Abstract
Estrogens and growth factors such as epidermal growth factor (EGF) act as mitogens promoting cellular proliferation in the breast and in the reproductive tract. Although it was considered originally that these agents manifested their mitogenic actions through separate pathways, there is a growing body of evidence suggesting that the EGF and estrogen-mediated signaling pathways are intertwined. Indeed, it has been demonstrated recently that 17β-estradiol (E2) can induce a rapid activation of mitogen-activated protein kinase (MAPK) in mammalian cells, an event that is independent of both transcription and protein synthesis. In this study, we have used a pharmacological approach to dissect this novel pathway in MCF-7 breast cancer cells and have determined that in the presence of endogenous estrogen receptor, activation of MAPK by E2 is preceded by a rapid increase in cytosolic calcium. The involvement of intracellular calcium in this process was supported by the finding that the presence of EGTA and Ca2+-free medium did not affect the activation of MAPK by E2 and, additionally, that this response was blocked by the addition of the intracellular calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate. Cumulatively, these data indicate that the estrogen receptor, in addition to functioning as a transcription factor, is also involved, through a nongenomic mechanism, in the regulation of both intracellular calcium homeostasis and MAPK-signaling pathways. Although nongenomic actions of estrogens have been suggested by numerous studies in the past, the ability to link estradiol and the estrogen receptor to a well defined signaling pathway strongly supports a physiological role for this activity.
The biological activity of estrogen is mediated through a specific high-affinity estrogen receptor (ER) located within target cell nuclei. In the absence of hormone, ER is associated with a host of proteins that prevent it from interacting with the cellular transcription apparatus. Upon binding estrogen, the receptor undergoes an activating conformational change, facilitating its association with target genes and permitting it to regulate gene transcription (1). In addition to this well established pathway, however, it has been shown that estrogen can induce extremely rapid increases in the concentrations of the intracellular second messengers calcium and cAMP (2–4). The time course of these events is similar to those elicited by growth factors and peptide hormones, lending support to the hypothesis that they do not involve the classical genomic action of estrogen through its receptor. These similarities, between the nongenomic actions of steroids and growth factor-signaling pathways, suggest that these two distinct, ligand-regulated pathways converge in such a manner to permit cross-talk. For instance, both estrogens and epidermal growth factor (EGF) are known to act as mitogens in promoting cellular proliferation in the breast and the reproductive tract (reviewed in ref. 1). Furthermore, the effects of these two agents sometimes overlap: estrogen has been shown to increase the uterine levels of both EGF and its receptor (5–7), and EGF has been shown to mimic the effects of estrogens in the mouse reproductive tract (8). Although the molecular details of this cross-talk remain to be elucidated, it is clear that ER itself is an important point of convergence. Specifically, it has been shown that ER transcriptional activity can be activated by binding to its cognate estrogenic ligand but also by a variety of other extracellular signals, among them EGF, transforming growth factor, insulin, and dopamine (9–11). The activation of ER by EGF has been demonstrated to involve direct phosphorylation by mitogen-activated protein kinase or extracellular-regulated kinase (MAPK/ERK) of serine residue 118 (12, 13). A further embodiment of this cross-talk was revealed when it was demonstrated that 17β-estradiol (E2) causes rapid activation of MAPK in mammalian cells in an ER-dependent fashion (14, 15). Thus, it appears that a feed-forward system exists where E2 activates MAPK, an event that, in turn, enhances the transcriptional activity of ER. Although the mechanism underlying estrogen-induced MAPK activation and its physiological significance remains to be explained, the activation of this signaling pathway may represent a potential mechanism by which estrogens regulate proliferation. Thus, because of the potential importance of this novel action of estrogen, we undertook a biochemical and pharmacological approach to define the specific mechanism by which estrogens activate MAPK.
MATERIALS AND METHODS
Cell Culture.
MCF-7 breast cancer cells, and HeLa cervical carcinoma cells (American Type Culture Collection, Manassas, VA) were cultured in DMEM/10% heat-inactivated FBS (GIBCO/BRL). Human recombinant EGF (GIBCO/BRL) was used at a concentration of 100 ng/ml, as indicated for each experiment. Treatments with estrogens were done by using E2 (Sigma) at a final concentration of 10−8 M and, when indicated, in combination with 10−6 M antiestrogen ICI 182,780 (a gift from Alan Wakeling, Zeneca Pharmaceuticals). All treatments were preceded by incubation with 0.05% charcoal-stripped FBS for 18 hr to down-regulate MAPK activity.
Western Blot Analysis.
Immunoblots were performed as described previously (16) by standard methods on crude protein extracts obtained from 5 × 106 cells untreated or treated with E2 or EGF for 10 min. Cell extracts containing 10 μg of total protein per sample (determined by using Bio-Rad protein assay) were analyzed by SDS/PAGE using 8% and 10% polyacrylamide gels, as indicated. Gels were transferred to nitrocellulose filters and probed with antibodies specific for either Raf-1 or p44/p42 MAPKs (rabbit polyclonal antibodies C-12 and Erk1, respectively; Santa Cruz Biotechnology) and anti-active p44/42 MAPKs (Promega). Filters then were washed and incubated with specific secondary peroxidase-conjugated antibodies and stained with enhanced chemiluminescence reagents (Amersham).
Indirect Immunofluorescence Staining.
For immunofluorescence analysis, MCF-7 cells were grown on glass coverslips, serum-starved for 24 hr, then either left untreated or treated with EGF or E2 for 30 min. Cells were fixed as described (17) and probed with either Erk2, recognizing both p44 and p42 isoforms (Santa Cruz Biotechnology), or anti-active MAPK (Promega) as specific primary antibody at the dilutions of 1:1,000 and 1:500, respectively, and fluorescein-conjugated donkey anti-rabbit as secondary antibody. Cells were then mounted in PBS/90% glycerol/0.1% p-phenylenediamine (pH 8) and examined under UV light.
Intracellular Free Calcium Measurements.
Ratiometric measurements. Intracellular Ca2+ concentrations ([Ca2+]i) were measured by using the ratiometric fluorescent indicator dye Fura 2-AM, the membrane-permeant acetoxymethyl ester form of Fura 2 (Fura 2-AM; Molecular Probes). Confluent MCF-7 cell monolayers grown on coverglasses were incubated at 37°C in Hanks’ balanced salt solution (HBSS) containing 10 μM Fura 2-AM and 0.05% BSA, pH 7.4, for 45 min. After the incubation period, coverslips were washed with the same buffer to remove excess Fura 2-AM and incubated in buffer for 15 min to allow hydrolysis of Fura 2-AM into its active-dye form, Fura 2. Each coverslip then was inserted into the cuvette of a double-beam spectrofluorimeter (Photon Technology International, Princeton) with the cell side exposed to the light beam at a 45° angle. Treatment with either ATP and bradykinin (1 μM final concentration), thapsigargin (TG, 0.2 μM final concentration; Molecular Probes), or E2 (10−7 M final) was carried out sequentially by adding the appropriate concentrations of each substance into the cuvettes in either HBSS buffer or Ca2+-free HBSS/2 mM EGTA as specified for each experiment. The excitation wavelength was alternated between 340 and 380 nm, and emission fluorescence was recorded at 510 nm. The fluorescence ratio was calculated as F340/F380, and the equivalent in Ca2+ concentration was calculated to range from 80 nM (basal levels) to 205 nM (after stimulation).
Individual cell measurements.
For intracellular calcium measurements at the single-cell level, the MCF-7 cells were plated on glass coverslips and cultured as described above to reach 60% confluence. At the beginning of each experiment the cells were incubated with the fluorescent calcium dye Fluo-3-AM (5 μM) for 20–30 min at 37°C, washed for 10 min to allow the deesterification of the dye, and, finally, transferred into a microincubator chamber. The calcium variations at the single-cell level were monitored by fluorescence confocal microscopy (Odyssey, Noran Instruments, Middleton, WI), recording a time series of images before and after cell stimulation. Time series of 70–100 confocal images were recorded for each experiment, each series containing images recorded at time intervals of 3 sec. For each treatment, maximal response to ATP stimulation (100 μM) was used as an internal positive control. The time series then were analyzed by using a Metamorph Imaging System (Universal Imaging, Westchester, PA). A 488-nm laser was used for the dye excitation, and a 515-nm long-pass filter was used for emission.
RESULTS
MAPK Activation in Response to E2 and EGF in MCF-7 and HeLa Cells.
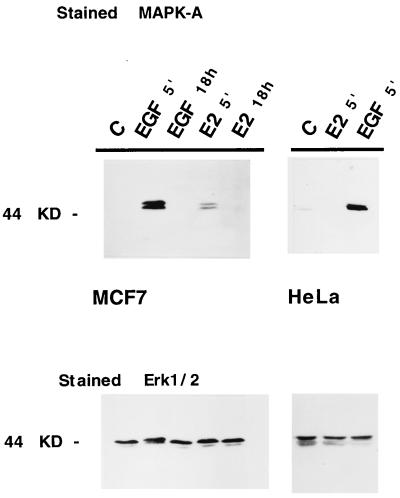
MAPK activity is tightly controlled by dual phosphorylation of intramolecular threonine and tyrosine residues (T 183 and Y 185). Both covalently bound phosphates are required for maximal activity (18, 19). Using a specific antibody recognizing the active, phosphorylated forms of p44/p42 MAPK (Erk1/Erk2), we have shown by Western immunoblot analysis that activation of MAPK in MCF-7 cells occurs after treatment with E2 (Fig. 1 Upper, lane 4). Under the conditions of this experiment, MAPK activation by E2 appears to be induced to a level that is about 20% of that observed in response to EGF. The kinetics of MAPK activation by both EGF and estradiol were similar, with maximal activity being observed 5 min after treatment, returning to basal levels by 60 min (data not shown) and remaining low for up to 18 hr after continuous stimulation with either EGF or E2 (Fig. 1, lanes 3 and 5), respectively. Similar experiments were conducted in HeLa cells, a cell line that does not express the classical ER. The results of this experiment are shown in Fig. 1 (Upper Left, lanes 6–8) and indicate that MAPK activation occurs in the presence of EGF but not E2; similar results were obtained in the ER-negative cell line 293 (data not shown). Importantly, Western immunoblot analysis of these same extracts from MCF-7 and HeLa cells, using an Erk1 antibody that recognizes both nonphosphorylated forms of p44/42 MAPK (shown in Fig. 1 Lower), confirmed that E2 and EGF specifically altered MAPK activity and not its expression level.
Figure 1.
Activation of MAPK in response to estrogen in MCF-7 cells. Western blot analysis of total lysates from MCF-7 and HeLa cells, stained with anti-active MAPK antibody (Upper) and with an antibody recognizing Erk1 and Erk2 (Lower). MCF-7 cells were mock-treated (C) or treated with either EGF for 5 min and 18 hr (lanes 2 and 3, respectively) or with estradiol (E2, lanes 4 and 5). HeLa cells were mock-treated (C) and treated for 5 min with E2 and EGF.
MAPK Translocation from the Cytoplasm to the Nucleus After E2 and EGF Treatment.
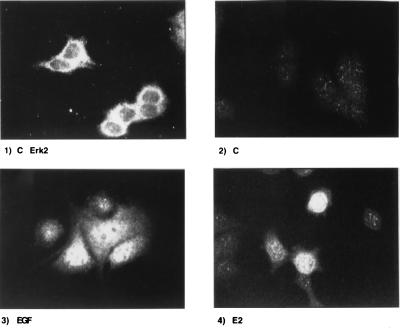
Activation of p44/p42 MAPK by growth factors and other extracellular stimuli results in its translocation, within minutes, from the cytoplasm to the nucleus, where these enzymes act on their target substrates (20–22). To determine whether this critical step also occurred in response to E2, we used indirect immunofluorescence to localize the activated form of MAPK in MCF-7 cells after EGF and E2 treatments. The results of a representative experiment are shown in Fig. 2. In this experiment, untreated cells were fixed and stained with either a primary antibody against p44/p42 MAPK (Fig. 2 Upper Left) or an antibody recognizing the active MAPK (Fig. 2 Upper Right and Lower). These studies reveal that Erk1/2 are located primarily in the cytoplasm of untreated cells (Fig. 2 Upper Left) and that these cells do not contain any significant amount of activated MAPK (Fig. 2 Upper Right). Treatment with either EGF or E2 (Lower Left and Right, respectively) results in translocation of the activated forms of p44/42 MAPK to the nuclear compartment of the cell. However, the intensity of the nuclear staining, as detected by indirect immunofluorescence, varies from cell to cell and differs in response to EGF and E2, the former being more intense than the latter, a result that is in agreement with that observed by Western immunoblot analysis.
Figure 2.
Nuclear translocation of MAPK in response to E2. Indirect immunofluorescence staining of MCF-7 cells using primary antibodies anti-Erk1/2 (Upper Left) and anti-active-MAPK antibodies (Upper Right and Lower). Cells either were left untreated (Upper) or treated with EGF (Lower Left) and estrogen (Lower Right) for 30 min before incubation with FITC secondary antibody.
E2-Induced MAPK Activation Does Not Involve the Classical Surface Receptor-Mediated Signaling Cascade.
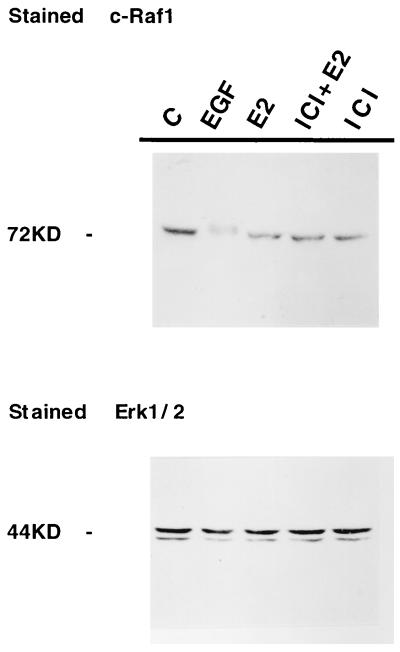
A recent study by Migliaccio et al. (14) demonstrated that the rapid estradiol-mediated activation of Erk1/2 paralleled a concomitant increase in p21 Ras-GTP, the activated form of Ras. This implied that the Ras cascade somehow was involved in mediating the actions of estradiol on the MAPK pathway. To pursue this hypothesis further and define the early events involved in the signaling of E2 through MAPK, we investigated whether the activation of MAPK in MCF-7 cells in response to E2 required activation of the protein kinase c-Raf, which is classically downstream of Ras in growth factor-induced, receptor-mediated transmembrane signaling (reviewed in ref. 23). This was accomplished by using Western immunoblot to assay for alterations in the electrophoretic mobility of Raf-1, which occurs as a consequence of an activating phosphorylation event (24, 25). The results of this experiment are shown in Fig. 3. After treatment of MCF-7 cells with EGF, activated Raf-1 was detected as an immunoreactive protein, which migrated slower than that extracted from untreated cells (Fig. 3, lane 2). In contrast, however, no change in Raf-1 mobility is observed after incubation of MCF-7 cells with either E2 alone (lane 3), the steroidal antiestrogen ICI 182,780, or a combination of the two treatments (lanes 5 and 4, respectively). Reprobing of the same filter with anti-Erk1/2 antibody, shown in Fig. 3 (Lower), subsequently was performed to confirm that comparable amounts of Erk1/2 protein was present in each lane.
Figure 3.
E2-induced MAPK activation is not mediated by cRaf-1. Immunoblot stained with anti-c-Raf1 antibody. MCF-7 cells were treated with either EGF or E2 as in Fig. 1, with the exception that cRAF-1 activation also was examined after treatment with E2 + the pure antiestrogen ICI 182,780 or ICI 82,780 alone. Extracts were analyzed on 7.5% SDS/PAGE. A change in mobility of the band corresponding to phosphorylated c-Raf 1 is clearly detectable after treatment with EGF. (Lower) Normalization for Erk1/2.
Hydrolysis of phosphatidylinositol 4,5-bisphosphate into inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] and 1,2-diacylglycerol frequently has been shown to occur as a consequence of transmembrane signaling (26–28). To establish whether this second messenger was involved in the E2-induced MAPK activation in MCF-7 cells, we undertook HPLC measurements of Ins(1,4,5)P3 (data not shown). A series of experiments was performed on MCF-7 cells after 20-sec and 1-min incubations with either E2 or EGF, in the absence and in the presence of LiCl, to block dephosphorylation. Under none of these conditions, however, did we observe any significant elevations in the amount of Ins(1,4,5)P3 produced in response to either agent (n = 5). This was not due to the inability to release Ins(1,4,5)P3 in this cell system, because a standard mixture of EGF and bradykinin (29) did lead to a 70% increase in the levels of Ins(1,4,5)P3 (n = 2). Thus, within the limits of our detection system, we were not able to show a link between estrogen and Ins(1,4,5)P3 hydrolysis.
MAPK Activation in Response to E2 is Calcium-Mediated and Involves Mobilization from Intracellular Ca2+ Stores.
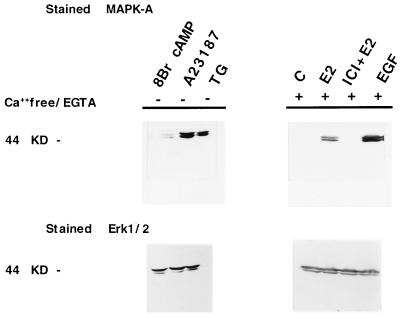
Activation of MAPK can be induced by a variety of extracellular stimuli mediated through receptor tyrosine kinases and cytokine receptors (reviewed in refs. 30 and 31), G proteins (32), and by alterations in [Ca2+]i (33–35). In support of a role for calcium in MAPK activation in MCF-7 cells, we have detected activated enzyme within 5 min of treatment with either the calcium ionophore A23187 or after incubation with TG, an agent that specifically mobilizes the intracellular calcium pools (Fig. 4, lanes 2 and 3, respectively). However, under the conditions of our assay, no activation of MAPK was observed in response to treatment with agents known to enhance cAMP levels such as forskolin (data not shown) or 8-bromo-cAMP (Fig. 4, lane 1). We then examined whether extracellular calcium entry or release of intracellular calcium was involved in estradiol-stimulated MAPK activation. Cells were treated with hormone in the presence or in the absence of Ca2+ in the medium and in the presence of the calcium chelator EGTA. Interestingly, the response to E2 but not to EGF (not shown) is abolished by simultaneous addition of the pure antiestrogen ICI 182,780 (Fig. 4, Upper Right, lane 3), indicating that ER participation in MAPK activation appears to be restricted to the estradiol-stimulated pathway. As shown in Fig. 4, removal of extracellular calcium does not affect the ability of E2 to activate MAPK, nor does it affect EGF activation in this assay (Fig. 4, treatment with EGF in lane 4).
Figure 4.
MAPK activation after treatments of MCF-7 cells with 8-bromo cAMP, calcium ionophore A23187, and TG. E2, E2/ICI, and EGF treatments were carried out in Ca2+/EGTA medium as indicated (Upper). The same blots then were stripped and probed with Erk1/2 as a control (Lower).
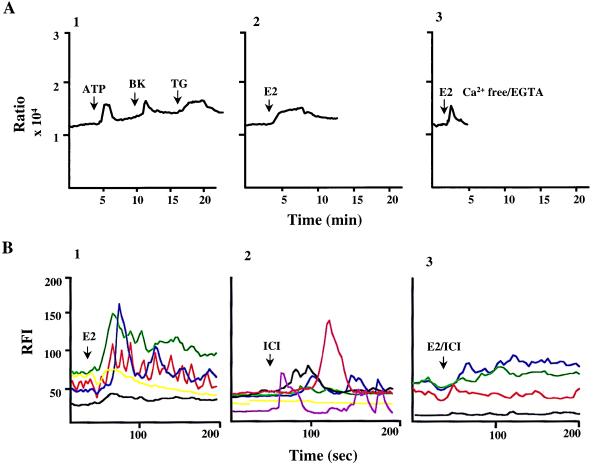
These findings indicate that estrogen-induced activation of MAPK is either a calcium-independent event or that it is due to the release of calcium from intracellular stores. To distinguish between these two possibilities, we measured the free cytosolic [Ca2+]i within MCF-7 cells by using the ratiometric dye Fura 2 after a short incubation with E2 in the presence or absence of extracellular calcium. The results, shown in Fig. 5A, demonstrate that E2 was able to facilitate a rapid increase in the level of free intracellular calcium (Fig. 5A2). This activity did not require extracellular calcium (Fig. 5A3), suggesting that release from intracellular Ca2+ stores was involved. TG as well as ATP and bradykinin also elevated intracellular Ca2+ in these cells (Fig. 5A1). As shown by these experiments, the E2-induced rise in calcium observed in Ca2+-free/EGTA buffer declines more rapidly than that seen in Ca2+ complete buffer (Fig. 5 A1 and A2, respectively) because of the presence of extracellular EGTA. Interestingly, when Ca2+-free/EGTA-containing buffer was replaced by Ca2+ complete buffer, MCF-7 cells responded to a second challenge with E2 in a manner indistinguishable from the response seen when E2 was applied initially (data not shown). This suggests that E2-mediated Ca2+ release does not desensitize rapidly. The oscillation in Ca2+ concentrations was calculated to rise from a basal level of 80 nM to about 205 nM after stimulation with the above-described treatments, consistent with the [Ca2+]i required for activation of the Erk1/2 pathway in other systems established recently (35). However, the relatively small amplitude of the calcium response combined with the heterogeneity observed in the intensity of the MAPK response in MCF-7 cells (see Fig. 2, compare 3 and 4) prompted us to examine the cytosolic calcium response at the level of individual cells by confocal microscopy. The results of single-cell calcium measurements are shown in Fig. 5B1–B3. In response to E2 treatment, an increase in the cytosolic calcium is visible within seconds and reaches its peak between 60 and 80 sec in a fashion similar to that observed by ratiometric measurements (Fig. 5, compare A2 with B1). However, with the confocal analysis, it was observed that the treated cells were heterogeneous with respect to their ability to respond to estrogen, because most cells demonstrated elevation in [Ca2+]i after E2 administration whereas, under identical conditions, some cells were unable to respond. This may be due to intrinsic properties of MCF-7 cells and may reflect heterogeneity in this tumor cell population. Furthermore, similar experiments were performed in HeLa cells, in the absence of the ER, and resulted in no change in the levels of cytosolic calcium concentration as measured by both ratiometric and confocal assay (data not shown), confirming a role for the ER in this process. To probe the pharmacology of this activity further, we performed experiments in MCF-7 cells after treatments with ICI and ICI/estradiol (Fig. 5 B2 and B3, respectively). Consistent with that observed by Western analysis, the simultaneous addition of estradiol and ICI does not result in any significant change in [Ca2+]i, and treatment with ICI alone results in only a small but detectable calcium oscillation. These results would be consistent with the hypothesis that in addition to elevation in [Ca2+]i, MAPK activation also requires ER to be induced by classical agonists.
Figure 5.
Cytosolic calcium oscillations in MCF-7 cells. (A) Free intracellular calcium measurements in response to treatment with ATP, bradykinin (BK), TG are shown in A1. (A2 and A3) The calcium measurements after estrogen treatment (E2) in regular medium and in Ca2+-free/EGTA medium, respectively. (B) Measurements of intracellular calcium at a single-cell level by fluorescence confocal microscopy. MCF-7 cells were stimulated as indicated in the text with estradiol (E2, B1), ICI (B2), and a combination of estradiol and ICI (E2/ICI, B3). The data shown are representative of a single experiment, and a minimum of four experiments were carried out for each treatment. Values are expressed as relative fluorescence units (RFI), with each color representing the calcium response in a single cell. In response to E2, 14 of 20 cells had an RFI >30% of the baseline; in the presence of ICI, it was 14 of 25, and in the presence of E2/ICI, it was 6 of 21 cells.
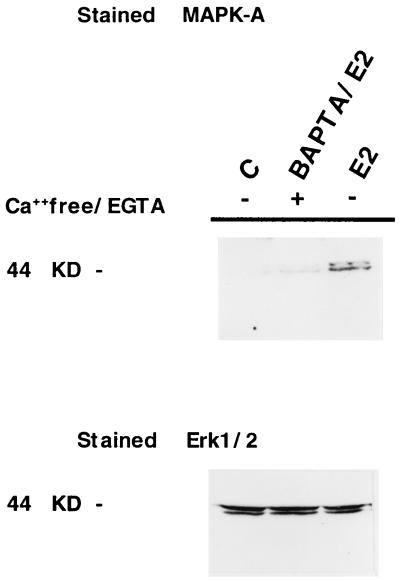
Together, these results provide strong evidence that estradiol activation of MAPK utilizes intracellular calcium as a second messenger. This hypothesis was tested further by assaying estrogen-induced MAPK activation in MCF-7 cells that were preincubated with the specific [Ca2+] chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA) for 45 min (Fig. 6). This study revealed that in the presence of BAPTA, the rapid activation of MAPK, observed in response to E2, is inhibited.
Figure 6.
Intracellular calcium requirement for E2-induced MAPK activation. Shown is Western blot analysis of MCF-7 cells in response to E2 in the presence and absence of the calcium chelator 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate. The blot was stained first with anti-active MAPK (Upper) and then subsequently stripped and restained with anti-Erk1/2 antibody.
DISCUSSION
Classically, estrogen is thought to manifest its biological activity in target cells by binding to the estrogen receptor and converting it into an active transcription factor. The ensuing effects on target gene transcription and, ultimately, on cellular phenotype occur over periods of hours or days. In addition to these delayed responses, however, estrogens exert a variety of rapid, nongenomic effects that may be critical for cell—cell communication (36). Although the mechanism underlying these rapid actions remains elusive, it appears that under certain circumstances estrogens may act directly at the cell membrane and exert effects on the activity of ligand-gated ion channels and G protein-coupled second messengers and also may be involved in the direct modulation of neurotransmitter synthesis and release. It has been considered that the nongenomic actions of estrogens may be of particular importance in the cardiovascular and nervous systems. Thus, although our understanding of the molecular pharmacology of ER is still in its infancy, it is apparent that the classical models, which describe this receptor as a ligand-dependent transcription factor, are incomplete and must be expanded to include ER as a component of other cell-signaling pathways.
In the present study we show that treatment of MCF-7 cells with estradiol leads to both a rapid activation of MAPK and a mobilization of intracellular calcium stores. Furthermore, the lack of MAPK activation and intracellular calcium response in the absence of estrogen receptor, as observed in HeLa cells, and the ability of the pure antiestrogen ICI 182,780 to inhibit E2-induced activity in MCF-7 cells strongly implicate ER in this pathway. Our initial observation that physiologically relevant concentrations of estradiol can mimic the activity of EGF in two different cell-based assays—immunoblot and immunohistochemistry—lends strong support to the hypothesis that estrogen-mediated activation of MAPK is likely to be biologically important. Furthermore, however, we must stress that the heterogeneity observed in both the intensity of the E2-mediated MAPK activation and in the variability of the cytosolic calcium response at the level of individual cell may be the result of a specific cellular phenotype; this variability may reflect important cell type-specific differences in the physiological role of the E2-mediated MAPK activation.
It has been suggested previously that E2 may interact with type I protein tyrosine kinase receptors, particularly Neu/erb-B2 coupled with the p21Ras/MAPK pathway, or, alternatively, that it may interact with a novel membrane receptor (14, 15, 37, 38). MAPK activation in response to growth factors such as EGF and platelet-derived growth factor classically proceeds via a Ras-mediated cascade that involves Raf-1 activation; in addition, receptors that couple to heterotrimeric G proteins also activate MAPK. Depending on cell type and the receptor involved, these signals have been shown to be either dependent or independent of Ras, phosphatidylinositol hydrolysis, and calcium influx (39). Within the limits of our assays, it appears as if Raf-1 activation is not an important component of E2-mediated MAPK activation in MCF-7 cells and that these two pathways must converge at another point. Indeed, experiments conducted in bone cells have indicated a role for G proteins in the early events induced by E2 (40, 41). The results of phosphoinositol measurements in MCF-7 cells after E2 and EGF treatments led us to conclude that either the release of Ins(1,4,5)P3 is not involved in this process or, alternatively, that the pool of Ins(1,4,5)P3 mobilized is very small and, thus, not detectable. The second messenger Ins(1,4,5)P3 is known to link surface hormone receptors to the release of intracellular calcium stores (42, 43) by binding to specific receptors on the endoplasmic reticulum. However, cytosolic calcium signals also are mediated by other mechanisms, i.e., acting through ryanodine receptors (see refs. 44 and 45 for review). One possibility is that the activation of MAPK is mediated by the ER in the cell cytoplasm by acting, either directly or indirectly, on the intracellular Ca2+ receptors and, therefore, resulting in the release of this second messenger. In this respect, the results obtained in response to TG treatment are particularly interesting, because TG also was shown to activate MAPK (Fig. 4, lane 3) and because the rise in [Ca2+]i in response to TG was similar to that seen with E2 (Fig. 5). In addition, TG is known selectively to inhibit the endoplasmic reticulum Ca2+ ATPase and to empty the pool by preventing Ca2+ reuptake independently of inositol phosphate formation (46, 47).
Previously, it has been shown that E2, in a rapid, nongenomic manner, can facilitate both uptake of extracellular calcium and release of intracellular calcium stores in cells derived from the reproductive system and bone (2, 3, 40, 41). Although Ca2+ elevation has been shown in the past to lead to MAPK activation, it was important, in light of our results, to link the two events. From our results we conclude that the estradiol-induced release of calcium from intracellular stores is the critical event that triggers the rapid activation of MAPK in MCF-7 cells and may be a physiologically important component of ER signaling in vivo.
Cumulatively, the data presented in this study strongly support the hypothesis that the rapid effect of E2 on intracellular calcium levels leads to an increase in MAPK, thus defining a potentially important link between estrogen and the cell cycle. This link between E2 and MAPK is intriguing and, although under the conditions of our assay this activity is not as robust as that manifested by EGF, it is likely that it is an important component of ER signaling and may help to explain some of the anomalous ER pharmacology that has been observed in breast cancer. These data beg a reevaluation of the relative contributions of genomic and nongenomic activities in ER biology, an activity that is likely to support the development of pharmaceutical agents that exert differential activities in the two pathways.
Acknowledgments
This work was supported by National Institutes of Health Grant DK48807 to D.P.M.
ABBREVIATIONS
- MAPK
mitogen-activated protein kinase
- EGF
epidermal growth factor
- E2
17β-estradiol
- ER
estrogen receptor
- [Ca2+]i
intracellular Ca2+ concentration
- Ins(1
4,5)P3, inositol 1,4,5-trisphosphate
- TG
thapsigargin
- ERK
extracellular-regulated kinase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.O’Malley B W, Tsai S Y, Bagchi M, Weigel N L, Schrader W T, Tsai M-J. Recent Prog Horm Res. 1991;47:1–26. doi: 10.1016/b978-0-12-571147-0.50005-6. [DOI] [PubMed] [Google Scholar]
- 2.Batra S. Eur J Pharmacol. 1986;127:37–42. doi: 10.1016/0014-2999(86)90203-7. [DOI] [PubMed] [Google Scholar]
- 3.Morley P, Whitfield J F, Vanderhyden B C, Tsang B K, Schwartz J L. Endocrinology. 1992;131:1305–1312. doi: 10.1210/endo.131.3.1505465. [DOI] [PubMed] [Google Scholar]
- 4.Aronica S M, Kraus W L, Katzenellenbogen B S. Proc Natl Acad Sci USA. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.DiAgustine R P, Petruez P, Bell G I, Brown C F, Korach K S, McLachlan J A. Endocrinology. 1988;122:2355–2363. doi: 10.1210/endo-122-6-2355. [DOI] [PubMed] [Google Scholar]
- 6.Mukku V R, Stancel C M. J Biol Chem. 1985;260:9820–9824. [PubMed] [Google Scholar]
- 7.Das S K, Tsukamura H, Paria B C, Andrews G K, Dey S K. Endocrinology. 1994;134:971–981. doi: 10.1210/endo.134.2.7507841. [DOI] [PubMed] [Google Scholar]
- 8.Nelson K G, Takahashi T, Bossert N L, Walmer D K, McLachlan J A. Proc Natl Acad Sci USA. 1991;88:21–25. doi: 10.1073/pnas.88.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ignar-Trowbridge D M, Teng C T, Ross K A, Parker M G, Korach K S, McLachlan J A. Mol Endocrinol. 1993;7:992–998. doi: 10.1210/mend.7.8.8232319. [DOI] [PubMed] [Google Scholar]
- 10.Aronica S M, Katzenellenbogen B S. Mol Endocrinol. 1993;7:743–752. doi: 10.1210/mend.7.6.7689695. [DOI] [PubMed] [Google Scholar]
- 11.O’Malley B W, Schrader W T, Mani S, Smith C, Weigel N L, Conneely O M, Clark J H. Recent Prog Horm Res. 1995;50:333–347. doi: 10.1016/b978-0-12-571150-0.50020-2. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Endoh H, Masuhiro Y, Kitamoto T, Uchiyama S, Sasaki H, Masushige S, Gotoh Y, Nishida E, Kawashima H, et al. Science. 1995;270:1491–1494. doi: 10.1126/science.270.5241.1491. [DOI] [PubMed] [Google Scholar]
- 13.Bunone G, Briand P-A, Miksicek R J, Picard D. EMBO J. 1996;15:2174–2183. [PMC free article] [PubMed] [Google Scholar]
- 14.Migliaccio A, Di Domenico M, Castoria G, deFalco A, Nola E, Auricchio F. EMBO J. 1996;14:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 15.Endoh H, Sasaki H, Maruyama K, Takeyama K, Waga I, Shmizu T, Kato S, Kawashima H. Biochem Biophys Res Commun. 1997;253:99–102. doi: 10.1006/bbrc.1997.6746. [DOI] [PubMed] [Google Scholar]
- 16.Improta T, Schindler C, Horvath C M, Kerr I M, Stark G R, Darnell J J. Proc Natl Acad Sci USA. 1994;91:4476–4480. doi: 10.1073/pnas.91.11.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Improta T, Pine R. Cytokine. 1997;9:383–393. doi: 10.1006/cyto.1996.0180. [DOI] [PubMed] [Google Scholar]
- 18.Seger R, Seger D, Lozeman F J, Ahn N G, Graves L M, Campbell J S, Ericsson L, Harrylock M, Jensen A M, Krebs E G. J Biol Chem. 1992;267:25628–25631. [PubMed] [Google Scholar]
- 19.Robbins D J, Zhen E, Owaki H, Vanderbilt C A, Ebert D, Geppert T D, Cobb M H. J Biol Chem. 1993;268:5097–5106. [PubMed] [Google Scholar]
- 20.Chen R, Sarnecki C, Blenis J. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzales F A, Seth A, Raden D L, Bowman D S, Fay F S, Davis R J. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenormand P, Sardet C, Pages G, L’Allemain G, Brunet A, Pouyssegur J. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egan S E, Weinberg R A. Nature (London) 1993;365:781–783. doi: 10.1038/365781a0. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Z Z, Tan Z J, Diltz C D, You M, Fischer E H. J Biol Chem. 1996;271:22251–22255. doi: 10.1074/jbc.271.36.22251. [DOI] [PubMed] [Google Scholar]
- 25.Ferrier A F, Lee M, Anderson W B, Benvenuto G, Morrison D K, Lowry D R, Declue J E. J Biol Chem. 1997;272:2136–2142. doi: 10.1074/jbc.272.4.2136. [DOI] [PubMed] [Google Scholar]
- 26.Berridge M J. Biochem J. 1984;220:345–360. doi: 10.1042/bj2200345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berridge M J, Irvine R F. Nature (London) 1989;341:197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- 28.Joseph S K, Williamson J R. Arch Biochem Biophys. 1989;273:1–15. doi: 10.1016/0003-9861(89)90156-2. [DOI] [PubMed] [Google Scholar]
- 29.Jamieson G A J, Villeral M L. Arch Biochem Biophys. 1987;252:478–486. doi: 10.1016/0003-9861(87)90054-3. [DOI] [PubMed] [Google Scholar]
- 30.Nishida E, Gotoh Y. Trends Biochem Sci. 1993;18:128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- 31.Blenis J. Proc Natl Acad Sci USA. 1993;90:5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crespo P, Xu N, Simonds W F, Gutkind J S. Nature (London) 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 33.Xia Z, Dudek H, Miranti C K, Greenberg M E. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J M, Plowman G D, Schlessinger J. Nature (London) 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 35.Dolmetsch R E, Lewis R S, Goodnow C C, Healy J I. Nature (London) 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 36.Moss R L, Gu Q, Wong M. Recent Prog Horm Res. 1997;52:33–68. [PubMed] [Google Scholar]
- 37.Matsuda S, Kadowaki Y, Ichino M, Akiyama T, Toyoshima K, Yamamoto T. Proc Natl Acad Sci USA. 1993;90:10803–10807. doi: 10.1073/pnas.90.22.10803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ben-Levy R, Paterson H F, Marshall C J, Yarden Y. EMBO J. 1994;13:3302–3331. doi: 10.1002/j.1460-2075.1994.tb06632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Della Rocca G J, van Biesen T, Daaka Y, Luttrell D K, Luttrell L M, Lefkowitz R J. J Biol Chem. 1997;272:19125–19132. doi: 10.1074/jbc.272.31.19125. [DOI] [PubMed] [Google Scholar]
- 40.Lieberherr M, Grosse B, Kachkache M, Balsan S. J Bone Miner Res. 1993;8:1365–1376. doi: 10.1002/jbmr.5650081111. [DOI] [PubMed] [Google Scholar]
- 41.Le Mellay V, Grosse B, Lieberherr M. J Biol Chem. 1997;272:11902–11907. doi: 10.1074/jbc.272.18.11902. [DOI] [PubMed] [Google Scholar]
- 42.Streb H, Irvine R F, Berridge M J, Schultz I. Nature (London) 1983;306:67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- 43.Burgess G M, Godfrey P P, McKinney J S, Berridge M J, Irvine R F, Putney J W., Jr Nature (London) 1984;309:63–66. doi: 10.1038/309063a0. [DOI] [PubMed] [Google Scholar]
- 44.Berridge M J. J Physiol (London) 1996;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marks A R. Am J Physiol. 1997;272:H597–605. doi: 10.1152/ajpheart.1997.272.2.H597. [DOI] [PubMed] [Google Scholar]
- 46.Takemura H A, Hughes A R, Thastrup O, Putney J W. J Biol Chem. 1989;264:12266–12271. [PubMed] [Google Scholar]
- 47.Thastrup O, Cullen P J, Drobak B K, Hanley M R, Dowson A P. Proc Natl Acad Sci USA. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]