weight of 0.476 g by using linear regression. For details see Materials and Methods.
Figure 1.
Classification of forebrain commissures in brains that were split in the midsagittal plane and stained with gold chloride. (A) C57BL/6 mouse with normal commissures. The anterior (ac) and ventral hippocampal (hcv) commissures are darkly stained, as are the genu (gcc), truncus (tcc), and splenium (scc) of the corpus callosum. There is no clear boundary between the dorsal hippocampal commissure (hcd) and the splenium. Fibers of the fornix (f) stain more weakly. They emerge along the ventral surface of the truncus, then run ventrally and rostrally parallel to the sectioning plane. Hb, Habenula; hbc, habenular commissure; pc, posterior commissure; Tha, thalamus. (B) Representative camera lucida drawings of commissures classified as normal: (Left) C57BL/6 (same brain as in A), (Right) a wild type of line βAPPΔ/Δ Sv. (C) Representative camera lucida drawings of commissures classified as abnormal. From top left to bottom right: slightly reduced corpus callosum (cc), strongly reduced corpus callosum, rudimentary corpus callosum, absent corpus callosum with still normal ventral hippocampal commissure, and absent corpus callosum with reduced ventral hippocampal commissure. (Bar in A = 1 mm. Bar in B = 1 mm, applies to B and C.) See text for details.
Figure 2.
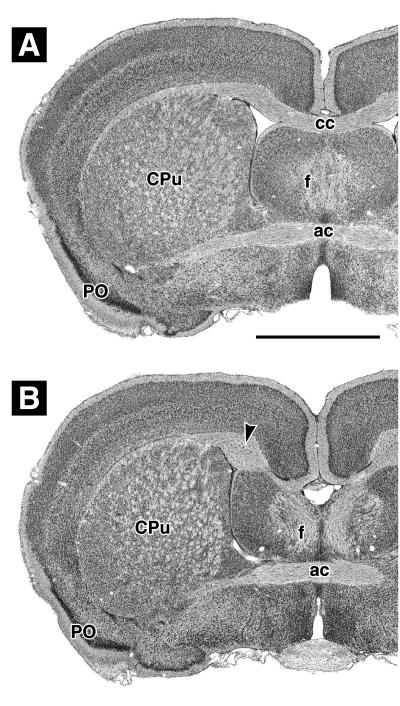
Cresyl violet-stained coronal sections through plastic-embedded brains, taken at a level showing the anterior commissure (ac), fornix columns (f), caudatoputamen (CPu), and primary olfactory cortex (PO). (A) βAPPΔ/Δ B6-Sv mouse with normal corpus callosum. (B) βAPPΔ/Δ Sv mouse with missing corpus callosum and well-developed Probst bundles (arrowhead). (Bar in A = 1 mm, applies to A and B.) See text for details.
Morphometry of Forebrain Commissures.
For this report, we have re-examined a subsample of 195 brains that had been stored in 4% buffered formalin at 4°C. Myelinated fiber tracts were stained by immersing the hemispheres in a gold chloride solution (22) according to a protocol modified by D. Wahlsten (University of Alberta, Edmonton, Canada). Images of the sagittal cutting face (Fig. 1A) were digitized by using a VIDEK Megaplus charge-coupled device camera equipped with a 50-mm Nikon lens and a macro extension tube. Images were optimized in Adobe Photoshop 4.0 for contrast and overlaid with quadratic counting grids for estimation of the cross section areas of the corpus callosum, ventral hippocampal, and anterior commissure. Because, especially in dyscallosal brains, the corpus callosum and the dorsal hippocampal commissure are difficult to separate, the two were measured together (12). Based on form factor and nugget variance estimates (23), appropriate point densities resulting in estimated coefficients of error around 5% were chosen for each individual commissure.
Statistical Analysis.
Areas and brain weights were compared by using the Mann–Whitney U test and adjusting P values by using the Bonferroni–Dunn correction for multiple comparisons. Two-way ANOVA with genotype and background as factors served to assess the influence of genetic background on mutation effects. The βAPPΔ and βAPP0 mutation effects were compared in a similar design with mutation and genotype as factors. Corpus callosum class frequencies (normal, reduced, and acallosal) were compared by using McNemar’s χ2, again applying the Bonferroni–Dunn correction. To validate the classification based on visual examination of the corpus callosum, 195 brains were reclassified by using the morphometrical data. They were classified as dyscallosal if the length of the corpus callosum was less than 3 mm and its area less than 0.85 mm2 (7–9, 12, 13, 24). Visual and morphometry-based classifications were almost identical and statistically indistinguishable.
Because commissure size correlates with brain weight, we recomputed statistics after normalizing commissure sizes to an average brain weight of 0.476 g by using linear regression (25). Because the regression slopes did not differ significantly between genotypes, respective group means were subtracted from brain weights, and commissure sizes and regression slopes were computed by using pooled data of all animals with a corpus callosum equal to or larger than 0.85 mm2. Corrected size values (Table 1) were obtained by using the formula area − b (weight − 0.476 g) with the following values for the slope b: anterior commissure, 0.0102 mm2/g; ventral hippocampal commissure, 0.4486 mm2/g; and corpus callosum, 0.8391 mm2/g. Commissure size also varies with age, and young adult mice may have smaller corpora callosa than 1-year-old mice for which classification criteria were designed (24). We did not apply age corrections, however, because all individuals younger than 250 days belonged to the βAPPΔ/Δ B6 line and already were classified as normal without age correction.
RESULTS
Brain Weight.
Independently of genetic background both underexpression of βAPP in a mutant form (βAPPΔ/Δ) and complete knock-out of βAPP (βAPP0/0) reduce adult brain weight by approximately 10%.
As shown in Table 1, multiple Mann–Whitney U tests detected significant genotype differences in all five lines examined. To assess the influence of background alleles, we compared the effect of the βAPPΔ mutation in the three lines (βAPPΔ/Δ B6, βAPPΔ/Δ B6-Sv, and βAPPΔ/Δ Sv) by using two-way ANOVA. The lines differed with respect to brain weight [line F(2,161) = 51.8, P < 0.0001], but the effect of genotype on brain weight was statistically independent of this line effect [genotype F(1,161) = 66.7, P < 0.0001, interaction not significant (ns)], indicating that the mutation effect was not influenced by the genetic background. A similar result was found when comparing the βAPP0 mutation effect in the lines βAPP0/0 B6-Ola and βAPP0/0 Sv-Ola [genotype F(1,71) = 24.0, P < 0.0001; line F(1,71) = 11.1, P < 0.0014, interaction ns]. We also compared the effects of the βAPPΔ and βAPP0 mutations in the respective lines with mixed background and found that the two mutations affected brain weight to the same extent, even though average brain weight differed slightly between the two lines [genotype F(1,101) = 31.7, P < 0.0001; mutation F(1,101) = 4.7, P < 0.0328, interaction ns].
Corpus Callosum.
The βAPPΔ and βAPP0 mutations both increase the frequency of callosal dysgenesis and agenesis in the lines whose background alleles are exclusively derived from the 129 strain. In the lines whose background on average contains 50% or more C57BL/6 alleles, they are associated with a size reduction that is more modest but about twice as severe as predicted by the brain weight reduction.
Defect frequencies are summarized in Table 1. We first examined the wild-type individuals of all five lines. In the congenic line with C57BL/6 background (βAPPΔ/Δ B6) and in the two mixed background lines (βAPPΔ/Δ B6-Sv and βAPP0/0 B6-Ola) all wild types had a normal corpus callosum, while, as expected, dyscallosal and acallosal wild-type individuals were found in both lines with 129 background (βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola). Compared with the βAPPΔ/Δ Sv line, the frequency of wild-type dyscallosal and acallosal animals was somewhat higher in the βAPP0/0 Sv-Ola line (χ2 = 7.5, df = 2, P < 0.0232). Subsequent examination of mutant animals revealed that the mutation effects were line dependent. In the lines with 129 background (βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola) all but one of the mutants were dyscallosal or acallosal (Fig. 2B), resulting in a highly significant statistical difference compared with the frequency in the respective controls. By contrast, all congenic βAPPΔ/Δ B6 mutants and all but three βAPPΔ/Δ B6-Sv and βAPPΔ/Δ B6-Ola mutants had normal corpus callosum morphology (Fig. 2A). Two dyscallosal and one acallosal individual were observed, but according to χ2 analysis these frequencies were not statistically different from controls. Mutant brains had no additional malformations, but acallosal animals often appeared to also have a smaller ventral hippocampal commissure (see below).
As shown in Table 1, multiple Mann–Whitney U tests on the morphometrically determined corpus callosum sizes confirmed the above mutation effects and, in addition, revealed a significant 7–13% size reduction in lines βAPPΔ/Δ B6-Sv and βAPPΔ/Δ B6. Wondering whether this reduction was a secondary consequence of the brain weight difference, we adjusted commissure size to an average brain weight of 0.476 g by linear regression and found a residual difference of 4–11%. The residual effect was no longer detected by multiple Mann–Whitney U tests but remained significant according to more powerful two-way ANOVA [genotype F(1,93) = 5.6, P < 0.0195, line ns, interaction ns].
Ventral Hippocampal Commissure.
Both the βAPPΔ and βAPP0 mutations entail a massive size reduction of the ventral hippocampal commissure in the lines whose background alleles are exclusively derived from the 129 strain. In the other lines, the size reduction is more modest but still at least twice as severe as predicted by the brain weight difference.
As shown in Table 1, both mutations entailed significant size reductions of the ventral hippocampal commissure: 71% and 83% in the lines with 129 background (βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola), 13% and 16% in lines βAPPΔ/Δ B6 and βAPPΔ/Δ B6-Sv. No controls were available in line βAPP0/0 B6-Ola, but the size of the ventral hippocampal commissure in mutants of that line was the same as in mutants of line βAPPΔ/Δ B6-Sv. To assess the influence of background alleles, we compared the βAPPΔ mutation effect in the lines βAPPΔ/Δ B6, βAPPΔ/Δ B6-Sv, and βAPPΔ/Δ Sv by using two-way ANOVA. We found a highly significant interaction between genotype and line confirming that the line with pure 129/SvEv background (βAPPΔ/Δ Sv) was more severely affected than the other lines [genotype F(1,141) = 109.8, P < 0.0001; line F(2,141) = 96.2, P < 0.0001; interaction F(2,141) = 27.5 P < 0.0001]. We also compared the effects of the βAPPΔ and βAPP0 mutations in the respective lines whose background alleles all were derived from the 129 strain (βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola) and found that ventral hippocampal commissure size was reduced to the same extent in both lines [genotype F(1,84) = 111.6, P < 0.0001, mutation ns, interaction ns]. In general, hippocampal commissures smaller than 0.12 mm2 were observed only in acallosal mice. Interestingly, acallosal mutants on average had a 60% smaller ventral hippocampal commissure than wild-type acallosal mice. This finding was true for both the βAPPΔ and βAPP0 mutation as indicated by two-way ANOVA analysis [genotype F(1,53) = 17.2, P < 0.0001, mutation ns, interaction ns].
When ventral hippocampal commissure size was adjusted to an average brain weight of 0.476 g by linear regression, the mutation effect was reduced to 50% and 64% in lines βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola, respectively, and to 9% and 8% in lines βAPPΔ/Δ B6 and βAPPΔ/Δ B6-Sv, respectively. Multiple Mann–Whitney U tests on adjusted sizes still detected a significant difference in all lines, except in βAPPΔ/Δ B6-Sv. According to two-way ANOVA, the residual effect was identical in lines βAPPΔ/Δ B6 and βAPPΔ/Δ B6-Sv [genotype F(93,1) = 11.1, P < 0.0012, line ns, interaction ns], but still larger in line βAPPΔ/Δ Sv [genotype F(141,1) = 71.6, P < 0.0001; line F(141,2) = 62.9, P < 0.0001; interaction F(141,2) = 26.5, P < 0.0001].
Anterior Commissure.
Underexpression or lack of βAPP entails a size reduction of the anterior commissure. The effect is twice as large as predicted by the brain weight difference, and evidence for a modulation by genetic background is lacking.
The βAPPΔ and βAPP0 mutations entailed size reductions between 7% and 21%, and multiple Mann–Whitney U tests detected significant genotype effects in all lines except βAPPΔ/Δ B6-Sv (Table 1). Two-way ANOVA comparison of lines βAPPΔ/Δ B6, βAPPΔ/Δ B6-Sv, and βAPPΔ/Δ Sv revealed no line dependence of the βAPPΔ mutation effect even though the three lines differed with respect to anterior commissure size [genotype F(1,140) = 23.8, P < 0.0001; line F(2,140) = 34.0 P, < 0.0001, interaction ns]. The βAPPΔ and βAPP0 mutations had indistinguishable effects as indicated by two-way ANOVA comparison of lines βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola [genotype F(1,84) = 39.7, P < 0.0001, mutation ns, interaction ns].
Adjusting commissure size to an average brain weight of 0.476 g by linear regression did not remove the genotype difference, but reduced it to 4–17%. Multiple Mann–Whitney U tests on adjusted sizes detected a significant effect only in lines βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola. To increase statistical power, we recomputed two-way ANOVA by using the adjusted sizes and obtained essentially the same result as with uncorrected values, both for the comparison of lines βAPPΔ/Δ B6, βAPPΔ/Δ B6-Sv, and βAPPΔ/Δ Sv [genotype F(1,140) = 12.4, P < 0.0006; line F(2,140) = 27.1, P < 0.0001, interaction ns] and for the comparison of the βAPPΔ and βAPP0 mutations [genotype F(1,84) = 27.3, P < 0.0001, mutation ns, interaction ns].
DISCUSSION
The βAPPΔ and βAPP0 Mutations Have Identical Effects.
βAPPΔ/Δ mice show 5% residual expression of a mutated βAPP, whereas βAPP0/0 mice lack βAPP completely. Because the two mutations result in identical reductions of brain weight and forebrain commissure size, the βAPPΔ mutation must be considered a functional knockout with respect to these variables. Moreover, our results argue against a toxic effect of the truncated βAPPΔ protein. Zheng et al. (16), who independently generated a line of βAPP−/− mice, reported no callosal defects (see Introduction). Because their animals had a mixed 129/SvEv × C57BL/6 background, their finding is in line with the results of the present study. The view that the βAPPΔ and βAPP0 mutations have similar effects gains additional support from our recent finding that the susceptibility to kainate-induced seizures is increased to a similar extent in both βAPPΔ/Δ and βAPP0/0 mice (26).
The Mutations Reduce Brain Weight Independently of Genetic Background.
Regardless of genetic background, underexpression of βAPP results in reduced brain weight. By contrast, we found no consistent reduction of body weight, except for line βAPP0/0 Sv-Ola in which mutants were significantly lighter than wild-type animals (data not shown). This finding is in accordance with our earlier observation that homozygous βAPPΔ/Δ B6-Sv mice show retarded gain of body weight during postnatal development, but then catch up as adults (15).
A reduction of adult brain size could result from degenerative processes or from a prenatal and/or postnatal developmental deficit. Degenerative processes appear unlikely because the mutation effect was the same in young adult βAPPΔ/Δ B6 mice and in 12- to 18-month-old βAPPΔ/Δ B6-Sv mice. A deficit in postnatal brain maturation is the most likely explanation given the absence of a difference in brain weight between newborn βAPPΔ/Δ mice and wild-type littermates (unpublished data). This view gains support from a study that monitored postnatal neurological and behavioral maturation in βAPPΔ/Δ mice. It found overall normal development during the first postnatal week, but significant deficits emerged during the second and third weeks (15).
Independently of genetic background, mutants had smaller anterior commissures than wild types. In addition, mutants of lines with B6 or mixed background had smaller ventral hippocampal commissures and corpora callosa, even if they did not suffer from callosal dysgenesis. Because the size of forebrain commissures correlates with brain weight (25), it is not surprising to find smaller commissures in mutants with smaller brains. Regression analysis revealed, however, that the mutation effect on commissure size was at least twice as large as predicted by the difference in overall brain weight. Such a discrepancy could result from a selective effect of the mutation on neuron populations contributing fibers to the commissures and/or from a shift in the size distribution of commissural fibers. These possibilities can be evaluated by morphometrical studies assessing volume and neuron number of involved brain areas, as well as number and diameter of commissural fibers. Volumetric pilot data indicate that the reduction of brain volume is not proportional, but spares the cerebellum and may preferentially affect the forebrain, with the largest size differences being observed in the hippocampal formation.
The Mutations Interact with Background Alleles of the 129 Strain.
Because none of 19 βAPPΔ/Δ B6 brains lacked a corpus callosum, underexpression of βAPP alone is not sufficient to induce callosal agenesis. On the other hand, it increased the frequency of corpus callosum defects to almost 100% penetrance in the lines βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola whose genetic background consists entirely of alleles from 129 strains. As outlined in the Introduction, 129 strains are known for spontaneous occurrence of corpus callosum defects, which show incomplete penetrance and are attributed to recessive multifactorial inheritance. Despite the fact that many litters were from mothers suckling a previous litter while pregnant, the defect frequency in wild-type mice of lines βAPPΔ/Δ Sv and βAPP0/0 Sv-Ola were not above the expected range. In the former, it was similar to the frequency we have found previously in inbred 129/SvEv mice obtained directly from the supplier (unpublished data, see Introduction). In the latter, it resembled that reported in the literature for the substrain 129/J to which 129/Ola are related (27). By contrast, near complete penetrance as found in the mutants of both lines has never been reported in any 129 substrain. Underexpression of βAPP not only boosted penetrance, but also increased the severity of the defect as evidenced by the 60% smaller ventral hippocampal commissures of mutant, as compared with wild-type acallosal mice. The idea that underexpression of βAPP enhances the effect of predisposing alleles in the 129 background is also compatible with our observation of occasional acallosal mutants in the lines βAPPΔ/Δ B6-Sv and βAPP0/0 B6-Ola with a segregating B6 × 129 background.
During the initial characterization of βAPPΔ/Δ mice (14), we found a markedly increased frequency of callosal agenesis in mutant animals with mixed background, even though their background was nominally the same as in line βAPPΔ/Δ B6-Sv of the present study. The reason for this discrepancy remains unclear. However, in view of the strong background dependence of this mutation effect, genetic drift of background alleles appears as the most likely explanation. Because of the small number of individuals and breeding pairs available for the initial characterization, genetic drift may have led to a rapid loss of C57BL/6 background alleles that are critical for corpus callosum development. Alternatively, penetrance may have been increased by environmental factors, such as large litter size, short intervals between subsequent litters, or asymptomatic infections during pregnancy.
The Mutations May Affect Commissural Axons or Midline Structures.
The developmental mechanisms causing the defects of the corpus callosum and hippocampal commissure in the 129 strains are not completely understood. The growth schedule of callosal axons before arrival at the midline is normal, indicating that the problem resides in the substrates of axon guidance at the midline (28, 29). Formation of the hippocampal commissure is retarded in embryos from mouse strains lacking a corpus callosum, and it has been suggested that callosal agenesis arises as a consequence of a developmental defect, which affects the formation of the hippocampal commissure before the arrival of callosal axons at midplane (5). The delayed closure of the midline cleft in the septal region is a major factor for the retarded formation of the hippocampal commissure. Although some early callosal axons fasciculate with the hippocampal commissure, their role in the guidance of subsequent callosal axons is not clear. A transitory glial sling (30) and specialized astroglia (31) also appear to play a role in the guidance of callosal axons. Thus, the successful formation of the corpus callosum and hippocampal commissure depends on the correct timing between the growth schedule of the crossing axons and the differentiation of midline structures. This requirement may render callosal development particularly vulnerable to genetic modifications. In fact, increased frequency of corpus callosum defects is not only observed in several targeted mutations of genes directly involved in axon guidance at the midline (32–34), but also in several mutations of widely expressed genes—mutations whose effects would not a priori be expected in forebrain commissure development (35–38).
βAPP, which is also widely expressed, may be involved in molecular interactions with one of the factors predisposing to commissure defects in the 129 strains. Alternatively, it may disturb developmental timing through an independent effect, to a degree that can be compensated as long as other disturbing factors are absent. Future studies should assess the precise developmental expression pattern of βAPP in the involved structures and should reveal whether underexpression of βAPP delays closure of the midline cleft, affects the differentiation of midline substrata, or alters the growth schedule or substratum responses of callosal and hippocampal commissural axons.
Acknowledgments
Rosmarie Lang and Inger Drescher provided valuable help with animal breeding and dissection of mouse brains. This work is part of a Ph.D. thesis supported by the Human Frontier Science Program and by Swiss National Science Foundation Grant 31–42347.94.
ABBREVIATIONS
- βAPP
β-amyloid-precursor protein
- ns
not significant
References
- 1.Livy D J, Wahlsten D. J Hered. 1991;82:459–464. doi: 10.1093/oxfordjournals.jhered.a111128. [DOI] [PubMed] [Google Scholar]
- 2.Wahlsten D. Brain Res. 1974;68:1–18. doi: 10.1016/0006-8993(74)90530-7. [DOI] [PubMed] [Google Scholar]
- 3.Probst M. Arch Psychiatr Nervenkr. 1901;34:709–786. [Google Scholar]
- 4.Ozaki H S, Shimada M. Brain Res. 1988;441:5–14. doi: 10.1016/0006-8993(88)91377-7. [DOI] [PubMed] [Google Scholar]
- 5.Livy D J, Wahlsten D. Hippocampus. 1997;7:2–14. doi: 10.1002/(SICI)1098-1063(1997)7:1<2::AID-HIPO2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Lipp H-P, Wahlsten D. In: Absence of the Corpus Callosum. Driscoll P, editor. Basel: Birkhäuser; 1992. pp. 217–252. [Google Scholar]
- 7.Wahlsten D. Experientia. 1989;45:828–838. doi: 10.1007/BF01954057. [DOI] [PubMed] [Google Scholar]
- 8.Wahlsten D. J Neurogenet. 1989;5:61–76. doi: 10.3109/01677068909167265. [DOI] [PubMed] [Google Scholar]
- 9.Wahlsten D, Smith G. J Hered. 1989;80:11–16. doi: 10.1093/oxfordjournals.jhered.a110781. [DOI] [PubMed] [Google Scholar]
- 10.Wahlsten D, Ozaki H S, Livy D J. Neurosci Lett. 1992;136:99–101. doi: 10.1016/0304-3940(92)90657-s. [DOI] [PubMed] [Google Scholar]
- 11.Wahlsten D, Schalomon P M. Behav Brain Res. 1994;64:111–117. doi: 10.1016/0166-4328(94)90123-6. [DOI] [PubMed] [Google Scholar]
- 12.Wahlsten D. Brain Res. 1982;239:329–347. doi: 10.1016/0006-8993(82)90513-3. [DOI] [PubMed] [Google Scholar]
- 13.Wahlsten D. Dev Brain Res. 1982;5:354–357. doi: 10.1016/0165-3806(82)90135-3. [DOI] [PubMed] [Google Scholar]
- 14.Müller U, Cristina N, Li Z-W, Wolfer D P, Lipp H-P, Rülicke T, Brandner S, Aguzzi A, Weissmann C. Cell. 1994;79:755–765. doi: 10.1016/0092-8674(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 15.Tremml P, Lipp H-P, Müller U, Ricceri L, Wolfer D P. Behav Brain Res. 1998;95:65–76. doi: 10.1016/s0166-4328(97)00211-8. [DOI] [PubMed] [Google Scholar]
- 16.Zheng H, Jiang M, Trumbauer M E, Sirinathsinghji D J S, Hopkins R, Smith D W, Heavens R P, Dawson G R, Boyce S, Conner M W, et al. Cell. 1995;81:525–531. doi: 10.1016/0092-8674(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 17.Grant S G N, Silva A J. Trends Neurosci. 1994;17:71–75. doi: 10.1016/0166-2236(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 18.Gerlai R. Trends Neurosci. 1996;19:177–181. doi: 10.1016/s0166-2236(96)20020-7. [DOI] [PubMed] [Google Scholar]
- 19.Silva A J, Simpson E M, Takahashi J S, Lipp H-P, Nakanishi S, Wehner J M, Giese K P, Tully T, Abel T, Chapman P F, et al. Neuron. 1997;19:755–759. [Google Scholar]
- 20.Lipp H-P, Wolfer D P. Curr Opin Neurobiol. 1998;8:272–280. doi: 10.1016/s0959-4388(98)80151-7. [DOI] [PubMed] [Google Scholar]
- 21.Li Z-W, Stark G, Götz J, Rülicke T, Müller U, Weissmann C. Proc Natl Acad Sci USA. 1996;93:6158–6162. doi: 10.1073/pnas.93.12.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmued L C. J Histochem Cytochem. 1990;38:717–720. doi: 10.1177/38.5.1692056. [DOI] [PubMed] [Google Scholar]
- 23.Gundersen H J G, Jensen E B. J Microsc (Oxford) 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 24.Wahlsten D. J Hered. 1982;73:281–285. doi: 10.1093/oxfordjournals.jhered.a109640. [DOI] [PubMed] [Google Scholar]
- 25.Bishop K M, Wahlsten D. Brain Res. 1998;815:358–366. doi: 10.1016/s0006-8993(98)01088-9. [DOI] [PubMed] [Google Scholar]
- 26.Steinbach J P, Müller U, Leist M, Li Z-W, Nicotera P, Aguzzi A. Cell Death Diff. 1998;5:858–866. doi: 10.1038/sj.cdd.4400391. [DOI] [PubMed] [Google Scholar]
- 27.Simpson E M, Linder C C, Sargent E E, Davisson M T, Mobraaten L E, Sharp J J. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- 28.Ozaki H S, Wahlsten D. J Comp Neurol. 1992;323:81–90. doi: 10.1002/cne.903230107. [DOI] [PubMed] [Google Scholar]
- 29.Ozaki H S, Wahlsten D. J Comp Neurol. 1993;336:595–604. doi: 10.1002/cne.903360411. [DOI] [PubMed] [Google Scholar]
- 30.Silver J, Lorenz S E, Wahlsten D, Coughlin J. J Comp Neurol. 1982;210:10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- 31.Silver J, Edwards M A, Levitt P. J Comp Neurol. 1993;328:415–436. doi: 10.1002/cne.903280308. [DOI] [PubMed] [Google Scholar]
- 32.Orioli D, Henkemeyer M, Lemke G, Klein R, Pawson T. EMBO J. 1998;15:6035–6049. [PMC free article] [PubMed] [Google Scholar]
- 33.Serafini T, Colamarino S A, Leonardo E D, Wang H, Beddington R, Skarnes W C, Tessierlavigne M. Cell. 1996;87:1001–1014. doi: 10.1016/s0092-8674(00)81795-x. [DOI] [PubMed] [Google Scholar]
- 34.Fazeli A, Dickinson S L, Hermiston M L, Tighe R V, Steen R G, Small C G, Stoeckli E T, Keino-Masu K, Masu M, Rayburn H, et al. Nature (London) 1997;386:796–804. doi: 10.1038/386796a0. [DOI] [PubMed] [Google Scholar]
- 35.Wu M, Chen D F, Sasaoka T, Tonegawa S. Proc Natl Acad Sci USA. 1996;93:2110–2115. doi: 10.1073/pnas.93.5.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stumpo D J, Bock C B, Tuttle J S, Blackshear P J. Proc Natl Acad Sci USA. 1995;92:944–948. doi: 10.1073/pnas.92.4.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu M, Anderson S, Chen S, Meneses J J, Hevner R, Kuwana E, Pedersen R A, Rubenstein J L. Dev Biol. 1996;178:174–178. doi: 10.1006/dbio.1996.0207. [DOI] [PubMed] [Google Scholar]
- 38.Tafuri A, Gass P, Hammerling G, Arnold B, Schütz G. Proc Natl Acad Sci USA. 1998;95:4481–4486. doi: 10.1073/pnas.95.8.4481. [DOI] [PMC free article] [PubMed] [Google Scholar]