Abstract
Islands are likely to differ in their susceptibility to colonization or invasion due to variation in factors that affect population persistence, including island area, climatic severity and habitat modification. We tested the importance of these factors in explaining the persistence of 164 introductions of six mammal species to 85 islands in the New Zealand archipelago using survival analysis and model selection techniques. As predicted by the theory of stochastic population growth, extinction risk was the greatest in the period immediately following introduction, declining rapidly to low probability by ca 25 years. This suggests that initially small populations were at greatest risk of extinction and that populations which survived for 25 years were likely to persist subsequently for much longer. Islands in the New Zealand archipelago become colder and windier with increasing latitude, and the probability of mammal populations persisting on islands declined steeply with increasing latitude. Hence, our results suggest that climatic suitability was an important determinant of the outcome of these invasions. The form of the relationship between latitude and persistence probability differed among species, emphasizing that the outcome of colonization attempts is species-environment specific.
Keywords: biological invasions, colonization, environmental stochasticity, extinction, island biogeography, stochastic population growth
1. Introduction
Colonization or invasion is a fundamental process shaping island biota and it plays a central role in controlling the number and the types of species found on islands, and their turnover through time (MacArthur & Wilson 1967). Despite its importance, the factors that determine whether a species will successfully colonize an island or not are difficult to study because the act of island colonization is rarely observed directly and many attempts by species to colonize islands might fail and go unrecorded (but see Boyett et al. 2000). Experimental introductions of species to islands provide a means of testing hypotheses about what determines the establishment and the persistence of island populations (Crowell 1973; Schoener & Schoener 1983; Schoener & Spiller 1995), but such studies will often be ethically or logistically impossible. An alternative is to use the record of past human-assisted introductions to investigate factors underlying island invasion success (e.g. Blackburn & Duncan 2001; Cassey et al. 2005). This approach has the disadvantage that the factors of interest are not under experimental control, and it relies on historical information that might be incomplete. The advantage is that there are numerous well-documented introductions of species to islands, providing a rich data source with wide geographic coverage.
Here, we use the record of introductions of six mammal species to islands in the New Zealand archipelago between 1769 and the early 1900s to examine factors hypothesized to influence the establishment and the persistence of island populations. Mammals were frequently introduced to islands around New Zealand to provide a food or a hunting resource, particularly on isolated islands where animals were released to provide food in case of shipwrecks. Since initially they provided a valuable resource, and subsequently many introduced populations became a conservation concern, the introductions and their outcomes were often well documented (Thomson 1922; King 1990).
We investigated why some populations persisted on islands following introduction while others went extinct in the absence of direct human intervention (i.e. they were not eradicated by humans). Here, we use data on the date of introduction and subsequent observations of population status (present on an island, extinct naturally or eradicated by humans) to estimate the persistence times of introduced mammal populations. We compare the distribution of persistence times with that predicted by theory and examine the roles of five factors hypothesized to affect persistence: (i) island area: larger islands should have more resources, favouring persistence because species can attain larger population sizes (MacArthur & Wilson 1967; Lomolino 1984), or a more diverse range of habitats increasing the probability that an introduced species will find a suitable habitat on the island (Smallwood 1994; Case 1996); (ii) habitat modification: since most mammal species introduced to New Zealand favour open habitats (King 1990), clearance of the original forest and the shrubland vegetation on islands should favour persistence by increasing the availability of suitable habitat (Brown 1989; Smallwood 1994; Case 1996); (iii) island latitude: species from temperate regions should be less likely to persist in increasingly colder and less productive environments, generating the prediction that persistence will decline with increasing latitude (Elton 1958; Chown et al. 1998; Sax 2001); and (v) body size: the impact of more severe climate on population persistence may depend on body size. Species with a larger body size should persist longer at higher latitudes than those with a smaller body size owing to improved heat conservation (Bergmann 1847 in Ashton et al. 2000) or buffering from environmental extremes (Lindstedt & Boyce 1985).
2. Material and methods
(a) Data collection
We consulted lists of mammal introductions (Thomson 1922; Taylor 1989; King 1990; Parkes 1990; Veitch & Bell 1990; Atkinson & Taylor 1992) and examined publications referred to in these lists, and systematically searched the journals New Zealand Journal of Ecology, New Zealand Journal of Zoology, Notornis and Tane for records of mammal introductions to islands in the New Zealand archipelago (excluding North and South Islands, but including Macquarie Island), and their outcomes. We included introductions that aimed to establish feral populations or where livestock were abandoned on islands. We excluded species that were introduced to fewer than 15 islands, and species in the Order Rodentia since these were mostly introduced inadvertently to islands (King 1990), which means the information required to estimate persistence times was not available. We included only introductions, where the year of introduction was recorded, excluding 32 introductions where this was unknown. Our final dataset comprised 164 introductions of six mammal species to 85 islands in the New Zealand archipelago. The species, mean body mass (King 1990) and the number of islands to which they were introduced (n) were: European rabbit (Oryctolagus cuniculus, 1.7 kg, n=41), goat (Capra hircus, 31.4 kg, n=33), cat (Felis catus, 2.5 kg, n=29), pig (Sus scrofa, 50.0 kg, n=26), brushtail possum (Trichosurus vulpecula, 2.5 kg, n=17) and sheep (Ovis aries, 39.5 kg, n=18). None of these species is inclined to swim open stretches of water and hence, once introduced to an island, the populations were isolated and could not have been maintained by natural immigration. For each island, we determined its area (ha), latitude (degrees south) and whether or not it was farmed by Europeans; farmed islands had at least some of the original forest or shrubland cleared. The 85 islands ranged in area from 0.8 to 175 000 ha and spanned 25° of latitude.
We determined the outcome of each introduction based on records from subsequent visits to, or surveys of, the islands. We were interested in the ‘success’ of each introduction, measured as the length of time for which an introduced population persisted on an island before natural extinction (i.e. in the absence of direct human intervention). Since the recorded visits to most islands were infrequent, our knowledge of persistence times for most introductions was coarse. For example, a species introduced to an island in 1870 and absent on a subsequent visit in 1890 could have persisted for any time in the interval 0–20 years. Similarly, a species introduced to an island in 1870 and present in 1990 had a minimum persistence time of 120 years. We used methods of survival analysis that incorporate such interval and right censoring to model these data (see below and the electronic supplementary material for details).
Each introduction had one of the three possible outcomes. First, a population could have gone extinct without direct human intervention at some time between recorded visits to an island. In most cases, this results in an estimate of persistence time (in years) that is an interval. If a population introduced to an island in 1870 was recorded as present in 1880, but absent in 1885 and subsequently, then the persistence time for the population is in the interval 10–15 years. In survival analysis terminology, these observations are interval censored; 42 introductions had this outcome. For eight of these introductions, the time to extinction was known to the year and we treated these observations as uncensored. Second, an extant population might have been present on the island at the last recorded visit. In this case, we have a minimum value for persistence time (the number of years from introduction to the last visit). In survival analysis terminology, these observations are right censored; the actual persistence time is greater than this minimum value, but the difference is unknown. Fifty-seven introductions had this outcome. Third, the introduced population may have been eradicated from the island by humans. These observations are also right censored because we again have a minimum value for persistence time—the number of years from introduction to eradication of the population, with persistence time to natural extinction being greater than this minimum value; 65 introductions had this outcome. All of the introductions, their outcomes and the relevant data sources are listed in the electronic supplementary material.
Climatic data were available for 22 of the 85 islands (National Institute of Water and Atmospheric Research, Wellington, New Zealand). To test whether increasing latitude was correlated with increasing climatic severity, we calculated Spearman's rank correlations between island latitude and each of mean annual temperature, mean annual days of ground frost, mean annual days of gale (wind more than 62 km h−1) and mean monthly rainfall.
(b) Statistical analysis
Our response variable was the number of years for which an introduced population persisted on an island (the number of years to natural extinction in the absence of direct human intervention). The probability that a population persists for a given time is a widely used measure of population viability and the subject of much theoretical work aimed at predicting the fate of endangered species (Lande & Orzack 1988; Dennis et al. 1991; Mangel & Tier 1993; Foley 1994). For small populations, such as the introductions considered here, fluctuations in population size due to demographic and environmental stochasticity are likely to be the major cause of extinction (Richter-Dyn & Goel 1972). For populations unaffected by density dependence and subject only to fluctuations caused by environmental stochasticity, stochastic population growth theory predicts that the distribution of persistence times will approach an inverse Gaussian distribution with parameters specifying the initial population size or viability, the long-term population growth rate and the variance due to environmental stochasticity (Lande & Orzack 1988; Dennis et al. 1991). The inverse Gaussian distribution is highly skewed with a long right tail, so that the theory predicts that populations will either go extinct quickly or persist for a long time (Claessen et al. 2005). For populations subject to fluctuations caused only by demographic stochasticity, a simple expression for the distribution of persistence times also exists (Engen et al. 2005), which we term the demographic distribution. We used these two distributions, which assume different types of stochasticity, to model the distribution of persistence times using maximum-likelihood methods (see electronic supplementary material for details). Ideally, we would have used the expected distribution of persistence times for a population subject to both demographic and environmental stochasticity, as they are both likely to be important, but a simple expression for this distribution is not available (Engen et al. 2005). We therefore compared the fit of the two available theoretical distributions to the data and selected the best-fitting based on log likelihoods.
A third distribution, the exponential, is also used to model population persistence times (Mangel & Tier 1993; Foley 1994; Vucetich & Waite 1998). The exponential persistence time distribution is likely to apply when populations are close to carrying capacity and regulated by density dependence (Middleton & Nisbet 1997). Since most introductions would have involved few individuals (Forsyth & Duncan 2001), this is unlikely to hold for our data. Nevertheless, we compared the fit of the exponential distribution, along with two further distributions widely used to model persistence times in other situations: the lognormal and the Weibull (Allison 1995; Tableman & Kim 2004). If the predictions of stochastic population growth theory hold, we expect the two distributions derived from this theory (the inverse Gaussian and demographic) to provide a good fit to the data.
To assess how well a chosen distribution fitted the data, we graphed the survival function (the probability that a population persisted beyond a given time following introduction) from the fitted model and compared this with a non-parametric survival function derived from the data using the methods described in Groeneboom & Wellner (1992) taking into account the interval and right censoring. We also plotted the hazard function, which describes the instantaneous risk that a population went extinct at a given time after introduction.
(c) Candidate models
Having chosen a distribution, we modelled the effect of explanatory variables on persistence time by assuming that these variables influenced the distribution's mean parameter. We formulated our hypotheses about the variables likely to affect persistence into a series of candidate models, and compared the fit of these models to the data using information-theoretic techniques (Burnham & Anderson 2002). Our hypotheses were translated into 20 candidate models (table 1) that included combinations of the explanatory variables: area (island area, a continuous variable that was log transformed), farm (a binary variable indicating whether an island had been farmed or not as a measure of habitat modification), species (a dummy variable coding for the six species), latitude (lat, a continuous variable) and the interaction between species and latitude (lat:species, which tests the hypothesis that the persistence times of species show different relationships with latitude as expected if large-bodied species persist longer at higher latitudes). We used the small sample version of Akaike's information criterion (AICc) to compare the fit of candidate models to the data (Burnham & Anderson 2002). AICc provides a measure of model fit accounting for the sample size and the number of estimated parameters in the model, with smaller values of AICc indicating a better-fitting model. For the set of candidate models, we calculated ΔAICc as the difference in AICc between a model and the best-fitting model in the candidate set, which has a ΔAICc of 0. A rule of thumb is that models having ΔAICc≤2 have substantial support, those in the range 4≤ΔAICc≤7, weaker support, and those with ΔAICc>10 virtually no support (Burnham & Anderson 2002).
Table 1.
Goodness-of-fit, as measured by the small sample version of Akaike's information criterion (AICc, where smaller values indicate a better-fitting model), for 20 candidate models used to explain variation in the persistence times of 164 introductions of six mammal species to 85 islands in the New Zealand archipelago. (Persistence time was modelled assuming an inverse Gaussian distribution with the following explanatory variables: area (island area (ha), log transformed), farm (a binary variable indicating whether an island had been farmed or not), species (a dummy variable coding for the six mammal species), latitude (degrees south) and lat:species (latitude by species interaction). For each model, the number of parameters estimated (k), log likelihood, AICc and ΔAICc (the difference in AICc between a model and the best-fitting model in the candidate set, which has a ΔAICc of 0) are shown. Models are listed from the most to the least supported, and the four models with substantial support are shown in bold.)
| model | k | log likelihood | AICc | ΔAICc |
|---|---|---|---|---|
| species+lat+lat:species+area | 14 | −96.8 | 196.5 | 0 |
| species+lat+lat:species+farm+area | 15 | −96.8 | 196.8 | 0.3 |
| species+lat+lat:species+farm | 14 | −97.8 | 198.4 | 2 |
| species+lat+lat:species | 13 | −98.3 | 199 | 2.6 |
| species+lat+farm+area | 10 | −102.8 | 207.1 | 10.6 |
| species+lat+area | 9 | −103 | 207.1 | 10.6 |
| species+lat+farm | 9 | −103.8 | 208.8 | 12.4 |
| species+lat | 8 | −104.5 | 210 | 13.5 |
| lat+area | 4 | −105.5 | 211.3 | 14.8 |
| lat+farm+area | 5 | −105.5 | 211.4 | 14.9 |
| lat+farm | 4 | −107.5 | 215.2 | 18.7 |
| lat | 3 | −108.2 | 216.5 | 20 |
| species+farm+area | 9 | −117.2 | 235.6 | 39.1 |
| species+farm | 8 | −117.8 | 236.5 | 40 |
| species | 7 | −122.5 | 245.6 | 49.2 |
| species+area | 8 | −122.5 | 245.8 | 49.4 |
| farm+area | 4 | −123.2 | 246.6 | 50.1 |
| farm | 3 | −123.5 | 247.2 | 50.7 |
| area | 3 | −127.9 | 256 | 59.5 |
| intercept only | 2 | −128 | 256.1 | 59.6 |
3. Results
Spearman's rank correlations between latitude and four climate variables for islands in the New Zealand archipelago were: mean annual temperature, r=0.97, n=16 islands, p<0.001; mean annual days of ground frost, r=0.91, n=9, p<0.001; mean annual days of gale, r=0.68, n=11, p=0.02; mean monthly rainfall, r=0.02, n=21, p=0.95. These data indicate that islands in the New Zealand archipelago become colder and windier, but not wetter, with increasing latitude.
Log likelihoods for the fit of different distributions to the observed persistence times were: inverse Gaussian distribution, derived from theory under the assumption of environmental stochasticity, −128.0; demographic distribution derived from theory under the assumption of demographic stochasticity, −129.7; lognormal, −137.2; Weibull, −140.8 and exponential, −148.4. The two distributions derived from theory had substantially larger log likelihoods than the other distributions, indicating a better fit to the data, with the inverse Gaussian, which assumes that extinction results from environmental stochasticity, providing a marginally better fit than the demographic distribution. We therefore used the inverse Gaussian distribution to model persistence times.
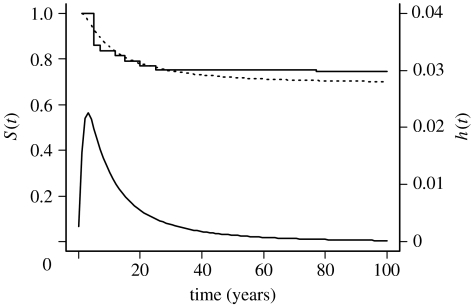
The survival function modelled using an inverse Gaussian distribution was a good fit to the observed persistence data (described using a non-parametric function to accommodate interval and right censoring; see Groeneboom & Wellner (1992)) in the interval 0–25 years (figure 1). The associated hazard function shows that the instantaneous probability of extinction was greatest in the years immediately following introduction and declined rapidly (figure 1), with most populations that became extinct persisting for less than 25 years. Beyond ca 25 years, the use of an inverse Gaussian distribution slightly underestimated persistence probability. Populations that persisted beyond 25 years had a very low probability of extinction and appeared likely to persist for a long time (at least decades to centuries) in the absence of human intervention.
Figure 1.
Survival functions (S(t), the probability that a population persists beyond a given time following introduction) and hazard function (h(t), the instantaneous probability of population extinction) for 164 mammal populations introduced to 85 islands in the New Zealand archipelago. The upper solid line is the observed data described using a non-parametric function to account for interval- and right-censoring (see text), the upper dashed line is a survival curve fitted by maximum likelihood assuming that persistence times follow an inverse Gaussian distribution, and the lower solid line is the corresponding hazard function showing the instantaneous probability of extinction at time t.
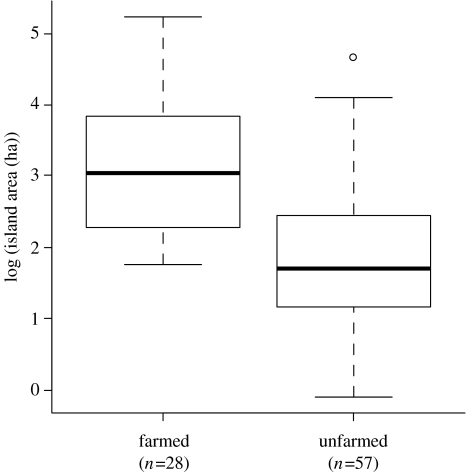
Four of the 20 candidate models had substantial support with ΔAICc≤2.6 (table 1). All four models included the terms species, latitude and the species by latitude interaction, but differed in whether they also include the variables farm, area or both. Inclusion of these last two variables produced a marginally better model, suggesting that they had some effect on persistence although this was not strongly supported. Moreover, inclusion of either farm, area or both produced a similar fitting model, implying that these two variables were essentially interchangeable. Indeed, the probability that an island was farmed or not was strongly correlated with island area; larger islands were more likely to have been farmed (figure 2). To illustrate the effect of these variables on persistence time, we considered the model that included the variables ‘farmed’ or ‘not farmed’, latitude, species, and the interaction between latitude and species (table 2), bearing in mind that farming and island area were strongly correlated (figure 2).
Figure 2.
Box and whisker plots showing island area (log transformed) in relation to whether islands had been farmed or not, for 85 islands in the New Zealand archipelago.
Table 2.
Parameter estimates and standard errors (s.e.) for the model ‘species+lat+lat:species+farm’ (see table 1) explaining variation in the persistence times of six mammal species introduced to islands in the New Zealand archipelago.
| parameter | estimate | s.e. | |
|---|---|---|---|
| intercept | 0.346 | 0.156 | |
| latitude | −0.006 | 0.003 | |
| farming | unfarmed | 0 | — |
| farmed | 0.018 | 0.019 | |
| species | cat | 0 | — |
| goat | 0.240 | 0.281 | |
| pig | 0.041 | 0.255 | |
| possum | 1.890 | 0.996 | |
| rabbit | −0.203 | 0.189 | |
| sheep | 0.517 | 0.376 | |
| lat:species | cat | 0 | — |
| goat | −0.006 | 0.006 | |
| pig | −0.001 | 0.006 | |
| possum | −0.040 | 0.022 | |
| rabbit | 0.004 | 0.004 | |
| sheep | −0.012 | 0.007 | |
| ln(scale) | −2.395 | 0.209 |
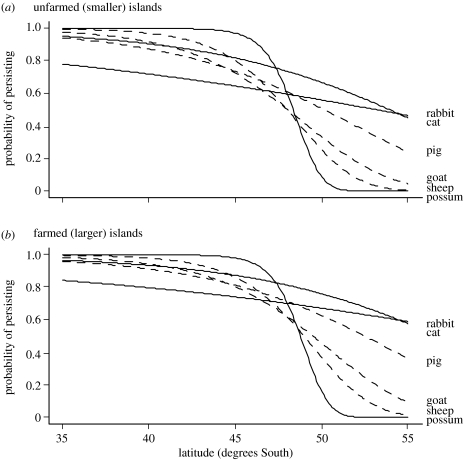
We used the model in table 2 to predict the probability that an introduced population persisted for at least 25 years under differing conditions (figure 3). The probability of persisting declined with latitude, although the form of this relationship differed among species. Pigs, goats and sheep had a high probability of persistence (close to 1) on islands at latitudes lower than 40 °S, with a steady decline in the probability of persisting at progressively higher latitudes. Possums also had a high probability of persisting at lower latitudes, but this declined steeply to almost zero between 45 and 50 °S. The probability of introductions persisting declined less steeply with latitude for small-bodied cats and rabbits. Hence, there was no evidence to support the prediction that larger-bodied mammals should have a higher probability of persistence than smaller-bodied mammals at higher latitudes (figure 3). Having accounted for species and latitude effects, introductions to farmed (larger) islands had a slightly higher probability of persisting than those introduced to unfarmed (smaller) islands, but this effect was less when compared to the effect of species and latitude.
Figure 3.
(a, b) Probability that a mammal population introduced to an island in the New Zealand archipelago persisted for at least 25 years as a function of species, latitude and whether the island had been farmed by Europeans or not. Large- and small-bodied species are shown as dotted and solid lines, respectively.
4. Discussion
Mammals introduced to islands around New Zealand faced the greatest risk of extinction in the years immediately following release, with extinction probability declining to low levels beyond ca 25 years (figure 1). Notably, the observed distribution of persistence times closely followed that predicted by stochastic population growth theory in which extinction results from fluctuations in population size caused by environmental and/or demographic stochasticity (Lande & Orzack 1988; Dennis et al. 1991; Engen et al. 2005; both the inverse Gaussian and the demographic distributions fitted the data well). Owing to the stochastic variation introduced by these two processes, extinction risk would have been greatest immediately following release when populations were small. For those introduced populations that survived the initial high-risk period and increased in size, the risk of extinction due to stochastic fluctuations would have correspondingly declined. Our results show that populations that survived for at least 25 years following release had increased in numbers sufficiently such that extinction risk became negligible and populations were likely subsequently to persist for a long time in the absence of human intervention.
Island latitude was a key factor influencing persistence: the probability that a population persisted for at least 25 years declined with increasing latitude across all species, although the decline became most pronounced at latitudes above 45° (figure 3). Declining survival and reproductive rates either due to increasingly colder and windier conditions (Picton 1984; Post & Stenseth 1999; Coulson et al. 2001) or due to reduced energy availability (Chown et al. 1998, 2005) could account for the lower persistence of populations on more southerly islands (see also Barnes 2002). The differing form of the species latitudinal response (figure 3) suggests that species differ in their tolerance of more severe climatic conditions and/or reduced energy availability, emphasizing that the outcome of introduction or colonization attempts depends on the particular combination of species and environment (Blackburn & Duncan 2001).
We hypothesized that larger-bodied species would better tolerate more climatically severe environments and were more likely to persist at higher latitudes, but this was not the case for the species examined here: small-bodied cats and rabbits had a higher probability of persisting at higher latitudes than large-bodied sheep, goats and pigs. Rabbits can protect themselves from inclement weather by remaining underground (King 1990), but there is no obvious mechanism for cats persisting better than other species at higher latitudes.
Larger islands are expected to support larger populations, thus increasing the probability of those populations persisting (MacArthur & Wilson 1967; Lomolino 1984). However, this is unlikely to be the mechanism underlying the inclusion of island area in the candidate models that received substantial support (table 1) because most failed populations went extinct soon after release (see also Schoener & Spiller 1995). Instead, larger islands were more likely to have been farmed (figure 2). This parallels the situation on other southern ocean islands, where larger (and also warmer) islands are more likely to have been occupied by humans, and to have had more visits, than smaller (and colder) islands (Chown et al. 1998, 2005). Although populations of four of the study species persist in intact native forest in New Zealand (cats, possums, goats and pigs), all except possums are more abundant in human-modified habitats, such as induced grassland, shrubland and second-growth forest, that result from human occupation and clearance for agriculture (King 1990). All the species, except for brushtail possums, are closely associated with humans in their native regions. Hence, the slightly greater success of these species on larger islands that had been farmed may reflect their ability to exploit these modified habitats (see also Russell et al. 2004), which may account for the success of several of these species in colonizing islands globally following introduction (Lever 1994; Long 2003).
Introduced mammals can have devastating impacts on island biota (e.g. Courchamp et al. 2003; Blackburn et al. 2004; Croll et al. 2005; Frenot et al. 2005). Our results suggest that islands below 45° in the New Zealand archipelago have an inherently high probability of successful colonization following introduction, and that success may be slightly higher on larger islands modified by farming. Since several species included in this study are problem invaders globally, and our results may also hold for other invasive species (Smallwood 1994; Case 1996; Chown et al. 1998, 2005), efforts to prevent introductions should be a priority on temperate islands similarly at risk from invasion. Many islands at latitudes above 45° currently contain relatively few introduced species (Chown et al. 1998), most probably due to their isolation and severe climate restricting colonization success. Here, along with increasing human visitation, global warming poses a risk through ameliorating climatic conditions and thereby increasing the likely persistence of introduced populations (Chown et al. 1998; Bergstrom & Chown 1999; Barnes 2002).
Acknowledgments
We thank P. Bellingham, T. Blackburn, S. Chown, J.-M. Gaillard, D. Peltzer, D. Wardle and three anonymous reviewers for their helpful comments on previous versions of the manuscript, and J. Parkes for helping locate introduction records.
Footnotes
Present address: Arthur Rylah Institute for Environmental Research, 123 Brown Street, Heidelberg, Victoria 3084, Australia.
Supplementary Material
References
- Allison P.D. SAS Institute, Inc; Cary, NC: 1995. Survival analysis using the SAS system. [Google Scholar]
- Ashton K.G, Tracy M.C, de Queiroz A. Is Bergmann's Rule valid for mammals? Am. Nat. 2000;156:390–415. doi: 10.1086/303400. doi:10.1086/303400 [DOI] [PubMed] [Google Scholar]
- Atkinson, I. A. E. & Taylor, R. H. 1992 Distribution of alien mammals on New Zealand islands Lower Hutt: DSIR Land Resources Contract Report No. 92/59.
- Barnes D.K.A. Invasions by marine life on plastic debris. Nature. 2002;416:808–809. doi: 10.1038/416808a. doi:10.1038/416808a [DOI] [PubMed] [Google Scholar]
- Bergstrom D.M, Chown S.L. Life at the front: history, ecology and change on southern ocean islands. Trends Ecol. Evo. 1999;14:472–477. doi: 10.1016/s0169-5347(99)01688-2. doi:10.1016/S0169-5347(99)01688-2 [DOI] [PubMed] [Google Scholar]
- Blackburn T.M, Duncan R.P. Determinants of establishment success in introduced birds. Nature. 2001;414:195–197. doi: 10.1038/35102557. doi:10.1038/35102557 [DOI] [PubMed] [Google Scholar]
- Blackburn T.M, Cassey P, Duncan R.P, Evans K.L, Gaston K.J. Avian extinction and mammalian introductions on oceanic islands. Science. 2004;305:1955–1958. doi: 10.1126/science.1101617. doi:10.1126/science.1101617 [DOI] [PubMed] [Google Scholar]
- Boyett W.D, Endries M.J, Adler G.H. Colonization-extinction dynamics of opossums on small islands in Panama. Can. J. Zool. 2000;78:1972–1979. doi:10.1139/cjz-78-11-1972 [Google Scholar]
- Brown J.H. Patterns, modes and extents of invasions by vertebrates. In: Drake J.A, Mooney H.A, di Castri F, Groves R.H, Kruger F.J, Rejmánek M, Williamson M, editors. Biological invasions: a global perspective. Wiley; Chichester, UK: 1989. pp. 85–109. [Google Scholar]
- Burnham, K. P. & Anderson, D. R. 2002 Model selection and multi-model inference 2nd edn. New York, NY: Springer.
- Case T.J. Global patterns in the establishment and distribution of exotic birds. Biol. Conserv. 1996;78:69–96. doi:10.1016/0006-3207(96)00019-5 [Google Scholar]
- Cassey P, Blackburn T.M, Duncan R.P, Gaston K.J. Causes of exotic bird establishment across oceanic islands. Proc. R. Soc. B. 2005;272:2059–2063. doi: 10.1098/rspb.2005.3193. doi:10.1098/rspb.2005.3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chown S.L, Gremmen N.J.M, Gaston K.J. Ecological biogeography of southern ocean islands: species–area relationships, human impacts, and conservation. Am. Nat. 1998;152:562–575. doi: 10.1086/286190. doi:10.1086/286190 [DOI] [PubMed] [Google Scholar]
- Chown S.L, Hull B, Gaston K.J. Human impacts, energy availability and invasion across Southern Ocean islands. Global Ecol. Biogeogr. 2005;14:521–528. doi:10.1111/j.1466-822x.2005.00173.x [Google Scholar]
- Claessen D, Gilligan C.A, Lutman P.J.W, van den Bosch F. Which traits promote persistence of feral GM crops? Part 1: implications of environmental stochasticity. Oikos. 2005;110:20–29. doi:10.1111/j.0030-1299.2005.13667.x [Google Scholar]
- Coulson T, Catchpole E.A, Albon S.D, Morgan B.J.T, Pemberton J.M, Clutton-Brock T.H, Crawley M.J, Grenfell B.T. Age, sex, density, winter weather, and population crashes in Soay sheep. Science. 2001;292:1528–1531. doi: 10.1126/science.292.5521.1528. doi:10.1126/science.292.5521.1528 [DOI] [PubMed] [Google Scholar]
- Courchamp F, Chapuis J.L, Pascal M. Mammal invaders on islands: impact, control and control impact. Biol. Rev. 2003;78:347–383. doi: 10.1017/s1464793102006061. doi:10.1017/S1464793102006061 [DOI] [PubMed] [Google Scholar]
- Croll D.A, Maron J.L, Estes J.A, Danner E.M, Byrd G.V. Introduced predators transform subarctic islands from grassland to tundra. Science. 2005;307:1959–1961. doi: 10.1126/science.1108485. doi:10.1126/science.1108485 [DOI] [PubMed] [Google Scholar]
- Crowell K.L. Experimental zoogeography: introductions of mice to small islands. Am. Nat. 1973;107:535–558. doi:10.1086/282857 [Google Scholar]
- Dennis B, Munholland P.L, Scott J.M. Estimation of growth and extinction parameters for endangered species. Ecol. Monogr. 1991;61:115–143. doi:10.2307/1943004 [Google Scholar]
- Elton C. Methuen; London, UK: 1958. The ecology of invasions by animals and plants. [Google Scholar]
- Engen S, Lande R, Sæther B.-E, Weimerskirch H. Extinction in relation to demographic and environmental stochasticity in age-structured models. Math. Biosci. 2005;195:210–227. doi: 10.1016/j.mbs.2005.02.003. doi:10.1016/j.mbs.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Foley P. Predicting extinction times from environmental stochasticity and carrying capacity. Conserv. Biol. 1994;8:124–137. doi:10.1046/j.1523-1739.1994.08010124.x [Google Scholar]
- Forsyth D.M, Duncan R.P. Propagule size and the relative success of exotic ungulate and bird introductions in New Zealand. Am. Nat. 2001;157:583–595. doi: 10.1086/320626. doi:10.1086/320626 [DOI] [PubMed] [Google Scholar]
- Frenot Y, Chown S.L, Whinam J, Selkirk P.M, Convey P, Skotnicki M, Bergstrom D.M. Biological invasions in the Antarctic: extent, impacts and implications. Biol. Rev. 2005;80:45–72. doi: 10.1017/s1464793104006542. doi:10.1017/S1464793104006542 [DOI] [PubMed] [Google Scholar]
- Groeneboom P, Wellner J.A. Birkhauser; Basel, Switzerland: 1992. Information bounds and nonparametric maximum likelihood estimation. [Google Scholar]
- King C.M, editor. The handbook of New Zealand mammals. Oxford University Press; Auckland, New Zealand: 1990. [Google Scholar]
- Lande R, Orzack S.H. Extinction dynamics of age-structured populations in a fluctuating environment. Proc. Natl Acad. Sci. USA. 1988;85:7418–7421. doi: 10.1073/pnas.85.19.7418. doi:10.1073/pnas.85.19.7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C. Cambridge University Press; Cambridge, UK: 1994. Naturalized animals. [Google Scholar]
- Lindstedt L.S, Boyce M.S. Seasonality, fasting endurance, and body size in mammals. Am. Nat. 1985;125:873–878. doi:10.1086/284385 [Google Scholar]
- Lomolino M.V. Mammalian island biogeography: effects of area, isolation and vagility. Oecologia. 1984;61:376–382. doi: 10.1007/BF00379638. doi:10.1007/BF00379638 [DOI] [PubMed] [Google Scholar]
- Long J.L. CSIRO Publishing; Victoria, Australia: 2003. Introduced mammals of the world: their history, distribution and influence. [Google Scholar]
- MacArthur R.H, Wilson E.O. Princeton University Press; Princeton, NJ: 1967. The theory of island biogeography. [Google Scholar]
- Mangel M, Tier C. A simple direct method for finding persistence times of populations and application to conservation problems. Proc. Natl Acad. Sci. USA. 1993;90:1083–1086. doi: 10.1073/pnas.90.3.1083. doi:10.1073/pnas.90.3.1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton D.A.J, Nisbet R.M. Population persistence time: estimates, models, and mechanisms. Ecol. Appl. 1997;7:107–117. [Google Scholar]
- Parkes J.P. Eradication of feral goats on islands and habitat islands. J. R. Soc. New Zeal. 1990;20:297–304. [Google Scholar]
- Picton H.D. Climate and the prediction of reproduction of three ungulate species. J. Appl. Ecol. 1984;21:869–879. doi:10.2307/2405052 [Google Scholar]
- Post E, Stenseth N.C. Climatic variability, plant phenology, and northern ungulates. Ecology. 1999;80:1322–1339. doi:10.2307/177078 [Google Scholar]
- Richter-Dyn N, Goel N.S. On the extinction of a colonizing species. Theor. Popul. Biol. 1972;3:406–433. doi: 10.1016/0040-5809(72)90014-7. doi:10.1016/0040-5809(72)90014-7 [DOI] [PubMed] [Google Scholar]
- Russell J.C, Clout M.N, McArdle B.H. Island biogeography and the species richness of introduced mammals on New Zealand offshore islands. J. Biogeogr. 2004;31:653–664. [Google Scholar]
- Sax D.F. Latitudinal gradients and geographic ranges of exotic species: implications for biogeography. J. Biogeogr. 2001;28:139–150. doi:10.1046/j.1365-2699.2001.00536.x [Google Scholar]
- Schoener T.W, Schoener A. The time to extinction of a colonizing propagule of lizards increases with island area. Nature. 1983;302:332–334. doi:10.1038/302332a0 [Google Scholar]
- Schoener T.W, Spiller D.A. Effect of predators and area on invasion: an experiment with island spiders. Science. 1995;267:1811–1813. doi: 10.1126/science.267.5205.1811. [DOI] [PubMed] [Google Scholar]
- Smallwood K.S. Site invasibility by exotic birds and mammals. Biol. Conserv. 1994;69:251–259. doi:10.1016/0006-3207(94)90424-3 [Google Scholar]
- Tableman M, Kim J.S. Chapman & Hall/CRC; Boca Raton, FL: 2004. Survival analysis using S: analysis of time-to-event data. [Google Scholar]
- Taylor, G. A. 1989 A register of northern offshore islands and a management strategy for island resources Auckland, New Zealand: Department of Conservation Northern Regional Technical Report Series 13.
- Thomson G.M. Cambridge University Press; Cambridge, UK: 1922. The naturalization of animals & plants in New Zealand. [Google Scholar]
- Veitch C.R, Bell B.D. Eradication of introduced animals from the islands of New Zealand. In: Towns D.R, Daugherty C.H, Atkinson I.A.E, editors. Ecological restoration of New Zealand islands. Department of Conservation; Wellington, New Zealand: 1990. pp. 137–146. [Google Scholar]
- Vucetich J.A, Waite T.A. On the interpretation and application of mean times to extinction. Biodivers. Conserv. 1998;7:1539–1547. doi:10.1023/A:1008808617633 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.