Abstract
Neuroplasticity in the vocal control system of songbirds is strongly influenced by seasonal fluctuations in circulating testosterone. These seasonally plastic telencephalic structures are implicated in the learning and production of song in songbirds. The role of the indoleamine melatonin in seasonal adaptations in birds has remained unclear. In this experiment, European starlings were castrated to remove the neuromodulating activity of gonadal steroids and were exposed to different photoperiods to induce reproductive states characteristic of different seasonal conditions. Long days increased the volume of the song-control nucleus high vocal center compared with its volume on short days. Exogenous melatonin attenuated the long-day-induced volumetric increase in high vocal center and also decreased the volume of another song-control nucleus, area X. This effect was observed regardless of reproductive state. To our knowledge, this is the first direct evidence of a role for melatonin in functional plasticity within the central nervous system of vertebrates.
European starlings (Sturnus vulgaris) are highly photoperiodic (1). Reproductive activity occurs in the spring as day length is increasing (i.e., photostimulation occurs), but is subsequently curtailed by the onset of photorefractoriness during exposure to long day lengths. During the onset of photorefractoriness, the hypothalamo-pituitary-gonadal (HPG) axis becomes inactive and the gonads regress (2). Starlings remain refractory to long day lengths until short days are experienced in the winter; the HPG axis slowly becomes responsive again in the absence of a long day photostimulus, in preparation for increasing day length and consequent full reproductive activity in the spring. It must be noted that these centrally mediated different reproductive states are not mediated by seasonal changes in gonadal steroids and still occur at the levels of the hypothalamus and the pituitary gland, even in the absence of gonads (2). Coincident with changes in reproductive activity, seasonal neuroplasticity now documented in several species of oscine songbirds occurs within discrete telencephalic nuclei that are involved in song learning and production (3–7). Increases in the volumes of these song-control nuclei largely depend on seasonal increases in circulating testosterone (T) and its metabolites (8–10) that are directly related to the annual reproductive cycles of these birds (11). These seasonal changes in volumes of the song-control nuclei are associated with changes in cell size and cell number in various song-control nuclei (12).
Recent studies suggest that there are gonad- and T-independent seasonal changes in the volumes of song nuclei (13–15). To date, it has been unclear what factors might be contributing to these T-independent neuronal changes. A complex suite of physiological events occurs during the onset of photorefractoriness (2). These include changes in circulating concentrations of photoperiodically controlled hormones other than T. There are also alterations in the responsiveness of the brain to hormones, and there could be other intrinsic changes in the brain associated with the different reproductive states of photosensitivity, photorefractoriness, and photostimulation (16).
One candidate for the regulation of T-independent changes in the song-control system is the photoperiodically controlled hormone melatonin. Melatonin concentrations in plasma are high during the dark phase of the circadian cycle among all vertebrate taxa including birds and mammals (16). This results in a seasonal change in the pattern of secretion; longer durations of high melatonin are characteristic of the short day lengths of the fall and winter, and short durations of high melatonin secretion are characteristic of the long days of the spring and summer (16). Although seasonal changes in the pattern of secretion of melatonin are identical in birds and mammals, birds, unlike mammals, do not use the melatonin signal to time their reproductive effort to an opportune time of year (17, 18). The function of annual fluctuation in the nocturnal melatonin signal in birds is unclear, but it has been implicated in the synchronization of circadian activity rhythms (19, 20) and seasonal changes in immune function (21). We propose that annual adjustments in melatonin secretion are also involved in the regulation of seasonal changes in the structure of the song-control system. Recent findings are consistent with this hypothesis. For example, the peak in the ratio of dying high vocal center (HVc) cells is preceded by a shortening day length (and hence is coincident with an increased duration of the melatonin signal) (22). In addition, melatonin binding sites have been described in the song-control system of three songbird species, including starlings (23–25). In starlings, the telencephalic nuclei HVc, the lateral magnocellular nucleus of the anterior neostriatum (lMAN), area X, and nucleus robustus archistriatalis (RA) all contain melatonin binding sites (24). To enable us to identify steroid-independent effects of changing photoperiod and of melatonin manipulation upon seasonal neuroplasticity within the starling song system, we used castrated male starlings. In this way, we removed the neuromodulating activity of seasonal changes in gonadal steroids and also any possible confounding effects of interactions of steroids with melatonin upon the song system.
MATERIALS AND METHODS
Animals.
Twenty-four photorefractory male starlings [held on 18L:6D (18 h light and 6 h darkness) per day] were castrated under anesthesia (intramuscular injection of 3.5 mg secobarbital sodium salt; Sigma, product no. S-1378), the testes removed through bilateral incisions between the last pair of ribs. Birds were then randomly allocated to one of four groups (n = six per group). They were housed in cages (49 × 95 × 51 cm; n = six per cage) and were supplied with food (turkey starter crumbs) and water ad libitum. All groups were held in cages at equivalent positions in separate cage racks. Photorefractory birds to be implanted with melatonin capsules (Prefr MEL) and photorefractory birds to be implanted with blank capsules (Prefr BLANK) remained on 18L:6D for 58 days. During this time, groups Pstim MEL (those eventually to be photostimulated and implanted with melatonin capsules), Pstim BLANK (those eventually to be photostimulated and implanted with blank capsules), and Short Day BLANK (to remain on short days, 8L:16D, throughout the experiment) were transferred to short days to regain photosensitivity.
Hormone Treatments.
Once all the birds were in the correct reproductive state for this experiment, the two MEL groups were implanted with silastic capsules containing melatonin. Silastic tubing (a total of 60 mm per bird, 1.47 mm i.d. × 1.96 mm o.d.) containing melatonin (Sigma, product no. M-5250) or left empty was implanted intraperitoneally, by using a technique similar to that described for castration. The amount of melatonin used was calculated to give a “high” dose, as described in ref. 26. The three BLANK groups were implanted with empty silastic capsules. Each group was transferred to its respective photoperiod on the day of implantation. Groups Prefr MEL and Prefr BLANK were maintained on 18L:6D; Pstim MEL and Pstim BLANK were transferred from short days to 18L:6D to photostimulate them, and the Short Day BLANK group remained on 8L:16D. Thus, of the four groups that experienced long day lengths, two of them were exposed to a long exogenous melatonin signal (akin to a very short day). In addition, these two groups were either photostimulated or photorefractory, so they were in different reproductive states. The fifth group, which experienced short days and thus also experienced a short-day melatonin signal, was photosensitive. The birds remained on their respective photoperiods for 24 days, at which point they were decapitated and the brains collected. A period of 24 days was chosen so that the photostimulated groups had time to become fully photostimulated but were not exposed to long days for a sufficient period of time to become photorefractory.
Volume Reconstruction.
Volumes of the song-control nuclei were reconstructed by four independent observers unaware as to the groups and manipulations involved, using NIH image 1.62 with an Apple Macintosh computer. Frozen brains were cut coronally at 25 μm. Every fourth section was collected for Nissl stain. Volumes were reconstructed by measuring the area of each nucleus on each section, summing the area measurements and multiplying by the distance between sampled sections.
Blood Sampling and Radioimmunoassay.
Blood samples were obtained immediately before the start of the experiment and again 3 days before its termination. Blood was collected during the daytime (10 a.m.) so that we could demonstrate that the melatonin implants had indeed elevated plasma melatonin in the implanted birds. At this time of day, endogenous concentrations of melatonin are minimal. By using this fact, we were able to determine to what extent our treatment had caused an increase in plasma melatonin, by comparison with the groups implanted with blank silastic capsules. A superficial wing vein was pricked and ca. 0.5 ml blood was collected into heparinized glass capillary tubes. The blood was centrifuged at 1,500 × g for 10 min, and the plasma was separated and stored at −20°C. Plasma was assayed for T via radioimmunoassay, as described in ref. 27. Melatonin was measured via radioimmunoassay as described in ref. 28 and that was validated for starlings as described in ref. 29.
Data Analysis.
Data were analyzed by using one-way ANOVA followed by Fisher’s protected least significant difference for multiple comparisons.
RESULTS
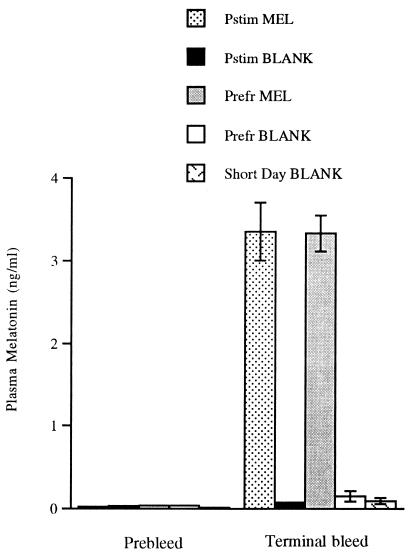
None of the birds had detectable plasma T. In confirmation of the radioimmunoassay, there were no signs of change of beak color from black to yellow in any of the birds, a sensitive bioassay for the presence of T (30). All of the birds were castrated when they were photorefractory (before the start of the experiment), and photoperiod was subsequently manipulated to induce the different reproductive states. In addition, all of the birds were the same age (first year), so all groups experienced similar previous exposure to T. The melatonin assay data presented in Fig. 1 demonstrate that the melatonin-implanted birds had elevated plasma melatonin as compared with birds with empty implants.
Figure 1.
Plasma melatonin before and during the experiment. Plasma melatonin concentrations in all groups of starlings were at or very close to the detection limit of the assay (0.01 ng/ml) before implantation. The graph demonstrates the rise in plasma melatonin over baseline concentrations in those groups implanted with melatonin (Pstim MEL and Prefr MEL).
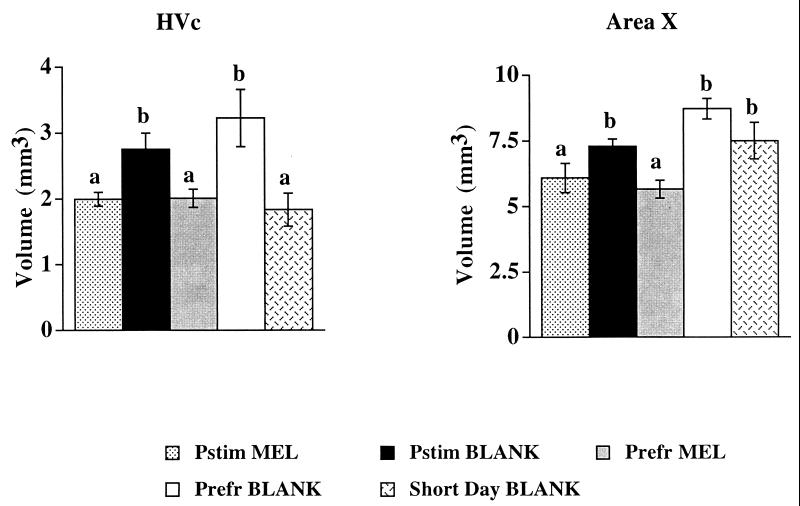
An effect of photoperiod was observed in the HVc, regardless of reproductive state (Figs. 2 and 3). Starlings with empty implants that were exposed to long days (18 h of light and 6 h of darkness per day, 18L:6D) had larger volumes of HVc than starlings with empty implants exposed to short days (8L:16D; Short Day BLANK). Thus, long days increased the volume of HVc, regardless of whether birds were photostimulated (Pstim) or photorefractory (Prefr). Melatonin treatment attenuated the long-day-induced increase in volume of HVc, also regardless of reproductive state (Figs. 2 and 3). HVc in melatonin-treated birds on long days (Pstim MEL and Prefr MEL) was similar in volume to that in short-day birds with blank implants. The latter observation suggests that even though the administration of melatonin may have been pharmacological in terms of duration (i.e., constant release vs. pulsatile) and concentration (on average, double the peak endogenous concentration observed in starlings), the observed effects were similar in magnitude to those seen in birds exposed to an increased endogenous melatonin signal, namely the Short Day BLANK group.
Figure 2.
Reconstructed volumes of song-control nuclei after treatment. Data were analyzed by using one-way ANOVA followed by Fisher’s protected least significant difference for multiple comparisons. ANOVA for area X: F = 7.695 (4, 21), P < 0.0007. ANOVA for HVc: F = 5.157 (4, 21), P < 0.006. The letters a or b above a particular column indicate statistically significant difference for that group in comparison to another group within a particular graph. They correspond to the following probability values (for HVc): Pstim MEL vs. Pstim BLANK, P = 0.0347; Pstim MEL vs. Prefr MEL, P = 0.9985; Pstim MEL vs. Prefr BLANK, P = 0.0088; Pstim MEL vs. Short Day Blank, P = 0.5038; Pstim BLANK vs. Prefr MEL P = 0.0353; Pstim BLANK vs. Prefr BLANK, P = 0.3671; Pstim BLANK vs. Short Day BLANK, P = 0.0123; Prefr MEL vs. Prefr BLANK, P = 0.0044; Prefr MEL vs. Short Day BLANK, P = 0.4634; Prefr BLANK vs. Short Day BLANK, P = 0.0018. For area X: Pstim MEL vs. Pstim BLANK, P = 0.0394; Pstim MEL vs. Prefr MEL, P = 0.4753; Pstim MEL vs. Prefr BLANK, P = 0.0006; Pstim MEL vs. Short Day Blank, P = 0.0406; Pstim BLANK vs. Prefr MEL P = 0.0059; Pstim BLANK vs. Prefr BLANK, P = 0.0572; Pstim BLANK vs. Short Day BLANK, P = 0.8378; Prefr MEL vs. Prefr BLANK, P < 0.0001; Prefr MEL vs. Short Day BLANK, P = 0.0077; Prefr BLANK vs. Short Day BLANK, P = 0.1209.
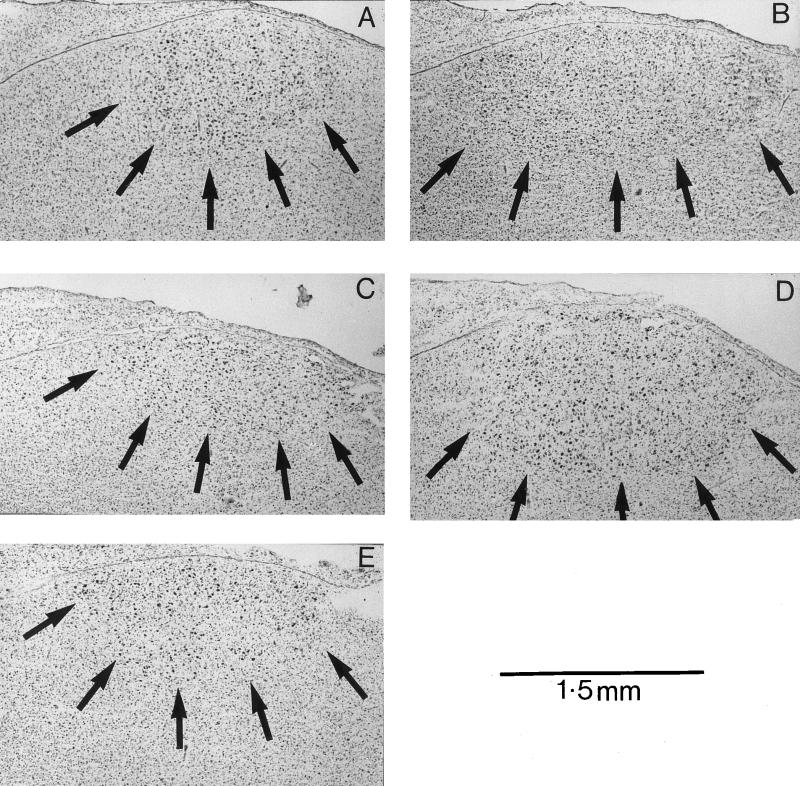
Figure 3.
Typical examples of Nissl-stained sections containing the HVc. (A) Pstim MEL; (B) Pstim BLANK; (C) Prefr MEL; (D) Prefr BLANK; (E) Short Day BLANK. Note the relatively small areas of HVc in the melatonin-treated and short-day birds.
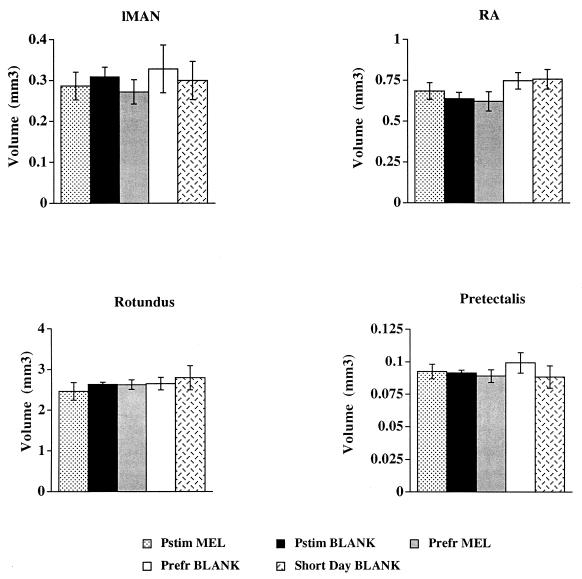
The volume of another song-control nucleus, area X, did not differ between long- and short-day birds, but it was significantly smaller in melatonin-treated starlings (Fig. 2). The lack of an effect of reproductive state upon HVc and area X volume presumably reflects the lack of circulating gonadal steroids. In intact birds, the Pstim BLANK group would presumably have had larger volumes of these nuclei and greater song output than the Prefr BLANK group (although we did not measure song output in this study). Two other song-control nuclei, the lMAN and RA, were unaffected by photoperiod, melatonin treatment, or reproductive state; the same is true for two non-song-control nuclei rotundus (Rt) and pretectalis (Pt) (Fig. 4).
Figure 4.
Reconstructed volumes of song-control nuclei and comparison control nuclei after treatment. Data were analyzed by using one-way ANOVA followed by Fisher’s protected least significant difference for multiple comparisons. No statistically significant differences were observed in lMAN, RA, nucleus rotundus, or nucleus pretectalis.
DISCUSSION
These studies confirm and extend previous work on seasonal plasticity of HVc in European starlings that had demonstrated an important role for T and reproductive state in regulating volumetric changes in HVc (31). To summarize, a change in photoperiod caused volumetric changes in HVc of European starlings, and this effect was independent of changes in circulating gonadal steroids. Exogenous melatonin administration attenuated the long-day-induced increase in the volume of HVc to a degree similar to that caused by transfer to a short photoperiod. Thus, natural and artificial increases in the duration of the melatonin signal have similar effects on HVc. In addition, the song-control nuclei HVc, lMAN, area X, and RA in starlings all contain melatonin receptors (24). Exogenous melatonin also decreased the volume of area X, indicating that this nucleus is responsive to a degree to changes in the melatonin signal, but less so than HVc. Thus, these data are strong evidence of a role for melatonin: involvement in seasonal neuroplasticity in telencephalic areas in songbirds. It is unclear as to why there are differential effects of melatonin and/or photoperiod on different song-control nuclei, even though they all contain melatonin binding sites. However, HVc and area X tend to be the more seasonally labile song-control nuclei in terms of volumetric changes (8–10), and the receptor subtype relative densities and population distributions within these nuclei require quantification. It is possible that melatonin is acting indirectly via an (as yet undescribed) action upon adrenal steroids and/or castration-resistant steroids, such as estradiol. Circulating estradiol is sometimes elevated in young castrated songbirds (32, 33), but it is unclear whether this is a seasonal phenomenon. Even though all the birds in this study were castrated, the surgery did not affect the endocrine state of the photorefractory birds, as these are essentially castrated as a result of photoperiod manipulation. Thus, if castration does increase the concentration of circulating adrenal steroids in adult starlings, then we would expect even gonad-intact photorefractory starlings to have high circulating adrenal estrogens. When administered in extremely high doses, melatonin can suppress the production of adrenal steroids in mammals (34). The only song-control nucleus that has a high number of estrogen receptors is HVc (35), and HVc morphology is affected by circulating estrogens. If increased melatonin secretion suppresses circulating estradiol, then this could be a mechanism by which melatonin is acting upon the song system even in gonad-intact birds, quite apart from the more likely direct action of melatonin upon melatonin receptors in the song-control nuclei. As the action of estrogens on HVc affects the volumes of other song-control nuclei, such as area X (36), then this could in some way explain the differential action of melatonin upon different song-control nuclei.
Whatever its mode of action, melatonin may be acting to “fine tune” the more dramatic effects of T on the song system, precisely timing the volumetric changes to a specific time of the year. Brain space for learned tasks such as singing is hypothesized to be energetically costly (37), thus it would be advantageous to an individual to time an increase in volume of brain areas to a narrow window of time when it will reap the maximum benefit. As spring progresses, starling plasma T concentrations rise, and elevated T causes increases in the volumes of song-control nuclei (5). It may well be that the nocturnal duration of melatonin secretion holds the T-induced increases in volumes of song-control nuclei in check at the start of the spring, but not later on in the spring. At this time of year, day length increases further, and it is more beneficial in terms of the effect of increased singing behavior on reproductive success to increase the size of these brain areas. Similarly in the fall, the increased melatonin signal associated with decreasing day length would cause the song-control nuclei to shrink to a greater extent than the termination of gonadal steroid secretion alone, as occurs at the onset of photorefractoriness. The mode of action of melatonin requires elucidation, however, and the activity of its receptors within the song-control nuclei needs to be quantified at different stages during the annual cycle. It is likely that fluctuations in plasma T alter the density of melatonin receptors within the brain, as in the pars tuberalis of mammals, where T has a negative effect on receptor density (38). Additionally, the action of other photoperiodically controlled hormones (e.g., thyroid hormones) within the song system demands investigation to clarify the full effects of changing photoperiod and hormone interactions on seasonal neuroplasticity in songbirds.
Acknowledgments
We thank Margaret McCarthy and Randy Nelson for their comments. This research was supported by the Biotechnology and Biological Sciences Research Council Wain Fellowship scheme, the National Science Foundation (IBN 95-14525), and the National Institute of Neurological Disorders and Stroke (NS 35467).
ABBREVIATIONS
- T
testosterone
- HVc
high vocal center
- lMAN
lateral magnocellular nucleus of the anterior neostriatum
- RA
nucleus robustus archistriatalis
References
- 1.Burger J W. J Exp Zool. 1939;80:249–257. [Google Scholar]
- 2.Nicholls T J, Goldsmith A R, Dawson A. Physiol Rev. 1988;68:133–176. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- 3.Sohrabji F, Nordeen E J, Nordeen K W. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- 4.Scharff C, Nottebohm F. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nottebohm F. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 6.Brenowitz E A, Nalls B, Wingfield J C, Kroodsma D E. J Neurosci. 1991;11:1367–1374. doi: 10.1523/JNEUROSCI.11-05-01367.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenowitz E A, Baptista L F, Lent K, Wingfield J C. J Neurobiol. 1998;34:69–82. doi: 10.1002/(sici)1097-4695(199801)34:1<69::aid-neu6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 8.Nottebohm F, Nottebohm M E, Crane L A, Wingfield J C. Behav Neural Biol. 1987;47:197–211. doi: 10.1016/s0163-1047(87)90327-x. [DOI] [PubMed] [Google Scholar]
- 9.Smith G T, Brenowitz E A, Wingfield J C, Baptista L F. J Neurobiol. 1995;28:114–125. doi: 10.1002/neu.480280110. [DOI] [PubMed] [Google Scholar]
- 10.Gulledge C C, Deviche P. J Neurobiol. 1997;32:391–402. doi: 10.1002/(sici)1097-4695(199704)32:4<391::aid-neu3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Dawson A. Gen Comp Endocrinol. 1983;49:286–294. doi: 10.1016/0016-6480(83)90146-6. [DOI] [PubMed] [Google Scholar]
- 12.Smith G T, Brenowitz E A, Beecher M D, Wingfield J C. J Neurosci. 1997;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernard D J, Wilson F E, Ball G F. Brain Res. 1997;760:163–169. doi: 10.1016/s0006-8993(97)00277-1. [DOI] [PubMed] [Google Scholar]
- 14.Smith G T, Brenowitz E A, Wingfield J C. J Neurobiol. 1997;32:426–442. doi: 10.1002/(sici)1097-4695(199704)32:4<426::aid-neu6>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Gulledge C C, Deviche P. J Neurobiol. 1998;36:550–558. [PubMed] [Google Scholar]
- 16.Cassone V M. Trends Neurosci. 1990;13:457–464. doi: 10.1016/0166-2236(90)90099-v. [DOI] [PubMed] [Google Scholar]
- 17.Storey C R, Nicholls T J. Gen Comp Endocrinol. 1978;34:468–470. doi: 10.1016/0016-6480(78)90288-5. [DOI] [PubMed] [Google Scholar]
- 18.Chakraborty S. Gen Comp Endocrinol. 1995;99:185–191. doi: 10.1006/gcen.1995.1100. [DOI] [PubMed] [Google Scholar]
- 19.Heigl S, Gwinner E. J Biol Rhythms. 1995;10:225–233. doi: 10.1177/074873049501000305. [DOI] [PubMed] [Google Scholar]
- 20.Menaker M. Proc Natl Acad Sci USA. 1968;59:414–421. doi: 10.1073/pnas.59.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bentley G E, Demas G E, Nelson R J, Ball G F. Proc R Soc London Ser B. 1998;265:1191–1195. doi: 10.1098/rspb.1998.0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirn J R, Schwabl H. J Neurobiol. 1997;33:223–321. doi: 10.1002/(sici)1097-4695(199709)33:3<223::aid-neu2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 23.Gahr M, Kosar E. J Comp Neurol. 1996;367:308–318. doi: 10.1002/(SICI)1096-9861(19960401)367:2<308::AID-CNE11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 24.Bentley G E, Ball G F. Soc Neurosci Abstr. 1998;24:665.8. [Google Scholar]
- 25.Whitfield-Rucker M G, Cassone V M. Horm Behav. 1996;30:528–537. doi: 10.1006/hbeh.1996.0056. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs J L. J Comp Physiol A. 1983;153:413–419. [Google Scholar]
- 27.Bernard D J, Ball G F. Gen Comp Endocrinol. 1997;105:276–283. doi: 10.1006/gcen.1996.6829. [DOI] [PubMed] [Google Scholar]
- 28.Fraser S, Cowen P, Franklin C, Arendt J. Clin Chem. 1983;29:396–397. [PubMed] [Google Scholar]
- 29.Zeman M, Gwinner E. J Comp Physiol A. 1993;172:333–338. [Google Scholar]
- 30.Witschi E, Miller R A. J Exp Zool. 1938;79:475–507. [Google Scholar]
- 31.Bernard D J, Ball G F. J Comp Neurol. 1995;360:726–734. doi: 10.1002/cne.903600415. [DOI] [PubMed] [Google Scholar]
- 32.Marler P, Peters S, Ball G F, Dufty A M, Wingfield J C. Nature (London) 1988;336:770–772. doi: 10.1038/336770a0. [DOI] [PubMed] [Google Scholar]
- 33.Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger M A. Gen Comp Endocrinol. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 34.Yamada K. Res Comm Chem Pathol Pharmacol. 1990;69:241–244. [PubMed] [Google Scholar]
- 35.Gahr M, Guttinger H R, Kroodsma D E. J Comp Neurol. 1993;327:112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- 36.Herrmann K, Arnold A P. J Neurobiol. 1990;22:29–39. doi: 10.1002/neu.480220104. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs L F. Trends Ecol Evol. 1996;11:82–86. doi: 10.1016/0169-5347(96)81048-2. [DOI] [PubMed] [Google Scholar]
- 38.Recio J, Pévet P, Masson-Pévet M. J Neuroendocrinol. 1998;10:303–308. doi: 10.1046/j.1365-2826.1998.00208.x. [DOI] [PubMed] [Google Scholar]