Abstract
Extant baleen whales (Cetacea, Mysticeti) are all large filter-feeding marine mammals that lack teeth as adults, instead possessing baleen, and feed on small marine animals in bulk. The early evolution of these superlative mammals, and their unique feeding method, has hitherto remained enigmatic. Here, I report a new toothed mysticete from the Late Oligocene of Australia that is more archaic than any previously described. Unlike all other mysticetes, this new whale was small, had enormous eyes and lacked derived adaptations for bulk filter-feeding. Several morphological features suggest that this mysticete was a macrophagous predator, being convergent on some Mesozoic marine reptiles and the extant leopard seal (Hydrurga leptonyx). It thus refutes the notions that all stem mysticetes were filter-feeders, and that the origins and initial radiation of mysticetes was linked to the evolution of filter-feeding. Mysticetes evidently radiated into a variety of disparate forms and feeding ecologies before the evolution of baleen or filter-feeding. The phylogenetic context of the new whale indicates that basal mysticetes were macrophagous predators that did not employ filter-feeding or echolocation, and that the evolution of characters associated with bulk filter-feeding was gradual.
Keywords: Cetacea, Mysticeti, Oligocene, Australia, evolution
1. Introduction
Recent discoveries of spectacular fossils, twinned with molecular data from extant taxa, have illuminated the origins of Cetacea (whales, dolphins and porpoises) and the evolutionary transition from a terrestrial to aquatic lifestyle (see Gingerich 1998, 2005; Thewissen & Williams 2002; Berta et al. 2006). In contrast, the origins and early evolution of the two extant suborders of Cetacea (Odontoceti and Mysticeti) remain poorly understood. Among the latter two groups, the origins of the Mysticeti (baleen whales), and their unique feeding mechanism, have proved particularly baffling. Living mysticetes are large (6–30 m body length) filter-feeding marine mammals (Bannister 2002), and include the largest animal to have ever lived, the blue whale (Balaenoptera musculus). They lack teeth (although foetal mysticetes possess vestigial teeth that are resorbed prior to birth), instead possessing baleen, which they use to filter small marine animals out of vast amounts of sea water (Pivorunas 1979; Slijper 1979; Sanderson & Wassersug 1993; Marshall 2002). Some fossil mysticetes possessed teeth, but are inferred to have fed in a similar manner to living Mysticeti, and therefore do not elucidate the origins and early evolution of baleen whales (Barnes et al. 1995; Fordyce & Muizon 2001; Ichishima 2005; Berta et al. 2006).
In this study, I report a new Late Oligocene toothed mysticete from Australia that is more basal than any previously described. Its bizarre morphology suggests it was a specialized macrophagous predator that did not filter-feed. This refutes the notions that: (i) all early mysticetes were filter-feeders; and (ii) that the origins and initial adaptive radiation of mysticete whales was tied to the evolution of filter-feeding (Fordyce 1980; Mitchell 1989; Fordyce & Barnes 1994; Barnes et al. 1995; Fordyce & Muizon 2001; Berta et al. 2006). It thus provides compelling evidence for the pattern of early mysticete evolution, and shows that archaic mysticetes were surprisingly unlike their modern relatives.
2. Systematic palaeontology
Order Cetacea Brisson, 1762
Suborder Mysticeti Flower, 1864
Family Janjucetidae fam. nov.
Janjucetus hunderi gen. et sp. nov.
(a) Holotype
NMV P216929 (Museum Victoria Palaeontology Collection, Melbourne, Australia); virtually complete skull, mandibles, teeth, basihyal, cervical vertebrae 1–3, two ribs, scapulae and radius.
(b) Etymology
Janju from Jan Juc (township near type locality; pronunciation: jan-juck) and cetus (Latin), whale; hunderi, in honour of Mr S. Hunder who discovered the holotype. Janjucetus pronunciation: jan-ju-see-tus.
(c) Diagnosis
Diagnosis for Janjucetidae is that of Janjucetus until other genera are described. Janjucetus is a mysticete cetacean with the following unique suite of apomorphies: premaxillae on rostrum adjacent and anterior to level of P2, or on anterior half of rostrum clearly overhang maxillae; anteriormost point on the posterior edge of the supraorbital process is medially positioned; and posteriormost end of ascending process of premaxilla in line with anterior half of supraorbital process of frontal.
Janjucetus differs from Mammalodon colliveri Pritchard 1939 by having: large caniniform upper incisors; having a high, sharply triangular rostrum that is tightly sutured to cranium and not dorsoventrally flattened; upper post-canine teeth with two unfused roots; robust premaxillae with expanded apices that contact one another at a median suture forming an anterior rosette; premaxillae that overhang the maxillae on the anterior half of the rostrum; only two facial infraorbital foramina (Mammalodon possesses five); ascending process of premaxilla that terminates at level within the anterior half of the orbit; orbital margin of postorbital process of frontal forms greater than 120° with sagittal plane; shorter intertemporal constriction; posteromedial temporal crest that does not overhang anteromedial temporal fossa; a more convex subtemporal crest; prominent sagittal crest; dorsally and posterolaterally flared nuchal crests; dorsal condyloid fossae; and a larger tympanic bulla with inflated involucrum that has a planar anterodorsal surface lacking deep grooves.
Janjucetus differs from Aetiocetidae by having: an upper tooth row that extends posteriorly to a point level with the anterior half of the orbit; a foreshortened rostrum that is less than 40% of CBL; premaxillae with expanded apices that form an incisor bearing anterior rosette, and overhang the maxillae on the anterior half of the rostrum; ascending process of premaxilla that terminates anterior to the mid-point (anteroposteriorly) of the orbit; premaxillae adjacent to and at posterior edge of nasal opening that do not overhang maxillae; high maxillae that are not dorsoventrally flattened; robust, inflated, zygomatic process of squamosal with attenuated apex; a broad basioccipital with bulbous basioccipital crests laterally elongated in an axis parallel with the external acoustic meatus; and mandibles that lack a distinct longitudinal groove for a fibrocartilaginous mandibular symphysis, and possess high, anteroposteriorly broad coronoid processes.
Janjucetus differs from Llanocetus denticrenatus Mitchell 1989 by having: lower teeth that are not separated from one another by a wide diastema; significantly smaller teeth that lack broadly palmate crowns and accessory denticles; significantly lesser skull length (Janjucetus CBL≤50 cm; Llanocetus CBL>180 cm); a high maxilla and rostrum that is not dorsoventrally thin; maxillae that lack nutrient grooves around tooth alveoli; and a mandible that is not dorsoventrally inflated.
(d) Locality, stratigraphy and age
Coastal cliff section SW of Torquay, central coastal Victoria, SE Australia (38°20′ S, 144°18′ E); Jan Juc Marl. The Jan Juc Marl consists of neritic, inner to mid-shelf, marine sediments deposited between 23.9 and 27.0 Myr (Chattian, Late Oligocene; Holdgate & Gallagher 2003). Marine vertebrates from the Jan Juc Marl are generally rare and fragmentary, and include: diverse sharks, rays and teleost fish; indeterminate avians; and mysticete (M. colliveri, and other undescribed forms) and odontocete (Prosqualodon sp. and a probable waipatiid) cetaceans (Fitzgerald 2004). The holotype of J. hunderi (NMV P216929) is exceptionally well-preserved, and represents the most complete Paleogene cetacean yet discovered in Australia (figures 1 and 2a). NMV P216929 was previously mentioned in a published abstract (Fitzgerald 2005).
Figure 1.
Janjucetus hunderi gen. et sp. nov. (NMV P216929) from the Upper Oligocene Jan Juc Marl, Victoria, Australia. Holotype skull and right mandible in: (a) dorsal view; (b) right lateral view; (c) anterior and slightly dorsal view; and (d) ventral view. Holotype left periotic and surrounding basicranium in (e) ventral view. Abbreviations: ap, anterior process of periotic; bo, basioccipital; boc, basioccipital crest; cm, coronoid process of mandible; eam, external acoustic meatus; eo, exoccipital; fer, fenestra rotunda; fpsq, falciform process of squamosal; fr, frontal; gl, glenoid fossa; jn, jugular notch; ju, jugal; la, lacrimal; lt, lateral tuberosity; m, mandible; mf, mallear fossa; mfo, mandibular foramen; mx, maxilla; na, nasal; nuc, nuchal crest; ol, outer lip of tympanic bulla; pa, parietal; pal, palatine; pc, pars cochlearis of periotic; pe, periotic; peo, paroccipital process of exoccipital; pmx, premaxilla; po, postorbital process of frontal; pp, posterior process of tympanoperiotic; pt, pterygoid; sac, sagittal crest; sgf, fossa on squamosal for sigmoid process of tympanic bulla; so, supraoccipital; spsq, spiny process of squamosal; sq, squamosal; st, stapes; sym, mandibular symphysis; t, loose left mandibular tooth; ty, tympanic bulla; vf, vascular foramen; vo, vomer; zmx, zygomatic process of maxilla; zsq, zygomatic process of squamosal. Scale bars: (a–d) 100 mm, (e) 10 mm.
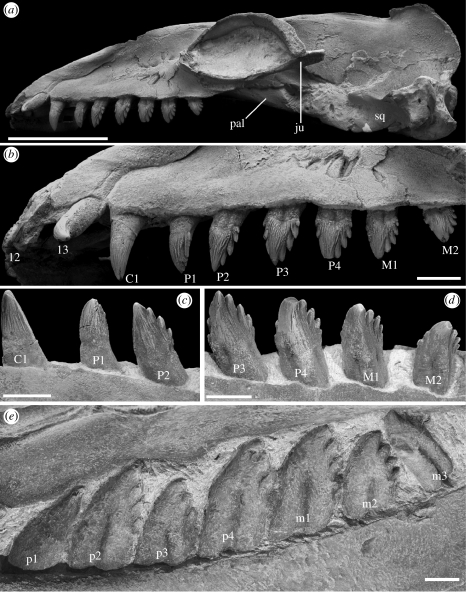
Figure 2.
Janjucetus hunderi gen. et sp. nov. (NMV P216929) from the Upper Oligocene Jan Juc Marl, Vic., Australia. Holotype skull in (a) left lateral view. Left upper dentition in (b) buccal view. Left C1–P2 in (c) lingual view. Left P3–M2 in (d) lingual view. Right lower post-canine dentition (p1–m3) in (e) lingual view. Note that the apex of the crown of P4 was lost during preparation. Abbreviations: ju, jugal; pal, palatine; sq, squamosal; I2, upper second incisor; I3, upper third incisor; C1, upper canine; P1, upper first premolar; p1, lower first premolar; P2, upper second premolar; p2, lower second premolar; P3, upper third premolar; p3, lower third premolar; P4, upper fourth premolar; p4, lower fourth premolar; M1, upper first molar; m1, lower first molar; M2, upper second molar; m2, lower second molar; m3, lower third molar. Scale bars: (a) 100 mm, (b–e) 20 mm.
3. Description
The holotype skull (NMV P216929) is undistorted except for right and left I3, which have been pushed upwards and laterally by dorsoventral diagenetic compression (figure 1a,c,d). All skull sutures are fused, and the C1–C3 epiphyses are fused to their respective vertebral centra, indicating that this specimen represents a subadult or adult individual. The skull and dental morphology differs markedly from other mysticetes (figure 3). Janjucetus was a relatively small cetacean compared to other mysticetes, with a preserved condylobasal length (CBL) of 46 cm, suggesting a total body length of no more than approximately 3.5 m. The rostrum is triangular, broad-based, foreshortened (its length being 38% of CBL) and high; unlike more derived mysticetes, the rostrum is not dorsoventrally flattened. The orbits open anterolaterally, are ovoid in outline, and relatively enormous for a cetacean (orbit diameter (OD)/CBL is 24%; figure 1b,c). The cranium is very broad, accommodating the voluminous temporal fossae. Frontals and parietals are extensively exposed in an elongated intertemporal constriction.
Figure 3.
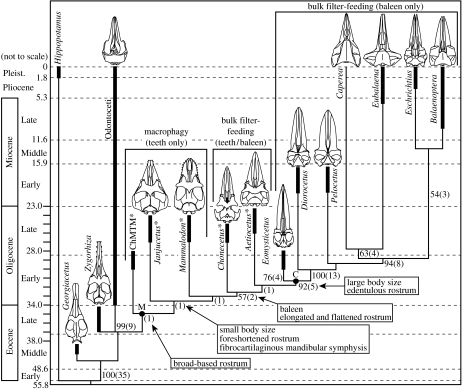
Phylogeny and stratigraphic record of Mysticeti, including Janjucetus, and the evolution of feeding ecology in mysticetes. Phylogenetic relationships of Janjucetus based on strict consensus of three trees derived from parsimony analysis of 266 characters in 26 genera (some taxa pruned from tree). Cetacean skull reconstructions shown in dorsal view. Characters relevant to the evolution of feeding in mysticetes are optimized on to the tree at nodes where they appear. Taxa marked with * represent toothed mysticetes. Numbers, and numbers in parentheses, at nodes represent bootstrap and branch support values, respectively. Solid circles denote named clades. Solid black bars on branches represent stratigraphic range error bars of their respective clade. Ages are in millions of years, with the time-scale being linear only for Late Eocene through Oligocene. Time-scale after Gradstein et al. (2004). Abbreviations: ChM TM, Charleston Museum toothed mysticetes; C, Chaeomysticeti; M, Mysticeti. (See electronic supplementary material for further information.)
The rostral portions of the premaxillae are robust, and on the anterior half of the rostrum, overhang the maxillae. The apical premaxillae form a distinctive incisor-bearing rosette (figure 1a,c). The maxilla represents 79% of rostral length, with its ascending process terminating anterior to the posterior edge of the nasal. The palatal surface of the maxilla (figure 1d) lacks any grooves and/or nutrient foramina correlated with the possession of baleen (Fordyce & Muizon 2001; Deméré 2005). The nasals are elongated and plate-like, with the external bony nares probably being located at a point level with M1.
The frontals are fused, and form a broad horizontal table that roofs the large orbits. Unusually for a mysticete, the orbits are positioned very high on the skull. The postorbital process of frontal is elongated ventrolaterally, with its distal end nearly contacting the zygomatic process of squamosal. A low sagittal crest forms the median suture between the parietals on the cranium (figure 1a,c). Lateral to the sagittal crest is a deep longitudinal groove (more prominent on the right parietal) that is interpreted as the site of origin for the deep temporalis musculature (figure 1a). In lateral view, the dorsal profile of the parietals is relatively low, as they do not rise posteriorly to meet the apex of the supraoccipital shield. The squamosal has a robust, dorsoventrally inflated, zygomatic process with an attenuated apex.
The periotic has extensive contact with the squamosal and exoccipital (figure 1d,e). However, the edges of the periotic are free of the squamosal. The tympanic bulla is basilosaurid-like in morphology (Kellogg 1936; Uhen 2004), articulating with the periotic and closely approximating the falciform process of squamosal. The outer lip of the tympanic bulla is fused to the anterior process of the periotic in a limited area immediately anterior to the mallear fossa (figure 1e). The pterygoid sinus fossa is well developed anterior to the periotic, but the peribullary and posterior sinus fossae are comparatively small (figure 1d,e). The roof of the pterygoid sinus fossa is formed primarily by alisphenoid, with the superior lamina of the pterygoid limited to the anterior extremity of the fossa. The basioccipital is broad and bears transversely expanded basioccipital crests, as in all mysticetes (Geisler & Sanders 2003). The vomer obscures the basioccipital/basisphenoid suture, and is exposed in the palate between the maxillae anterior to the level of P3.
The right mandible is nearly complete, robust and slightly bowed medially. The coronoid process is high and long. The mandibular symphysis is represented by a shallow longitudinal fossa, which indicates that it was not sutural but fibrocartilaginous, as in extant Mysticeti (Lambertsen et al. 1995). The mandibular foramen is large (figure 1d), and the mandible wall lateral to the foramen is transversely thin.
The dental formula of Janjucetus is I2 or I3/I2 or I3. C1/C1. P4/P4. M2/M3 (figure 2a–e). The crown enamel bears coarse longitudinal ridges, and cheek teeth (equal to post-canine dentition) possess accessory denticles. Upper teeth possess sharp mesial and distal carinae, are broad-based, and deeply rooted. All cheek teeth possess two roots. The upper incisors and canines are recurved, strongly caniniform and larger than post-canine teeth. Among other described toothed mysticetes only two taxa, Aetiocetus polydentatus Barnes et al. 1995 and M. colliveri, have reasonably complete dentitions. The incisors and canines of A. polydentatus are recurved, but are relatively smaller and more gracile than the anterior teeth of Janjucetus. Until recently, the anterior teeth of M. colliveri were unknown. Further examination of the matrix surrounding the holotype skull yielded a presumed upper incisor. This unique specimen shows that the upper incisors of Mammalodon were vestigial, being reduced to small peg-like teeth.
4. Phylogenetic relationships
To investigate the systematic position of Janjucetus within Cetacea, a data matrix consisting of 266 morphological characters in 26 taxa (adapted with modifications from Geisler & Sanders 2003), was analysed using parsimony in PAUP* v. 4.0b (Swofford 2002). The heuristic search option yielded three equally parsimonious trees of 1167 steps [consistency index (CI) 0.4790; retention index (RI) 0.5679; rescaled consistency index (RC) 0.2720], the strict consensus of which is shown in figure 3 (see electronic supplementary material for data matrix, analysis protocol and further discussion). This analysis posits Janjucetus within a stem-based Mysticeti (figure 3). Janjucetus shares two key synapomorphies with all other mysticetes: (i) laterally directed zygomatic process of maxilla with a steep anterior face clearly separating it from the rostral portion of the maxilla; and (ii) wide and bulbous basioccipital crests. Janjucetus represents a basal clade within Mysticeti, but is not the most basal-branching mysticete (Barnes & Sanders 1996; figure 3 and electronic supplementary material).
This analysis resolves the phylogenetic position of Mammalodon, showing that it is clearly an archaic mysticete, being the sister group of Chonecetus goedertorum and all more derived Mysticeti. Unlike all previous studies (Barnes et al. 1995; Geisler & Sanders 2003; Berta & Deméré 2005; Deméré et al. 2005; Ichishima 2005), this hypothesis suggests that the diverse Aetiocetidae are a paraphyletic taxon.
Among described toothed mysticetes, J. hunderi is similar to C. goedertorum, A. cotylalveus and M. colliveri, but only in morphological features that are interpreted here as plesiomorphies (see electronic supplementary material). The distinctive suite of features possessed by Janjucetus, are unique among Mysticeti, if not all Cetacea. In addition to the latter, its lack of derived features diagnostic of other toothed mysticete clades, and its position in this phylogenetic hypothesis, warrants the placement of Janjucetus in a new family, the Janjucetidae. Therefore, at least six (including Llanocetus) distinct lineages of toothed archaic Mysticeti existed during the Oligocene.
5. Feeding ecology
Was Janjucetus hunderi a filter-feeder? This fundamental question, within the context of the phylogenetic position of Janjucetus, has broad implications for interpreting mysticete evolution. Several morphological features are functional correlates of bulk filter-feeding in mysticetes: (i) elongated and broadened rostrum and mandibles in proportion to total skull length (increase in buccal volume and area of filtering structure); (ii) reduced orbit and eye size, and orbits directed laterally (vision not critical in prey selection and capture); (iii) large, anteriorly sloping supraoccipital shield (increased area of attachment of epaxial musculature for dorsiflexion of head and resistance of downward torque on the head when the mouth is opened); (iv) reduced/absent dentition; and (v) rostrum and mandibles not thickened or heavily ossified (regulators of water flow into oral cavity but not active elements in prey capture; Sanderson & Wassersug 1993). Janjucetus lacks all of the latter characteristics functionally correlated with a bulk filter-feeding prey capture method. The palatal surface of the maxilla of Janjucetus lacks the nutrient foramina and sulci associated with vascular supply to baleen plates (Slijper 1979; Fordyce & Muizon 2001; Deméré 2005). This is strong evidence against Janjucetus possessing baleen, even in an incipient form.
In addition, the following morphological features possessed by Janjucetus indicate that it was not a planktivorous bulk filter-feeder, but a macrophagous predator that captured single prey items: large, sharp, recurved, broad-based and deeply rooted anterior teeth; upper incisors arranged in a robust premaxillary rosette; incisors and canines possess mesial and distal carinae and sharp longitudinal ridges on their labial and lingual surfaces; cheek teeth distal accessory denticles have sharp, carinate edges; temporalis muscle origin on cranium very large, with a well-developed coronoid process for extensive temporalis insertion on mandible, thus enabling powerful adduction of mandibles; and robust mandibular rami (figures 1 and 2).
The pattern of tooth wear (figure 2c–e), suggests that anterior teeth interlocked, and cheek teeth sheared against one another during occlusion. It has been hypothesized that toothed mysticetes used their denticulate teeth in a filtering function (Barnes et al. 1995; Fordyce & Muizon 2001; Ichishima 2005), analogous to that employed by crabeater seals (Lobodon carcinophaga; Sanderson & Wassersug 1993; Adam & Berta 2002; Marshall 2002). The presence of heavy shear and apical wear facets on its teeth (which Lobodon teeth lack), and absence of interdigitating lattice-like dentition, indicates that the teeth of Janjucetus did not serve a suspension-feeding function (see electronic supplementary material for further discussion).
The robust skull, foreshortened rostrum with buttressed apex, relatively elongated cranium and strong development of caniniform anterior dentition (figure 2a–c), along with other features noted above, of Janjucetus are all morphological features observed in the leopard seal Hydrurga leptonyx and some extinct marine reptiles (e.g. the pliosauroid Rhomaleosaurus; Massare 1987; Taylor 1992; Cruickshank 1994; Adam & Berta 2002). It is hypothesized that the masticatory system of J. hunderi is convergent on that of the leopard seal and pliosauroid marine reptiles. Janjucetus thus probably captured relatively large individual prey items, such as fish, in a manner analogous to those marine tetrapods, perhaps using a grip and tear feeding method like the leopard seal, and inferred for Rhomaleosaurus, to dismember larger prey (Taylor 1992; Cruickshank 1994; Adam & Berta 2002).
Extant mysticetes and odontocetes differ markedly in their auditory adaptations for perceiving their environment and detecting prey (Wartzok & Ketten 1999). Living mysticetes produce low frequency sounds, and probably have acute infrasonic (less than 20 Hz; but not ultrasonic) hearing, whereas odontocetes can hear ultrasonic sounds (greater than 20 kHz; Ketten 1992; Wartzok & Ketten 1999). In addition, odontocetes echolocate, whereby they sense their environment by analysing echoes from self-generated ultrasonic signals (Ketten 1992; Cranford et al. 1996; Wartzok & Ketten 1999, p. 125). The ultrasonic outgoing component of odontocete echolocation is produced by the nasal sac system in the blowhole, and propagated via the melon (a mass of low density lipids) situated anterior to the blowholes (Ketten 1992; Cranford et al. 1996; Berta et al. 2006). The ability to produce ultrasonic sounds, and hence echolocate, has been inferred for almost all fossil odontocetes (Fordyce & Muizon 2001).
Extant mysticetes possess a mass of adipose tissue anterior to the blowholes that has been interpreted as a structure homologous with the melon of odontocetes (Heyning & Mead 1990). The latter authors suggested that the original function of the melon in cetaceans was to facilitate free movement of the nasal plugs during retraction of nasal plug muscles in respiration. Thus, the possession of a hypertrophied melon involved in sound production would represent a synapomorphy of Odontoceti. Contrary to this interpretation, Milinkovitch (1995) proposed that the adipose tissue in extant mysticetes represents a vestigial melon, and that mysticetes secondarily lost ultrasonic echolocation capabilities. In the latter scenario, echolocation would be present in the common ancestor of mysticetes and odontocetes.
Was ultrasonic sound reception and production present in basal Mysticeti and subsequently lost in more crownward clades? The basal position of Janjucetus in mysticete phylogeny provides an opportunity to evaluate this question. It is not possible to determine whether or not Janjucetus possessed a hypertrophied melon. Nonetheless, the skull of Janjucetus lacks other unambiguous osteological features (e.g. premaxillary sac fossae; strongly concave facial region anterior to bony external nares) correlated with possession of odontocete-like nasofacial soft tissue implicated with production of ultrasonic signals. Janjucetus has an enlarged mandibular foramen, with the mandible wall lateral to the mandibular foramen consisting of mediolaterally thin and dense pan bone, as in odontocetes and basilosaurid archaeocetes, but unlike extant mysticetes, which lack those features (Ketten 1992; Uhen 2004). The enlarged mandibular foramen of Janjucetus perhaps housed a fat body medial to the thin pan bone (as in extant odontocetes; Ketten 1992), which acted as a low-density sound channel conducting incoming sound from the mandible to the middle ear. These data suggest that Janjucetus was probably not capable of echolocation, although reception of ultrasonic signals cannot be ruled out. Evidence furnished by Janjucetus corroborates the hypothesis of Heyning & Mead (1990) that ultrasonic echolocation is a synapomorphy of Odontoceti, and never evolved in mysticetes.
The relatively enormous orbit of Janjucetus provides additional support for the latter hypothesis. Among cetaceans, only the odontocete Odobenocetops peruvianus Muizon 1993 has an OD approaching that of Janjucetus (see electronic supplementary material). Apart from Odobenocetops, which either lacked or had greatly reduced echolocation capabilities, all odontocetes possess relatively small orbits and eyes (see electronic supplementary material). Given that orbit and eye size reflects importance of vision in vertebrates (Motani et al. 1999; Motani 2005), it is likely that Janjucetus relied on well-developed vision as its primary sense in perception of its environment. This probably compensated for its lack of echolocation capabilities. In the latter respects, Janjucetus could be interpreted as being convergent on Odobenocetops, extant phocid pinnipeds and Mesozoic ichthyosaurs, which all have large orbits and eyes, but do/did not echolocate (McGowan 1973; Wartzok & Ketten 1999; Schusterman et al. 2000; Muizon et al. 2002; Kear 2005).
6. Patterns in the evolution of mysticeti
Evidence for how mysticetes and their remarkable feeding adaptations evolved has proved elusive. Filter-feeding en masse, possession of baleen, lack of teeth in adults and large body size typify extant and fossil mysticete whales in the clade Chaeomysticeti (Pivorunas 1979; Sanderson & Wassersug 1993; Fordyce & Muizon 2001; Marshall 2002; Geisler & Sanders 2003; figure 3). The geologically oldest recognized mysticete, Llanocetus denticrenatus is known from the Eocene/Oligocene boundary (approx. 34 Myr) of Antarctica, possessed widely spaced teeth with broad palmate denticles, and perhaps ‘proto-baleen’, which supplemented the hypothesized filtering function of its teeth (Fordyce 2003; see electronic supplementary material for further discussion). Other toothed mysticetes include the diverse Aetiocetidae, which are thought to have been incipient bulk filter-feeders and possessed baleen, and Mammalodon colliveri, which lacked baleen but whose feeding ecology remains enigmatic (Barnes et al. 1995; Fordyce & Muizon 2001; Berta & Deméré 2005; Deméré 2005; Ichishima 2005; Sawamura et al. 2005; Berta et al. 2006).
Janjucetus hunderi is morphologically and ecologically unlike all other Mysticeti, and indeed all other known Cetacea: it was small (relative to other mysticetes), had a robust skull with foreshortened rostrum, possessed large eyes and exhibits adaptations for predatory macrophagy, not bulk filter-feeding. This is perhaps not unexpected, as macrophagy is presumably primitive for all Cetacea (Barnes et al. 1995). Nevertheless, Janjucetus clearly demonstrates that archaic mysticetes were surprisingly disparate from both their archaeocete (stem Cetacea) sister taxa and later diverging baleen whales.
The pattern of evolution of mysticete feeding adaptations was reconstructed within the framework of the phylogeny of Janjucetus (figure 3). This suggests that the evolution of filter-feeding adaptations was stepwise, with unequivocal adaptations for bulk filter-feeding not being present in basal mysticetes. It also implies that large body size evolved once in mysticetes. If L. denticrenatus proves to be a more basal branching mysticete than Janjucetus, it is plausible that large body size may have evolved twice in Mysticeti. Janjucetus, Mammalodon, Chonecetus and Aetiocetus all possess relatively large orbits, with a trend of decreasing OD/CBL ratio from Janjucetus through Aetiocetus, culminating in the relatively tiny orbits (and eyes) of Chaeomysticeti (see electronic supplementary material). It is hypothesized that this trend correlates with the evolution of pelagic bulk filter-feeding and large body size, and implies a fundamental shift from capture of single prey items, where acute vision is critical, to pelagic engulfment of small prey in bulk, where such heightened vision is less important. Macrophagous basal mysticetes probably relied on underwater vision for capture of individual prey items. This corroborates the hypothesis that mysticetes never evolved sophisticated echolocation, as used by odontocetes.
The phylogeny of Mysticeti presented here agrees with the concept of an explosive radiation of crown group cetaceans during the Oligocene, particularly the Early Oligocene (28.5–34.0 Myr (ago); Fordyce 1992; Nikaido et al. 2001; Sasaki et al. 2005). This adaptive radiation was contemporaneous with global climate change, and increased productivity and heterogeneity of oceanic environments, associated with the establishment of the Antarctic Circumpolar Current following the final break-up of Gondwana and isolation of Antarctica (Fordyce 1980, 1992; Nikaido et al. 2001). Previous studies have emphasized the primacy of filter-feeding as the impetus for the initial radiation of mysticetes (Fordyce 1980; Fordyce 1992; Fordyce & Barnes 1994; Barnes et al. 1995; Fordyce & Muizon 2001; Nikaido et al. 2001; Berta et al. 2006; but the novel morphology and basal position of Janjucetus (and Mammalodon) in mysticete phylogeny, which are both hypothesized to have not been filter-feeders, suggests that the initial radiation of mysticetes was not linked to the evolution of filter-feeding.
Prior to the evolution of bulk filter-feeding, a modest radiation of stem Mysticeti included the evolution of small cetaceans that were convergent on some Mesozoic marine reptiles, and extant phocid seals. The Oligocene evolution of mysticetes therefore appears to have been characterized by greater diversity and disparity, and thus complexity, than previously thought. Mysticetes evidently occupied a wide range of ecological roles during their early evolutionary history, being substantially more disparate than their Miocene to recent representatives would otherwise indicate.
Acknowledgments
I thank S. Hunder and the Dixon family for discovering the holotype of Janjucetus and saving this remarkable fossil for science. D. P. Domning, B. P. Kear, J. A. Long, T. H. Rich and P. Vickers-Rich are thanked for their critical comments and discussion of earlier versions of this manuscript, L. G. Barnes, T. A. Darragh, A. E. Sanders, J. H. Geisler and F. C. Whitmore, Jr, for discussions and R. Start for superb photography. R. E. Fordyce and two anonymous referees are thanked for their thoughtful and constructive reviews of the manuscript. L. G. Barnes, D. J. Bohaska, R. E. Fordyce, T. H. Rich and A. E. Sanders provided access to specimens. Preparation was facilitated and supported by Museum Victoria. This work was supported by an Australian Postgraduate Award, Monash University and Museum Victoria.
Supplementary Material
References
- Adam P.J, Berta A. Evolution of prey capture strategies and diet in the Pinnipedimorphia (Mammalia, Carnivora) Oryctos. 2002;4:83–107. [Google Scholar]
- Bannister J.L. Baleen whales. In: Perrin W.F, Würsig B, Thewissen J.G.M, editors. Encyclopedia of marine mammals. Academic Press; San Diego, CA: 2002. pp. 62–72. [Google Scholar]
- Barnes L.G, Sanders A.E. The transition from archaeocetes to mysticetes: Late Oligocene toothed mysticetes from near Charleston, South Carolina. Paleontol. Soc. Spec. Publ. 1996;8:24. [Google Scholar]
- Barnes L.G, Kimura M, Furusawa H, Sawamura H. Classification and distribution of Oligocene Aetiocetidae (Mammalia; Cetacea; Mysticeti) from western North America and Japan. The Island Arc. 1995;3:392–431. doi:10.1111/j.1440-1738.1994.tb00122.x [Google Scholar]
- Berta A, Deméré T.A. Phylogenetic relationships among the diverse toothed mysticete clade the Aetiocetidae and reconsideration of the filter feeding niche. Cranbrook Inst. Sci. Misc. Publ. 2005;1:9. [Google Scholar]
- Berta A, Sumich J.L, Kovacs K.M. 2nd edn. Academic Press; San Diego, CA: 2006. Marine mammals: evolutionary biology. [Google Scholar]
- Cranford T.W, Amundin M, Norris K.S. Functional morphology and homology in the odontocete nasal complex: implications for sound generation. J. Morphol. 1996;228:223–285. doi: 10.1002/(SICI)1097-4687(199606)228:3<223::AID-JMOR1>3.0.CO;2-3. doi:10.1002/(SICI)1097-4687(199606)228:3<223::AID-JMOR1>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- Cruickshank A.R.I. Cranial anatomy of the Lower Jurassic pliosaur Rhomaleosaurus megacephalus, Stuchbury (Reptilia: Plesiosauria) Phil. Trans. R. Soc. B. 1994;343:247–260. [Google Scholar]
- Deméré T.A. Palate vascularization in an Oligocene toothed mysticete (Cetacea: Mysticeti: Aetiocetidae); implications for the evolution of baleen. Cranbrook Inst. Sci. Misc. Publ. 2005;1:21. [Google Scholar]
- Deméré T.A, Berta A, McGowen M.R. The taxonomic and evolutionary history of fossil and modern balaenopteroid mysticetes. J. Mammal. Evol. 2005;12:99–143. doi:10.1007/s10914-005-6944-3 [Google Scholar]
- Fitzgerald E.M.G. A review of the Tertiary fossil Cetacea (Mammalia) localities in Australia. Mem. Mus. Vic. 2004;61:183–208. [Google Scholar]
- Fitzgerald E.M.G. Toothed mysticetes (Mammalia: Cetacea) from the Late Oligocene of Australia. Cranbrook Inst. Sci. Misc. Publ. 2005;1:25. [Google Scholar]
- Fordyce R.E. Whale evolution and Oligocene Southern Ocean environments. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1980;31:319–336. doi:10.1016/0031-0182(80)90024-3 [Google Scholar]
- Fordyce R.E. Cetacean evolution and Eocene/Oligocene environments. In: Prothero D.R, Berggren W.A, editors. Eocene–Oligocene climatic and biotic evolution. Princeton University Press; Princeton, NJ: 1992. pp. 368–381. [Google Scholar]
- Fordyce R.E. Early crown group Cetacea in the Southern Ocean: the toothed archaic mysticete Llanocetus. J. Vertebr. Paleontol. 2003;23(Suppl. 3):50A. [Google Scholar]
- Fordyce R.E, Barnes L.G. The evolutionary history of whales and dolphins. Annu. Rev. Earth Planet. Sci. 1994;22:419–455. doi:10.1146/annurev.ea.22.050194.002223 [Google Scholar]
- Fordyce R.E, Muizon C. de. Evolutionary history of cetaceans: a review. In: Mazin J.-M, de Buffrénil V, editors. Secondary adaptation of tetrapods to life in water. Verlag Dr. Friedrich Pfeil; München, Germany: 2001. pp. 169–233. [Google Scholar]
- Geisler J.H, Sanders A.E. Morphological evidence for the phylogeny of Cetacea. J. Mammal. Evol. 2003;10:23–129. doi:10.1023/A:1025552007291 [Google Scholar]
- Gingerich P.D. Paleobiological perspectives on Mesonychia, Archaeoceti, and the origin of whales. In: Thewissen J.G.M, editor. The emergence of whales. Plenum Press; New York, NY: 1998. pp. 423–429. [Google Scholar]
- Gingerich P.D. Cetacea. In: Rose K.D, Archibald J.D, editors. The rise of placental mammals. Johns Hopkins University Press; Baltimore, MD: 2005. pp. 234–252. [Google Scholar]
- Gradstein F, Ogg J, Smith A, editors. A geologic time scale 2004. Cambridge University Press; Cambridge, UK: 2004. [Google Scholar]
- Heyning J.E, Mead J.G. Evolution of the nasal anatomy of cetaceans. In: Thomas J, Kastelein R, editors. Sensory abilities of cetaceans. Plenum Press; New York, NY: 1990. pp. 67–79. [Google Scholar]
- Holdgate G.R, Gallagher S.J. Tertiary: a period of transition to marine basin environments. In: Birch W.D, editor. Geology of Victoria. Geology Society Australia; Sydney, NSW: 2003. pp. 289–335. Spec. Publ. 23. [Google Scholar]
- Ichishima H. Notes on the phyletic relationships of the Aetiocetidae and the feeding ecology of toothed mysticetes. Bull. Ashoro Mus. Paleontol. 2005;3:111–117. [Google Scholar]
- Kear B.P. Cranial morphology of Platypterygius longmani Wade, 1990 (Reptilia: Ichthyosauria) from the Lower Cretaceous of Australia. Zool. J. Linn. Soc. 2005;145:583–622. doi:10.1111/j.1096-3642.2005.00199.x [Google Scholar]
- Kellogg A.R. A review of the Archaeoceti. Carnegie Inst. Washington Publ. 1936;482:1–366. [Google Scholar]
- Ketten D.R. The marine mammal ear: specializations for aquatic audition and echolocation. In: Webster D.B, Fay R.R, Popper A.N, editors. The evolutionary biology of hearing. Springer; New York, NY: 1992. pp. 717–750. [Google Scholar]
- Lambertsen R, Ulrich N, Straley J. Frontomandibular stay of Balaenopteridae: a mechanism for momentum recapture during feeding. J. Mammal. 1995;76:877–899. [Google Scholar]
- Marshall C.D. Morphology, functional. In: Perrin W.F, Würsig B, Thewissen J.G.M, editors. Encyclopedia of marine mammals. Academic Press; San Diego, CA: 2002. pp. 759–774. [Google Scholar]
- Massare J.A. Tooth morphology and prey preference of Mesozoic marine reptiles. J. Vertebr. Paleontol. 1987;7:121–137. [Google Scholar]
- McGowan C. The cranial morphology of the Lower Liassic latipinnate ichthyosaurs of England. Bull. Br. Mus. (Nat. Hist.) Geol. 1973;24:1–109. [Google Scholar]
- Milinkovitch M.C. Molecular phylogeny of cetaceans prompts revision of morphological transformations. Trends Ecol. Evol. 1995;10:328–334. doi: 10.1016/s0169-5347(00)89120-x. doi:10.1016/S0169-5347(00)89120-X [DOI] [PubMed] [Google Scholar]
- Mitchell E.D. A new cetacean from the Late Eocene La Meseta Formation, Seymour Island, Antarctic Peninsula. Can. J. Fish. Aquatic Sci. 1989;46:2219–2235. [Google Scholar]
- Motani R. Evolution of fish-shaped reptiles (Reptilia: Ichthyopterygia) in their physical environments and constraints. Annu. Rev. Earth Planet. Sci. 2005;33:395–420. doi:10.1146/annurev.earth.33.092203.122707 [Google Scholar]
- Motani R, Rothschild B.M, Wahl W., Jr Large eyeballs in diving ichthyosaurs. Nature. 1999;402:747. doi: 10.1016/s0002-9394(00)00518-3. doi:10.1038/45435 [DOI] [PubMed] [Google Scholar]
- Muizon C. de. Walrus-like feeding adaptation in a new cetacean from the Pliocene of Peru. Nature. 1993;365:745–748. doi:10.1038/365745a0 [Google Scholar]
- Muizon C. de, Domning D.P, Ketten D.R. Odobenocetops peruvianus, the walrus-convergent delphinoid (Mammalia: Cetacea) from the Early Pliocene of Peru. Smithsonian Contrib. Paleobiol. 2002;93:223–261. [Google Scholar]
- Nikaido M, et al. Retroposon analysis of major cetacean lineages: the monophyly of toothed whales and the paraphyly of river dolphins. Proc. Natl Acad. Sci. USA. 2001;98:7384–7389. doi: 10.1073/pnas.121139198. doi:10.1073/pnas.121139198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivorunas A. The feeding mechanisms of baleen whales. Am. Sci. 1979;67:432–440. [Google Scholar]
- Pritchard G.B. On the discovery of a fossil whale in the older Tertiaries of Torquay, Victoria. Vic. Nat. 1939;55:151–159. [Google Scholar]
- Sanderson S.L, Wassersug R. Convergent and alternative designs for vertebrate suspension feeding. In: Hanken J, Hall B.K, editors. The skull. vol. 3. Chicago University Press; Chicago, IL: 1993. pp. 37–112. [Google Scholar]
- Sasaki T, et al. Mitochondrial phylogenetics and evolution of mysticete whales. Syst. Biol. 2005;54:77–90. doi: 10.1080/10635150590905939. doi:10.1080/10635150590905939 [DOI] [PubMed] [Google Scholar]
- Sawamura H, Ichishima H, Ito H, Ishikawa H. The baleen of mysticetes grows on the alveolar process of maxilla: comparative anatomy of the fetus of the minke whale. Cranbrook Inst. Sci. Misc. Publ. 2005;1:76–77. [Google Scholar]
- Schusterman R.J, Kastak D, Levenson D.H, Reichmuth C.J, Southall B.L. Why pinnipeds don't echolocate. J. Acoust. Soc. Am. 2000;107:2256–2264. doi: 10.1121/1.428506. doi:10.1121/1.428506 [DOI] [PubMed] [Google Scholar]
- Slijper E.J. 2nd edn. Hutchinson; London, UK: 1979. Whales. [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, MA: 2002. PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. [Google Scholar]
- Taylor M.A. Functional anatomy of the head in the large aquatic predator Rhomaleosaurus zetlandicus (Plesioauria, Reptilia) from the Toarcian (Lower Jurassic) of Yorkshire, England. Phil. Trans. R. Soc. B. 1992;335:247–280. [Google Scholar]
- Thewissen J.G.M, Williams E.M. The early radiations of Cetacea (Mammalia): evolutionary pattern and developmental correlations. Annu. Rev. Ecol. Syst. 2002;33:73–90. doi:10.1146/annurev.ecolsys.33.020602.095426 [Google Scholar]
- Uhen M.D. Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): an archaeocete from the Middle to Late Eocene of Egypt. Univ. Michigan Paper Paleontol. 2004;34:1–222. [Google Scholar]
- Wartzok D, Ketten D.R. Marine mammal sensory systems. In: Reynolds J.E III, Rommel S.A, editors. Biology of marine mammals. Melbourne University Press; Melbourne, Australia: 1999. pp. 117–175. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.