Abstract
Mechanisms leading to down-regulation of activated microglia and astrocytes are poorly understood, in spite of the potentially detrimental role of activated glia in neurodegeneration. Prostaglandins, produced both by neurons and glia, may serve as mediators of glial and neuronal functions. We examined the influence of cyclopentenone prostaglandins and their precursors on activated glia. As models of glial activation, production of inducible nitric-oxide synthase (iNOS) was studied in lipopolysaccharide-stimulated rat microglia, a murine microglial cell line BV-2, and IL-1β-stimulated rat astrocytes. Cyclopentenone prostaglandins were potent inhibitors of iNOS induction and were more effective than their precursors, prostaglandins E2 and D2. 15-Deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) was the most potent prostaglandin among those tested. In activated microglia, 15d-PGJ2 suppressed iNOS promoter activity, iNOS mRNA, and protein levels. The action of 15d-PGJ2 does not appear to involve its nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) because troglitazone, a specific ligand of PPARγ, was unable to inhibit iNOS induction, and neither troglitazone nor 15d-PGJ2 could stimulate the activity of a PPAR-dependent promoter in the absence of cotransfected PPARγ. 15d-PGJ2 did not block nuclear translocation or DNA-binding activity of the transcription factor NFκB, but it did inhibit the activity of an NFκB reporter construct, suggesting that the mechanism of suppression of microglial iNOS by 15d-PGJ2 may involve interference with NFκB transcriptional activity in the nucleus. Thus, our data suggest the existence of a novel pathway mediated by cyclopentenone prostaglandins, which may represent part of a feedback mechanism leading to the cessation of inflammatory glial responses in the brain.
An increased number of reactive astrocytes and activated microglia is one of the prominent features of Alzheimer’s disease (AD) pathology (1–3). Moreover, the idea of active glial participation in the progression of the disease is gaining increasing attention (reviewed in ref. 4). Chronic glial activation as seen in AD may be directly related to a propagation of neuroinflammatory events and oxidative stress because glial activation leads to the induction of proinflammatory cytokines such as IL-1, as well as the augmented production of neurotoxic free radicals, mediated in part by the increased activity of inducible nitric-oxide synthase (iNOS). The resulting neuronal damage consequently can lead to still further glial activation and, thus, a self-propagation of a neurodegenerative “cytokine cycle” (4). Obviously, an increased understanding of the mechanisms regulating glial activation is of significant biomedical importance. In particular, elucidating the pathways involved in the down-regulation of activated glia may offer new drug discovery targets for treatment of AD as well as other neurodegenerative diseases.
The importance of understanding the chronic inflammatory processes observed in neurodegeneration is strengthened further by clinical and epidemiological studies that demonstrate that the use of antiinflammatory compounds such as nonsteroidal antiinflammatory drugs reduces the incidence of AD (5, 6). These results also raise the question of the role of cyclooxygenases and their products, prostaglandins, in the development of AD. Here, we have investigated the influence of two major prostaglandins, PGE2 and PGD2, and their cyclopentenone derivatives, PGA2, 15-deoxy-Δ12,14-prostaglandin A2 (15d-PGA2), PGJ2, and 15-deoxy-Δ12,14-prostaglandin J2 (15d-PGJ2) on the production of iNOS in activated glia.
Various inflammatory mediators, such as endotoxins, cytokines, and AD-associated peptides such as amyloid β, can activate glia and induce iNOS expression both in vitro and in vivo (7–9). The expression of iNOS also is detected in activated glia from AD brain (10). Our focus here is on the signaling mechanisms involved in iNOS induction in endotoxin-stimulated microglia and astrocytes. We report that cyclopentenone prostaglandins, notably 15d-PGJ2, suppress the production of iNOS in activated microglia and astrocytes. Moreover, we demonstrate that 15d-PGJ2 may inhibit iNOS and, potentially, other NFκB-dependent transcriptional activities via a novel pathway not related to the inhibition of nuclear translocation of NFκB or the activation of the nuclear receptor for 15d-PGJ2, peroxisome proliferator-activated receptor γ (PPARγ).
MATERIALS AND METHODS
Reagents.
Prostaglandins were from Cayman Chemicals (Ann Arbor, MI). PGE2 and PGD2 were prepared from powder as ethanol solutions. PGA2, PGJ2, 15d-PGJ2, and 15d-PGA2 were supplied in methyl acetate. Before the experiments, methyl acetate solutions were evaporated in a SpeedVac concentrator and the resulting clear oil was dissolved in absolute ethanol. Troglitazone was provided by Parke-Davis and dissolved in cell culture-grade DMSO (Sigma). Lipopolysaccharide (LPS) from Salmonella typhimurium (Sigma) was resuspended in sterile PBS and stored at −20°C. Rat IL-1β (R&D) was dissolved in PBS containing 0.1% BSA and stored in aliquots at −80°C.
Plasmids.
The PPRE-luciferase construct containing three PPAR-responsive elements (PPREs) cloned upstream of thymidine kinase promoter, the mouse PPARγ expression vector (11), and control vectors were generously provided by J. K. Reddy (Department of Pathology, Northwestern University Medical School). 3xRel-luc plasmid, containing three NFκB consensus sequences, was described previously (9). The luciferase reporter construct pGLH/H2, containing the murine iNOS promoter (12), was a gift from W. J. Murphy (University of Kansas Medical Center). The plasmid iNOS/mutNFκB-luc was prepared by changing GG at positions −84 to −83 in the iNOS promoter to AA (GGG ACT CTC C to GAA ACT CTC C) by using the QuikChange mutagenesis kit (Stratagene). These mutations were confirmed by nucleotide sequencing.
Cell Culture.
Rat primary glial cultures and tertiary astrocyte cultures were prepared and maintained as described (13). Rat primary microglia were isolated from the glial cultures. Briefly, astrocytes were cultured in 75-mm2 flasks for 10–14 days in αMEM (Gibco) containing 10% FBS (HyClone)/100 units/ml of penicillin G/100 μg/ml of streptomycin (pen-strep). To separate microglia, flasks were shaken for 2 h at 250 rpm in a rotary shaker at 37°C. Detached cells were filtered through a 36-μm mesh and plated into four-well chamber slides at a density of 1 × 105 cells per well. After 20 min of incubation at 37°C, nonadherent cells were removed and fresh medium supplemented with 10 ng/ml of macrophage colony-stimulating factor (M-CSF; R&D) was added. The purity of microglial cultures was assessed by using OX42 antibody (Serotec); >95% of cells stained positively. Cells were cultured for 2 days before treatment. Before treatment, serum-containing medium was removed, cells were washed twice with warm αMEM, and αMEM containing N2 media supplement (Gibco) and 10 ng/ml of M-CSF was added.
BV-2 cells, a murine microglial cell line from M. McKinney (Mayo Clinic, Jacksonville, FL), were cultured in αMEM containing 10% FBS and pen-strep. For experiments, cells were washed twice with warm αMEM and then treated in serum-free medium. In all experiments, cells were pretreated with prostaglandins for 30 min before the addition of activating agent. Prostaglandins and troglitazone were added to cells as ethanol and DMSO solutions, respectively, with the final concentration of ethanol or DMSO never exceeding 0.1%. Control samples contained the same concentration of diluent as experimental samples.
Thiazolyl blue (MTT) assay, trypan blue exclusion assay, and determination of nitrite concentrations in cell-conditioned medium were performed as described (13–15), except that cells were dissolved in DMSO for MTT assay.
Cell Lysates.
Nuclear extracts were prepared as described (9), except that nuclear pellets were washed one more time with the low-salt buffer before resuspension in the high-salt buffer.
For preparation of whole-cell lysates, cells were washed with PBS and lysed with a buffer containing 20 mM Tris⋅HCl, pH 7.5/6 M urea/2% SDS/10% glycerol (vol/vol)/0.1% 2-mercaptoethanol (vol/vol)/1 mM Na3VO4/1 mM NaF. Lysates were sonicated and stored at −20°C before use.
Western Blotting.
Five micrograms of total protein from nuclear extracts and 10 μl of whole cell lysate per lane were used. Western blots were done as described (16) by using LumiGlo chemiluminescence detection system (New England Biolabs). Monoclonal antimurine iNOS (Transduction Laboratories) and polyclonal anti-human p65 (Upstate Biotechnology) antibodies were used.
RNA Isolation and Northern Slot Blots.
Total RNA was isolated from cells, and slot blots were done as described (8) by using a rat iNOS cDNA probe (17). Equal loading of RNA was verified by stripping the membranes and reprobing with pTRI-GAPDH probe (Ambion).
Transfections.
Transfections were done with SuperFect reagent (Qiagen) at a 1:2.5 (μg of DNA/μl of reagent) ratio, and all plasmids were purified by using the EndoFree plasmid purification kit (Qiagen). Cells were plated 24 h before transfection in six-well plates at 3 × 105 cells per well (PPRE-luc and iNOS promoter transfections) or in 24-well plates at 6 × 104 cells per well (3xRel-luc transfections) and incubated with DNA/SuperFect mixtures for 2 h in αMEM containing 0.5% FBS. Medium was changed to αMEM containing 20% FBS, incubation continued for an additional 4 h, and then medium was changed to αMEM containing 10% FBS. At 12–20 h posttransfection, cells were treated for 6–24 h in serum-free medium. At the end of the incubation, cells were washed with cold PBS and lysed, and luciferase activity in cell lysates was measured as described (9).
Electrophoretic Mobility-Shift Assays.
Gel-shift assays to characterize the activation state of NFκB by DNA-binding activity was done with a consensus NFκB-binding shift probe (Promega) as described (9).
RESULTS
15d-PGJ2 Down-Regulates iNOS in Activated Glia.
To determine the effect of prostaglandins on iNOS induction in activated glia, we used three different cell-model systems (murine microglial cell line BV-2, rat microglia, and rat astrocytes) stimulated with LPS or IL-1β and monitored iNOS activity by measuring the levels of the stable NO metabolite nitrite in the conditioned medium. The effects of two major prostaglandins, PGE2 and PGD2, and their cyclopentenone derivatives, PGA2, 15d-PGA2, PGJ2, and 15d-PGJ2, were examined. Fig. 1 shows the structures and relationships among the different prostaglandins used. Preincubation of BV-2 cells with the prostaglandins before LPS treatment leads to a dose-dependent decrease in nitrite accumulation (Fig. 2 A and B). Interestingly, treatment of cells with PGE2 only partially inhibited LPS-stimulated nitrite production even at high PGE2 concentrations (Fig. 2B). The IC50 values for the inhibition in BV-2 cells were 0.2 μM (15d-PGJ2), 0.8 μM (PGJ2), 2 μM (PGA2 and 15d-PGA2), and >4 μM (PGD2). Similar effects were observed in LPS-treated rat microglia, i.e., 15d-PGJ2 was the most potent inhibitor of nitrite production (Fig. 2C) and PGE2 was a weak inhibitor (not shown). Arachidonic acid, the precursor of prostaglandins, was also significantly less effective than cyclopentenone prostaglandins in the inhibition of iNOS, displaying an IC50 of ≈4 μM (data not shown).
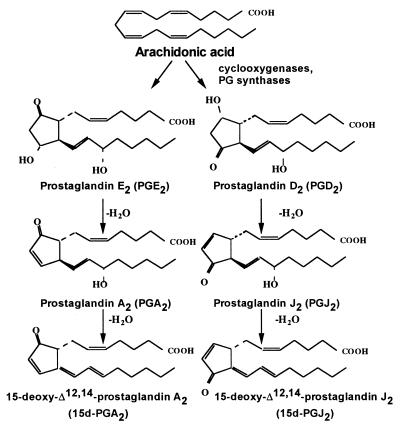
Figure 1.
Structures and relationships among the prostaglandins. PGE2 and PGD2, major prostaglandin products of activated microglia, are produced enzymatically from arachidonic acid by the action of cyclooxygenase-2 and corresponding synthases, whereas cyclopentenone prostaglandins PGA2, 15d-PGA2, PGJ2, and 15d-PGJ2 are the products of nonenzymatic conversion of the precursor prostaglandins.
Figure 2.
Influence of prostaglandins on nitrite production in the murine microglial cell line BV-2 (A and B), rat microglial cells (C), and rat astrocytes (D). Cells pretreated with the indicated concentrations of prostaglandins were stimulated with 0.4 ng/ml of LPS (microglia), 80 ng/ml of LPS (BV-2 and astrocytes), or 100 ng/ml of IL-1β (astrocytes). Control cultures were treated with diluent alone (C) or prostaglandins alone. Conditioned medium was collected at 12 h (microglia), 24 h (BV-2), or 48 h (astrocytes), and nitrite level was determined. Results are the mean ± SEM of n = 3 (BV-2) and n = 4 (microglia) independent experiments. For astrocytes, results of one of three representative experiments are shown. For BV-2 experiments, inhibition is expressed as percentage of control values, where the control is the nitrite level in cells stimulated with LPS alone.
The ability of 15d-PGJ2 to suppress iNOS induction in rat astrocytes also was determined. 15d-PGJ2 inhibited nitrite accumulation in LPS-stimulated astrocytes but with an IC50 of ≈2 μM (Fig. 2D), suggesting that it is less efficient than in microglia, where 85% inhibition was achieved by 0.5 μM 15d-PGJ2 (Fig. 2C). Importantly, not only LPS- but also IL-1β-stimulated iNOS activity in astrocytes was decreased, demonstrating that 15d-PGJ2 suppression of iNOS is not specific to LPS stimulation.
15d-PGJ2 Treatment Is Not Toxic to Microglia.
To address the possibility that 15d-PGJ2 was inhibiting iNOS through a toxic effect on the cells, we investigated its influence on the viability of BV-2 cells. The concentrations that inhibit completely the iNOS induction by LPS were nontoxic to cells as judged by the MTT assay (Fig. 3A), which serves as an early indicator of cell dysfunction. Moreover, 15d-PGJ2 even protected the cells from the LPS-induced cell death, which becomes evident after 24 h of treatment (Fig. 3). In addition to the MTT assay, we confirmed the protective action of 15d-PGJ2 against LPS-induced death by the trypan blue exclusion assay (data not shown). Therefore, we conclude that the observed inhibition of LPS-stimulated iNOS by 15d-PGJ2 is not a result of general toxicity, but, rather, that a specific pathway may be involved in prostaglandin modulation of iNOS activity in microglia.
Figure 3.
15d-PGJ2 is not toxic to microglia. (A) MTT reduction assay. BV-2 cells were treated with 1 μM 15d-PGJ2, 80 ng/ml of LPS, 15d-PGJ2 + LPS, or the control buffer for 24 h, then the MTT assay was performed. Results are mean ± SEM of n = 3 independent experiments, each of which was done in triplicate. Asterisk indicates significantly different from other samples (P < 0.05). Statistics here and throughout have been calculated by using Student’s t test with significance determined as P < 0.05. (B) Phase-contrast images of BV-2 cells after treatment with LPS or LPS + 0.5 μM 15d-PGJ2 for 24 h. (Bar = 100 μm.)
15d-PGJ2 Inhibits Production of iNOS mRNA and Protein.
Based on the finding that 15d-PGJ2 was the most potent inhibitor of iNOS activity in microglia, we explored the mechanism of its action in more detail. Because the response to 15d-PGJ2 was similar for both primary microglia and the BV-2 microglial cell line, BV-2 cells were used for all subsequent experiments. This cell line was reported to reproduce many microglial responses (18).
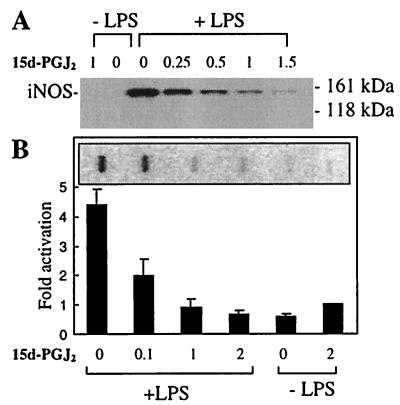
To determine how the inhibition of iNOS occurs, iNOS protein and mRNA levels were measured in BV-2 cells treated with LPS or LPS plus 15d-PGJ2. Treatment with 15d-PGJ2 leads to a significant decrease of both iNOS protein (Fig. 4A) and mRNA levels (Fig. 4B) in a dose-dependent manner.
Figure 4.
15d-PGJ2 inhibits production of iNOS protein and mRNA in LPS-stimulated BV-2 cells. (A) Cells were incubated with 80 ng/ml of LPS alone, LPS + 0.25, 0.5, 1, or 1.5 μM 15d-PGJ2, 15d-PGJ2 alone, or control buffer. Cell lysates were prepared at 9 h after treatment, and iNOS protein levels were determined by Western blotting. (B) Cells were incubated with 80 ng/ml of LPS alone, LPS + 2, 1, or 0.1 μM 15d-PGJ2, the control buffer, or 2 μM 15d-PGJ2 and total RNA isolated at 12h. One representative slot blot is shown. Levels of iNOS mRNA were normalized to glyceraldehyde-3-phosphate dehydrogenase levels and expressed as relative increase compared with the control. Values correspond to the mean ± SEM of four independent experiments.
15d-PGJ2 Regulates iNOS Production at the Transcriptional Level.
Suppression of iNOS mRNA production by 15d-PGJ2 suggests that the prostaglandin may modulate iNOS expression at the transcriptional level. Therefore, we studied the effect of 15d-PGJ2 on iNOS promoter activity in BV-2 cells by using a murine macrophage iNOS promoter–luciferase construct. Stimulation of cells with LPS leads to a 5-fold increase in luciferase activity whereas pretreatment with 1 μM 15d-PGJ2 inhibited luciferase activity by ≈70% (Fig. 5A). Therefore, 15d-PGJ2 may act as a transcriptional inhibitor of iNOS production in microglia.
Figure 5.
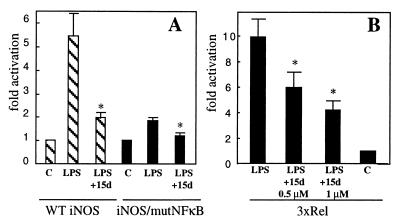
15d-PGJ2 inhibits iNOS and 3xRel reporter activity. (A) Cells were transfected with murine wild-type (WT) iNOS-luc plasmid or iNOS/mutNFκB-luc plasmid, pretreated with 1 μM 15d-PGJ2 (15d) or vehicle, and stimulated with 80 ng/ml of LPS for 9 h. Results are the mean ± SEM of n = 7 (WT) and n = 5 (iNOS/mutNFκB) independent experiments, each done in triplicate. (B) Cells were transfected with 3xRel-luc plasmid, pretreated with the indicated concentrations of 15d-PGJ2 or vehicle, and stimulated with 80 ng/ml of LPS for 7 h. Results are mean ± SEM of n = 6 independent experiments. Control values in A and B are normalized to 1.0. Asterisk indicates significantly different from LPS-treated samples (P < 0.05).
A potential pathway that might mediate this effect of PGJ2 on iNOS expression involves the transcription factor NFκB. The murine iNOS promoter contains two known NFκB-response elements, and the TATAA-box-proximal NFκB sequence is important in the modulation of the response to LPS (12, 19). We confirmed this observation for LPS-stimulated iNOS transcription in microglia by showing that mutating the proximal NFκB site in the iNOS promoter decreased the luciferase activity in response to LPS by ≈75% (Fig. 5A). Interestingly, 15d-PGJ2 still partially inhibited the remaining activity of the mutated promoter, suggesting that other pathways involved in the induction of iNOS by LPS may be inhibited by this prostaglandin or, alternatively, the remaining upstream NFκB-response element contributes to the residual activity of the mutant promoter and becomes inhibited by 15d-PGJ2.
In view of the significant contribution of NFκB to the iNOS transcription, we have studied the influence of 15d-PGJ2 by using a reporter construct, 3xRel-luc, that contains three tandem NFκB-response elements. LPS stimulation of BV-2 cells transiently transfected with 3xRel-luc leads to a 10-fold increase in luciferase activity compared with control, whereas pretreatment of cells with 0.5–1 μM 15d-PGJ2 before the addition of LPS resulted in a 45–60% decrease in LPS-stimulated luciferase activity (Fig. 5B). These data strongly suggest that the observed inhibition of iNOS mRNA by 15d-PGJ2 is mediated, at least in part, by the suppression of iNOS transcription via decreased NFκB-dependent gene transcription. However, it cannot be excluded that 15d-PGJ2, in addition to the direct suppression of NFκB-dependent activity, modulates iNOS mRNA levels by influencing the activity of other transcription factors important for iNOS induction such as AP-1 (20) or at the posttranscriptional level.
Inhibition of NFκB Transcriptional Activity Is Not Due to Inhibition of Its Nuclear Translocation or DNA Binding.
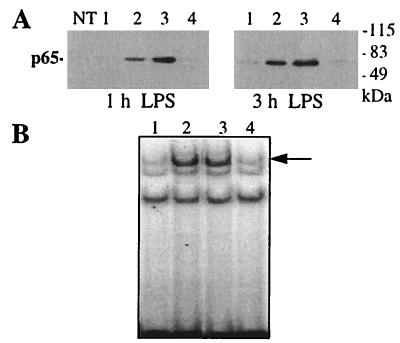
One mechanism by which NFκB transcriptional activity can be inhibited is by preventing nuclear translocation and the subsequent DNA-binding activity of NFκB. We analyzed whether this mechanism occurred in BV-2 cells treated with 15d-PGJ2. Western blots of nuclear extracts demonstrate that the p65 subunit of NFκB is virtually absent in nuclei of nonstimulated cells but is found at high levels in nuclear extracts of cells treated with LPS (Fig. 6A). 15d-PGJ2 alone did not induce nuclear translocation of p65, and pretreatment of cells with 15d-PGJ2 did not inhibit LPS-induced nuclear accumulation of p65 but, rather, slightly stimulated it. In parallel experiments, we verified that treatment with 15d-PGJ2 led to a complete inhibition of LPS-induced iNOS activity (not shown).
Figure 6.
15d-PGJ2 does not inhibit nuclear translocation or DNA-binding ability of NFκB. (A) Nuclear extracts were prepared from untreated BV-2 cells (NT) or BV-2 cells treated with the control buffer (1), 80 ng/ml of LPS (2), LPS + 1 μM 15d-PGJ2 (3), or 15d-PGJ2 alone (4) for 1 and 3 h. p65 protein levels were determined by Western blotting. (B) The same nuclear extracts as in A were analyzed for the presence of NFκB-binding activity in a gel-shift assay. Arrow indicates the position of the shifted band. Experiments were repeated three (A) and two (B) times with similar results, and data from one representative of each experiment are shown.
We tested next whether the DNA-binding ability of NFκB complex was affected by 15d-PGJ2 treatment. Gel-shift experiments using an NFκB consensus-response element showed that LPS rapidly induces binding activity in nuclear extracts from BV-2 cells (Fig. 6B, arrow). 15d-PGJ2 alone did not produce the NFκB mobility shift and was unable to block the LPS-stimulated response. Altogether, these data strongly suggest that 15d-PGJ2 suppresses NFκB transcriptional activity via a mechanism that does not involve a decreased availability or DNA-binding activity of NFκB in the nucleus, but is, instead, a result of direct interference with transcriptional activation by NFκB.
Inhibition of Microglial iNOS by 15d-PGJ2 Is Not Mediated by PPARγ.
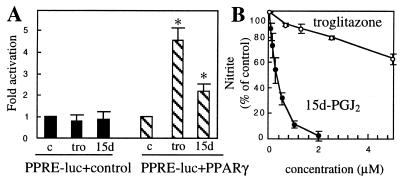
15d-PGJ2 is a naturally occurring ligand for PPARγ (21, 22) and can inhibit iNOS activity and tumor necrosis factor α production in mouse thioglycolate-elicited macrophages and human monocytes by a PPARγ-dependent mechanism (23, 24). This fact, together with the observed order of prostaglandin activity in BV-2 cells (15d-PGJ2 > PGJ2 > PGD2), suggests that PPARγ could be involved in the mediation of the effects of 15d-PGJ2 in microglial cells. We tested this hypothesis in transient transfection experiments by using a reporter construct containing three PPREs (PPRE-luc) and an expression vector for mouse PPARγ. As a positive control we used troglitazone, an antidiabetic drug described as a specific ligand of PPARγ (25). In BV-2 cells transfected with PPRE-luc alone, treatment with troglitazone or 15d-PGJ2 did not result in an increase of luciferase activity (Fig. 7A). However, when cells were cotransfected with PPRE-luc and the PPARγ expression construct, a significant activation was observed in 15d-PGJ2- and troglitazone-treated cells in comparison with the control (Fig. 7A). These data suggest that endogenous PPARγ is absent or its level is very low in BV-2 cells and that the inhibition of microglial iNOS by 15d-PGJ2 is not mediated by PPARγ. In control experiments with COS-7 cells that express endogenous PPARγ (Y. Zhu, personal communication), we observed strong stimulation of PPRE-luc in the absence of cotransfected PPARγ, both by troglitazone and 15d-PGJ2 treatment (data not shown).
Figure 7.
Action of 15d-PGJ2 in BV-2 cells is PPARγ-independent. (A) Cotransfection with PPARγ expression vector is necessary to observe PPRE-luc activity in troglitazone- or 15d-PGJ2-stimulated BV-2. Cells were transfected with PPRE-luc plasmid + control vector or PPARγ expression vector and treated with 5 μM troglitazone (tro), 2 μM 15d-PGJ2 (15d), or control buffer (c) for 24 h. Results are mean ± SEM of n = 7 independent experiments. Control values are normalized to 1.0. Asterisk indicates significantly different from the control (P < 0.05). (B) Troglitazone is not an effective inhibitor of LPS-stimulated nitrite production in BV-2 cells compared with 15d-PGJ2. Cells were preincubated with troglitazone or 15d-PGJ2 and then treated with 80 ng/ml of LPS for 24 h, and nitrite level in conditioned medium was determined. Results are mean ± SEM of n = 3 (15d-PGJ2) and n = 2 (troglitazone) independent experiments, with each point done in triplicate. Data are expressed as percentage of control nitrite levels, where control is the nitrite level from cells stimulated with LPS alone.
Supporting the idea of a PPARγ-independent inhibition of iNOS in microglia by 15d-PGJ2 is the observation that troglitazone was relatively ineffective in the inhibition of LPS-stimulated nitrite accumulation (Fig. 7B), although the same concentrations of the drug elicited a strong response in PPRE-luc/PPARγ-transfected cells. Taken together, these results strongly suggest that, at least in the microglial cell line used here, the inhibition of iNOS transcription by 15d-PGJ2 is not mediated by PPARγ.
DISCUSSION
In this study, we investigated the effect of cyclopentenone derivatives of the “classical” prostaglandins, PGD2 and PGE2, such as PGJ2, 15d-PGJ2, PGA2, and 15d- PGA2, on the induction of iNOS in glia. All the cyclopentenone prostaglandins were able to suppress the iNOS activity in BV-2 cells stimulated with LPS. The cyclopentenone prostaglandins were more potent than their precursors, PGD2 and PGE2, and the common precursor for all the prostaglandins, arachidonic acid. 15d-PGJ2 was the most efficient inhibitor, with an IC50 of ≈0.2 μM. The only other report to date of an inhibitory effect of prostaglandins in microglia showed a partial suppression of iNOS by PGE2 (26), and we observed a similar effect of PGE2 in BV-2 cells. The complete inhibition of iNOS activity by cyclopentenone prostaglandins suggests the existence of a novel mechanism for the modulation of iNOS, uncoupled from the activation of surface prostanoid receptors. Relatedly, it has been reported (ref. 27; reviewed in ref. 28) that cyclopentenone prostaglandins, unlike PGE2 and PGD2, do not stimulate the production of cAMP or bind to cell membranes but, instead, are actively transported into cells, where they accumulate in the nucleus and endoplasmic reticulum.
We have studied in more detail the action of the most potent cyclopentenone prostaglandin, 15d-PGJ2. 15d-PGJ2 suppresses LPS-induced iNOS mRNA and protein accumulation in BV-2 cells, as well as iNOS promoter activity, demonstrating that the inhibition occurs at the transcriptional level. Moreover, the prostaglandin significantly decreased the activity of an NFκB reporter construct. That 15d-PGJ2 acts as a transcriptional inducer in the case of PPRE-luc/PPARγ-transfected BV-2 cells (Fig. 7A) strongly suggests that the prostaglandin is not a general inhibitor of transcription. Similarly, we have found that 15d-PGJ2 did not decrease TPA-induced luciferase activity in PPRE-luc transfected cells (data not shown). We conclude, therefore, that the observed down-regulation of iNOS and 3xRel promoters by 15d-PGJ2 may be explained, at least in part, by specific suppression of NFκB transcriptional activity.
In many instances, inhibition of NFκB transcriptional activity results from the prevention of nuclear translocation and DNA binding of NFκB complex as was shown for Jurkat cells treated with 15d-PGJ2 (29). However, 15d-PGJ2 did not inhibit nuclear accumulation of NFκB complex nor its DNA-binding ability in stimulated microglia, suggesting that its action is mediated by a distinct mechanism resulting in a direct interference with NFκB transcriptional activity. Our data show, therefore, that in addition to the pathway(s) regulating NFκB translocation from cytoplasm to nucleus, there may be another important pathway modulating NFκB transcriptional activity directly in the nucleus, which, at least in some cell types such as microglia, may be influenced by naturally occurring substances, the prostaglandins. Maximal NFκB transcriptional activity appears to require interaction with other components of transcriptional machinery, such as p300/CBP (30), as well as phosphorylation of NFκB itself (31). One can speculate that 15d-PGJ2 might interfere with these pathways, thus impairing NFκB-dependent transcription without affecting NFκB nuclear translocation or DNA binding. This idea of a putative nuclear role of prostaglandins is substantiated by the fact that cyclooxygenase-2, the enzyme responsible for the production of prostaglandins, was shown to be localized both in the nuclear envelope and the endoplasmic reticulum (32).
A prostaglandin-regulated pathway in the nucleus could be mediated by a nuclear receptor for 15d-PGJ2, PPARγ, shown recently to be important in the modulation of inflammatory responses in peripheral macrophages and monocytes (23, 24). However, in contrast to thioglycolate-elicited mouse macrophages, the action of 15d-PGJ2 in microglial cells appears to be PPARγ-independent, because there was no activation of the PPARγ-dependent reporter in microglia nor was the selective PPARγ agonist troglitazone able to inhibit significantly the iNOS activity in LPS-stimulated cells. Interestingly, another PPARγ activator, BRL49653, was able to suppress iNOS production in macrophages, but at much higher drug concentrations than those required for the PPARγ activation (23). However, we cannot rule out the possibility that PPARγ may be expressed in activated microglia under other conditions and consequently play a role in microglia functioning similar to the one suggested for peripheral macrophages.
Clearly, the exact mechanism of action of 15d-PGJ2 in microglia remains undefined. One possible clue is the induction of heme oxygenase-1, BiP, and hsp70 by 15d-PGJ2 as observed in other cell types (29, 33, 34). However, the doses of 15d-PGJ2 used in those studies were about 10-fold higher than in this study, and it remains to be seen whether these effects are relevant to the inhibition of iNOS in microglia. Another interesting observation made here is that the isomer of 15d-PGJ2, 15d-PGA2, is a much weaker inhibitor of iNOS activity (IC50 = 2 μM for 15d-PGA2 vs. 0.2 μM for 15d-PGJ2), which suggests a significant stereospecificity of 15d-PGJ2 action. In turn, it is tempting to speculate that this observation might reflect the need for the interaction of 15d-PGJ2 with a specific component of cellular machinery. Further studies are necessary to investigate this and other possibilities, which may lead to the identification of new mechanisms regulating the activated state of glia.
An important consideration is whether the effects described here are physiologically significant. 15d-PGJ2 was shown to be formed from PGD2 in vivo (35), and PGD2 can be converted readily to J2 prostaglandins in the presence of plasma (36). Although the production of 15d-PGJ2 was not studied in activated microglia and astrocytes, there are many precedents suggesting that 15d-PGJ2 may be present in the brain at relevant concentrations. Indeed, PGD2 is one of the most abundantly produced prostaglandins in brain (37, 38), and it is produced by microglia upon activation (39). Taken together, these data strongly suggest that levels of PGD2 derivatives may reach functionally significant levels in the brain.
In summary, we have demonstrated here that 15d-PGJ2 is a potent inhibitor of iNOS in activated microglia and that it acts, at least in part, by suppressing NFκB transcriptional activity via a novel pathway not related to the inhibition of nuclear translocation of NFκB or the activation of PPARγ. Our results suggest that the role of prostaglandins is, in reality, more complex than simply being a “proinflammatory” stimulus. Some prostaglandins may be important mediators of glial “deactivation,” a potentially important step preventing the development of chronic inflammation and neurodegeneration after an acute traumatic episode in the brain. This role appears to be specific to cyclopentenone prostaglandins, because they are significantly more effective than their precursors. The production of PGD2 derivatives, such as 15d-PGJ2, at late stages of glial activation may represent a part of a feedback mechanism that ensures the return of reactive glia to the resting state and leads to the cessation of inflammatory responses. This role extends the array of prostaglandin functions in brain, such as regulation of fever response, cerebral blood flow, and nociception. Our findings also suggest the importance of studying cyclopentenone prostaglandins and their analogs as potential therapeutical agents to reduce microglial activation.
Acknowledgments
We thank Drs. E. Turkington and L. Guo for assistance with the astrocyte cultures and Drs. D. M. Watterson and J. Haiech for critical reading of the manuscript and helpful suggestions. This work was supported in part by National Institutes of Health Grant AG13939 (L.V.E.), National Institutes of Health Training Grant GM08061 (K.A.), and by a postdoctoral fellowship from the Swiss National Science Foundation (T.P.).
ABBREVIATIONS
- 15d-PGA2
15-deoxy-Δ12,14-prostaglandin A2
- 15d-PGJ2
15-deoxy-Δ12,14-prostaglandin J2
- AD
Alzheimer’s disease
- iNOS
inducible nitric-oxide synthase
- LPS
lipopolysaccharide
- MTT
thiazolyl blue
- PGA2
PGD2, PGE2, and PGJ2, prostaglandins A2, D2, E2, and J2, respectively
- PPAR
peroxisome proliferator-activated receptor
- PPRE
peroxisome proliferator response element
References
- 1.Itagaki S, McGeer P L, Akiyama H, Zhu S, Selkoe D. J Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 2.Dickson D W, Farlo J, Davies P, Crystal H, Fuld P, Yen S H. Am J Pathol. 1988;132:86–101. [PMC free article] [PubMed] [Google Scholar]
- 3.Griffin W S, Stanley L C, Ling C, White L, MacLeod V, Perrot L J, White C L, III, Araoz C. Proc Natl Acad Sci USA. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Griffin W S, Sheng J G, Royston M C, Gentleman S M, McKenzie J E, Graham D I, Roberts G W, Mrak R E. Brain Pathol. 1998;8:65–72. doi: 10.1111/j.1750-3639.1998.tb00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stewart W F, Kawas C, Corrada M, Metter E J. Neurology. 1997;48:626–632. doi: 10.1212/wnl.48.3.626. [DOI] [PubMed] [Google Scholar]
- 6.McGeer P L, Schulzer M, McGeer E G. Neurology. 1996;47:425–432. doi: 10.1212/wnl.47.2.425. [DOI] [PubMed] [Google Scholar]
- 7.Weldon D T, Rogers S D, Ghilardi J R, Finke M P, Cleary J P, O’Hare E, Esler W P, Maggio J E, Mantyh P W. J Neurosci. 1998;18:2161–2173. doi: 10.1523/JNEUROSCI.18-06-02161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu J, Akama K T, Krafft G A, Chromy B A, Van Eldik L J. Brain Res. 1998;785:195–206. doi: 10.1016/s0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 9.Akama K T, Albanese C, Pestell R G, Van Eldik L J. Proc Natl Acad Sci USA. 1998;95:5795–5800. doi: 10.1073/pnas.95.10.5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace M N, Geddes J G, Farquhar D A, Masson M R. Exp Neurol. 1997;144:266–272. doi: 10.1006/exnr.1996.6373. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Y, Qi C, Calandra C, Rao M S, Reddy J K. Gene Exp. 1996;6:185–195. [PMC free article] [PubMed] [Google Scholar]
- 12.Lowenstein C J, Alley E W, Rava P, Snowman A M, Snyder S H, Russell S W, Murphy W J. Proc Natl Acad Sci USA. 1993;90:9730–9734. doi: 10.1073/pnas.90.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu J, Castets F, Guevara J L, Van Eldik L J. J Biol Chem. 1996;271:2543–2547. doi: 10.1074/jbc.271.5.2543. [DOI] [PubMed] [Google Scholar]
- 14.Suzumura A, Sawada M, Itoh Y, Marunouchi T. J Neuroimmunol. 1994;53:209–218. doi: 10.1016/0165-5728(94)90031-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banker G, Goslin K. In: Culturing Nerve Cells. Banker G, Goslin K, editors. Cambridge, MA: MIT Press; 1991. pp. 51–52. [Google Scholar]
- 16.Burgess W H, Watterson D M, Van Eldik L J. J Cell Biol. 1984;99:550–557. doi: 10.1083/jcb.99.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galea E, Reis D J, Feinstein D L. Neurosci Res. 1994;37:406–414. doi: 10.1002/jnr.490370313. [DOI] [PubMed] [Google Scholar]
- 18.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 19.Xie Q W, Kashiwabara Y, Nathan C. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]
- 20.Marks-Konczalik J, Chu S C, Moss J. J Biol Chem. 1998;273:22201–22208. doi: 10.1074/jbc.273.35.22201. [DOI] [PubMed] [Google Scholar]
- 21.Kliewer S A, Lenhard J M, Willson T M, Patel I, Morris D C, Lehmann J M. Cell. 1995;83:813–819. doi: 10.1016/0092-8674(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 22.Forman B M, Tontonoz P, Chen J, Brun R P, Spiegelman B M, Evans R M. Cell. 1995;83:803–812. doi: 10.1016/0092-8674(95)90193-0. [DOI] [PubMed] [Google Scholar]
- 23.Ricote M, Li A C, Willson T M, Kelly C J, Glass C K. Nature (London) 1998;391:79–82. doi: 10.1038/34178. [DOI] [PubMed] [Google Scholar]
- 24.Jiang C, Ting A T, Seed B. Nature (London) 1998;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- 25.Lehman J M, Moore L B, Smith-Oliver T A, Wilkinson W, Willson T M, Kliewer S A. J Biol Chem. 1995;270:12953–12956. doi: 10.1074/jbc.270.22.12953. [DOI] [PubMed] [Google Scholar]
- 26.Minghetti L, Nicolini A, Polazzi E, Creminon C, Maclouf J, Levi G. Glia. 1997;19:152–160. [PubMed] [Google Scholar]
- 27.Takahashi S, Odani N, Tomokiyo K, Furuta K, Suzuki M, Ichikawa A, Negishi M. Biochem J. 1998;335:35–42. doi: 10.1042/bj3350035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Negishi M, Koizumi T, Ichikawa A. J Lipid Mediat Cell Signal. 1995;12:443–448. doi: 10.1016/0929-7855(95)00029-p. [DOI] [PubMed] [Google Scholar]
- 29.Rossi A, Elia G, Santoro M G. Proc Natl Acad Sci USA. 1997;94:746–750. doi: 10.1073/pnas.94.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerritsen M E, Williams A J, Neish A S, Moore S, Shi Y, Collins T. Proc Natl Acad Sci USA. 1997;94:2927–2932. doi: 10.1073/pnas.94.7.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhong H, SuYang H, Erdjument-Bromage H, Tempst P, Ghosh S. Cell. 1997;89:413–424. doi: 10.1016/s0092-8674(00)80222-6. [DOI] [PubMed] [Google Scholar]
- 32.Morita I, Schindler M, Regier M K, Otto J C, Hori T, DeWitt D L, Smith W L. J Biol Chem. 1995;270:10902–10908. doi: 10.1074/jbc.270.18.10902. [DOI] [PubMed] [Google Scholar]
- 33.Koizumi T, Odani N, Okuyama T, Ichikawa A, Negishi M. J Biol Chem. 1995;270:21779–21784. doi: 10.1074/jbc.270.37.21779. [DOI] [PubMed] [Google Scholar]
- 34.Odani N, Negishi M, Takahashi S, Kitano Y, Kozutsumi Y, Ichikawa A. J Biol Chem. 1996;271:16609–16613. doi: 10.1074/jbc.271.28.16609. [DOI] [PubMed] [Google Scholar]
- 35.Hirata Y, Hayashi H, Ito S, Kikawa Y, Ishibashi M, Sudo M, Miyazaki H, Fukushima M, Narumiya S, Hayaishi O. J Biol Chem. 1988;263:16619–16625. [PubMed] [Google Scholar]
- 36.Kikawa Y, Narumiya S, Fukushima M, Wakatsuka H, Hayaishi O. Proc Natl Acad Sci USA. 1984;81:1317–1321. doi: 10.1073/pnas.81.5.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdel-Halim M S, Hamberg M, Sjoquist B, Anggard E. Prostaglandins. 1977;14:633–643. doi: 10.1016/0090-6980(77)90190-3. [DOI] [PubMed] [Google Scholar]
- 38.Ogorochi T, Narumiya S, Mizuno N, Yamashita K, Miyazaki H, Hayaishi O. J Neurochem. 1984;43:71–82. doi: 10.1111/j.1471-4159.1984.tb06680.x. [DOI] [PubMed] [Google Scholar]
- 39.Minghetti L, Levi G. J Neurochem. 1995;65:2690–2698. doi: 10.1046/j.1471-4159.1995.65062690.x. [DOI] [PubMed] [Google Scholar]