Abstract
Animals that live in cooperative societies form hierarchies in which dominant individuals reap disproportionate benefits from group cooperation. The stability of these societies requires subordinates to accept their inferior status rather than engage in escalated conflict with dominants over rank. Applying the logic of animal contests to these cases predicts that escalated conflict is more likely where subordinates are reproductively suppressed, where group productivity is high, relatedness is low, and where subordinates are relatively strong. We tested these four predictions in the field on co-foundress associations of the paper wasp Polistes dominulus by inducing contests over dominance rank experimentally. Subordinates with lower levels of ovarian development, and those in larger, more productive groups, were more likely to escalate in conflict with their dominant, as predicted. Neither genetic relatedness nor relative body size had significant effects on the probability of escalation. The original dominant emerged as the winner in all except one escalated contest. The results provide the first evidence that reproductive suppression of subordinates increases the threat of escalated conflict, and hence that reproductive sharing can promote stability of the dominant–subordinate relationship.
Keywords: aggression, fighting, reproductive skew, dominance hierarchies
1. Introduction
Cooperatively breeding animals typically form dominance hierarchies in which high-status individuals reap a disproportionate share of the benefits of group cooperation over reproduction, and can expect to produce many more offspring over their lifetime than individuals that remain subordinate (Wilson 1971; Stacey & Koenig 1990; Emlen 1995; Ratnieks et al. 2006). Dominant status thus represents a major ‘prize’ in the life history of social animals, and we can expect strong selection on strategies that help an individual reach the top of the hierarchy. In cooperatively breeding vertebrates and many social insects, subordinates can inherit dominance if they outlive those above them in the hierarchy (Strassmann & Meyer 1983; Wiley & Rabenold 1984; Field et al. 1999; Monnin & Peeters 1999; Monnin & Ratnieks 1999; Cant & Field 2001; Field et al. 2006). Alternatively, they may try to seize the dominant position by fighting (Davies 1992; Gust 1995; Monnin & Peeters 1999; Alberts et al. 2003). The stability of the dominant–subordinate relationship therefore requires that subordinates prefer to queue peacefully rather than engage in escalated conflict over rank.
The decision of a subordinate to initiate a fight over rank is a specific instance of the more general question of when to enter into an escalated conflict over a valuable resource (Maynard Smith & Price 1973; Parker 1974; Enquist & Leimar 1983, 1987). This question was first addressed in detail by Parker (1974). He modelled a contest between two individuals in different social roles: a holder of a resource and a challenger that could either fight for the resource or withdraw to look for alternative resources elsewhere. The model predicts that escalated conflict is more likely where challengers and holders are of similar resource holding potential (RHP), since in these circumstances the outcome of the contest is uncertain. In addition, the decision to fight or not will depend on the net value of winning to the two players, which may be different for animals in different social roles. In many cases, a holder of a resource has more to lose than the challenger stands to gain. For example, where a challenger must re-invest in the resource before realizing any gain from the patch, the absolute value of winning will be lower for the challenger than for a holder that has already provided the necessary investment (Parker 1974). Together, asymmetries in RHP and in net payoffs can account for observations across diverse taxa that the holders of a resource typically win against challengers (Davies 1978; Sigurjonsdottir & Parker 1981; Haley 1994; Johnson et al. 1999; Bridge et al. 2000).
Here, we apply the logic of these analyses to the potential conflict between a dominant and a subordinate in a social hierarchy. In this case the contested resource is social status and the attendant benefits this brings. To understand the factors influencing the subordinate's decision of whether to enter into an escalated fight over rank, we must take into account three additional features of the conflict between dominant and subordinate:
the contestants may be relatives;
subordinates may eventually obtain the resource without fighting, i.e. they may inherit if they outlive the dominant; and
subordinates may share the resource with the dominant without fighting, i.e. they may obtain a share of current reproduction.
The latter feature has led to the suggestion that dominants might offer a share of a resource to reduce a subordinate's incentive to challenge (Reeve & Ratnieks 1993). This ‘peace incentive’ model solves for the fraction of reproduction that would be necessary to appease a subordinate challenger, and the conditions under which it would pay a dominant to grant this share. The peace incentive model therefore assumes that reproductive skew is causally influenced by the payoffs to the subordinate of fighting. An alternative approach is to assume that skew is somehow determined first, and this determines the payoff of fighting. In this case, the payoffs of fighting are causally influenced by the level of skew. Here, we simply test whether there is an association between the degree of reproductive sharing and the frequency of escalated contests, so we are not able directly to tease out the direction of causality. However, we are able to test the central assumption of the peace incentive model that increased reproductive suppression should increase the risk of escalated fighting, and to test whether conflict over social status conforms to the general logic of animal contests (Parker 1974; Enquist & Leimar 1987).
In §2, we use a simple model to make explicit the expected effects of reproductive suppression, group productivity, relatedness and relative RHP on the payoffs of fighting. In §§3–5, we report an experiment on paper wasps to test these predictions. Part of the difficulty of studying within-group conflict is that actual fights are usually rare and difficult to observe, and it is sometimes hard to pin down exactly what the group members are fighting over. We circumvented this problem by inducing contests over dominance rank experimentally.
2. Escalated conflict over dominance rank
To help quantify (and visualize) the interaction between the factors that determine the payoff of fighting, it helps to construct a simple model. Consider a dominant and a single subordinate, symmetrically related by a coefficient, r. The two individuals form a social queue in which the subordinate individual can ascend to the rank 1 position upon the death of the dominant. Grouping is assumed to be stable because subordinates prefer queuing to dispersal, as is frequently the case where there are future benefits of group membership (Kokko & Johnstone 1999; Cant & English 2006). We assume that the outcome of an escalated conflict over rank is the subordination of the loser. This assumption fits with our empirical observations, as we report in §5.
For simplicity, let the dominant and subordinate experience the same constant mortality rate, m. When both breeders are alive, offspring are produced at a constant rate k (greater than 1). We assume that reproductive skew is determined in some unspecified way first, such that the dominant obtains a constant share, (1−p), and the subordinate obtains constant share, p, of the k offspring produced in each time unit (where p<0.5). If one of the females dies, the other breeder monopolizes reproduction and the group productivity drops to a standardized level of 1. Qualitatively identical results are obtained if instead we assume that a new subordinate (obtaining new share p′, which may differ from p) is recruited upon the death of one of the females (see electronic supplementary material). This means that the predictions of the model will also hold for conflicts between a dominant and a rank 2 subordinate in a larger group.
With these assumptions, the expected direct fitness of a dominant individual in a peaceful group is proportional to
| 2.1 |
and that of a subordinate is proportional to
| 2.2 |
where the constant of proportionality is half the expected lifespan 1/2m (see electronic supplementary material). The first term in these two expressions is the expected productivity of the dominant or the subordinate, respectively, for each instant that the two females are alive; the second term equal to unity is the expected productivity for each instant that the focal female is alive and its partner is dead. In the case of the subordinate, this means that it has inherited the nest.
We assume that escalated contests involve a fitness cost to both the players of c units, reflecting the time and effort wasted in fighting, and the potential for injury. The inclusive fitness payoff to a subordinate of fighting versus waiting can then be written.
In this expression, f is the probability that the subordinate wins an escalated conflict over rank. Thus, f is a measure of the relative RHP of the two individuals.
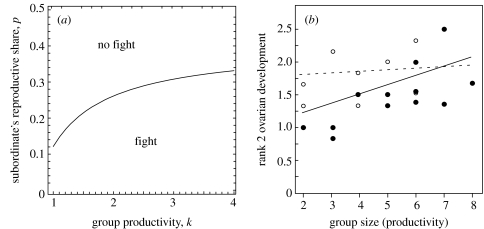
Subordinates will favour fighting over peaceful queuing, where the challenger's payoff W is greater than 0. This condition is illustrated graphically in figure 1a, which shows the zones for which the challenger's payoff is positive or negative as a function of group productivity k and the subordinate's share p. The contour line shown is that for which W=0. Two main predictions, evident from the figure, are that escalated conflict becomes more likely as the subordinate's share of reproduction decreases, and as group productivity increases. This is because both increased productivity and reduced subordinate share lead to an increase in the value of being dominant rather than subordinate. Two further intuitive predictions are that the probability of escalated conflict will increase with increasing subordinate strength, f, and with decreasing subordinate relatedness, r. Increasing f and decreasing r both have the effect of shifting the pitch of the zero-contour line in figure 1a upwards. These results are presented more formally in the electronic supplementary material.
Figure 1.
(a) Zones for which the challenger's payoff is positive (‘fight’) or negative (‘no fight’), as a function of group productivity and the subordinate's fraction of reproduction. In the example shown, the dominant and the subordinate are full sisters (r=0.75), of equal strength (f=0.5) and the cost of fighting is c=0.05 fitness units. (b) Results of the dominant removal–reintroduction experiment. Mean ovarian development of rank 2 subordinates is plotted as a function of group size, which is an index of group productivity. Solid circles, rank 2 individuals that engaged in an escalated contest with the returning dominant; open circles, rank 2s that immediately submitted to the returning dominant. The significant least-squares regression for all rank 2s is shown (solid line). In addition, the non-significant least-squares regression of dominant ovarian development is plotted against group size (dashed line). Both the level of subordinate ovarian development and group size had significant effects on the probability of an escalated contest.
To summarize, we can make four testable predictions about the frequency of escalated conflict between a dominant and a subordinate in a social hierarchy. Escalated conflict becomes more likely as: (i) the subordinate's share of reproduction decreases; (ii) total group productivity increases; (iii) relatedness decreases; and (iv) the relative strength of the subordinate increases. In §§3–5, we describe the field experiment that we used to test these four predictions.
3. Experimentally induced contests over rank in Polistes dominulus
Our experiment involved the removal of dominant individuals from co-foundress associations of Polistes dominulus to allow a subordinate to inherit the nest. Once the replacement dominant had become established, we reintroduced the original dominant and recorded the resulting interaction between the two wasps vying for the rank 1 position. We then collected the two wasps and measured them for ovarian development, genetic relatedness and relative size (as an index of RHP). Group size and number of cells in the nest were taken as indices of group productivity. We employed this experiment as a test of our predictions because the magnitude of the challenger's payoff, measuring as it does the inclusive fitness differential between fighting for the role of dominant versus accepting subordinate status, also measures the extent to which a newly promoted individual would benefit from fighting to retain dominant status rather than accept demotion to the subordinate role.
4. Study site and methods
The study was carried out in farmland near Conil de la Frontera, Cadiz, Spain, between March and May 2003. Associations between co-foundresses form in early spring. Nests were located on hedges of Opuntia cactus in a 100 m2 area of fallow pasture. The groups comprise a single dominant individual and 1–10 subordinates that form a linear hierarchy in which each wasp directs most of its aggression to those individuals at adjacent rank (Cant et al. 2006). Approximately 30% of dominants die in the two-month founding phase prior to the emergence of workers, in which case the rank 2 subordinate can inherit the nest (Cant & Field 2001). A rank 2 subordinate can therefore inherit by outliving the dominant, but escalated fights over dominance do also occur (Reeve 1991; M. Cant and J. Field 2003, personal observations).
All foundresses (range 2–8 individuals) on 35 nests were captured and marked with enamel paint using methods described elsewhere (Cant & Field 2001; Shreeves et al. 2003; Cant et al. 2006). Twenty-eight nests were assigned at random as experimental nests in which the dominant individual was to be removed and later reintroduced; seven nests were assigned at random as controls in which a known non-dominant wasp was to be removed and reintroduced. This was to control the possibility that resident wasps were responding to a wasp potentially perceived as foreign after separation from the nest rather than to the return of the dominant per se.
The dominant individual on each P. dominulus nest can be readily identified from daytime censuses because it rarely leaves the nest, whereas subordinates spend most of their time away from the nest foraging (Reeve 1991; Cant & Field 2001). Following previous studies (Cant & Field 2001; Cant et al. 2006), we classed as dominant those wasps that were present on the nest for more than 66% of daytime censuses (mean time on nest of dominants±s.e.=94±1.9%). After identifying the dominant foundress, we videotaped each nest continuously for 3 h on a warm day (shade temperature range 19.5–27.0°C) between the hours of 11.00 and 17.00 for the later scoring of aggression. Aggression rates were scored as the number of aggressive acts directed by the dominant towards the rank 2 wasp per minute that the two were together on the nest (see Cant et al. 2006).
On the morning after each nest had been videoed, we captured the dominant individual (or a known non-dominant, in the case of the control nests) and transferred it to a clear plastic tube with a perforated cap. The tube containing the removed wasp was then stored in a refrigerator at 5°C for a period ranging from 3 to 8 days. This is within the range of night temperatures at the site during early spring, and wasps in the refrigerator became inactive in much the same way as co-foundresses on the nest become inactive overnight. When removed from the refrigerator, all the wasps regained full activity within a few minutes of their return to ambient day temperature, and we were unable to detect any adverse effects of the time spent in storage on mobility or flying ability.
During the period for which dominants were held in storage, we censused nests repeatedly (average 14 censuses) and identified the replacement dominant (i.e. the rank 2 wasp) as the individual (one per nest) that was present on the nest the most (mean time on nest for replacement dominants=92±3.7%, versus 50±4.0% for the same individuals when at rank 2). In all the cases, the rank 2 wasp identified from census data matched the rank 2 inferred from later videos of the dominant reintroductions, confirming the reliability of our method based on the census data. Once the rank 2 individual had been identified, the original dominant was taken from storage and released 1 m from the nest between 11.00 and 18.00 on a sunny warm day. In 25 out of 28 trials, dominants were released on day 3, 4 or 5 after their initial removal, but due to bad weather two trials were delayed to day 6 and a single trial to day 8. All the trials were conducted on nests in the founding phase, prior to the emergence of workers.
Video cameras were used to record the return of the dominant to the nest (median time from release to return=8 min, range<1–55 min), and releases were timed so that the rank 2 individual was present on the nest at the time of release. Nests were videoed for the duration of any dominance interactions or aggressive activity following the arrival of the released wasp, and recording continued for 10 min after the cessation of dominance activity. Returning dominants immediately initiated a dominance interaction with the individual that had newly inherited the nest. These interactions began with mutual antennation followed immediately either by the submission of one of the wasps, or by an escalated contest involving biting, prolonged grappling with the forelegs, and in some cases repeated ‘falling fights’ in which the duelling wasps attempted to sting each other. Dominance interactions were classed as ‘escalated’ if they involved grappling lasting more than 4 s, or a falling fight and ‘non-escalated’ if they involved only antennation followed by submission. In practice, escalated and non-escalated interactions were qualitatively different and easy to categorize. Only one interaction (lasting 2.5 s) was ambiguous, so we checked that the categorization of this data point did not qualitatively affect our results. The duration of escalated interactions was measured from the video recordings using a stopwatch.
(a) Ovarian development and genetic relatedness
The original dominant and the rank 2 individual were collected together on the morning after the reintroduction experiment and immediately frozen at −5°C. Some samples became degraded, but we were able to obtain data on ovarian development in both rank 1 and rank 2 wasps for 20 of the 28 trials of the experiment. These wasps were dissected and measured for ovarian development in July and August 2004. Abdomens were dissected in 1% saline solution and measurements taken through a dissecting microscope at either 25× or 50× magnification. Ovarian development in each of the six ovarioles of each female was scored on a scale 0–3 according to the size and the number of eggs present. The scores for each ovariole were assigned as follows: threadlike ovariole, no recognizable eggs=0; eggs<1 mm long present=1; eggs>1 mm long present, no chorion (shell)=2; eggs>1 mm with chorion=3. A single ‘ovarian development score’ for each wasp was calculated as the mean of these six ovariole scores. Body size was taken as wing length measured under a dissecting microscope, and the relative body size calculated as log (DWL/SWL), where DWL and SWL are the wing-length measurements of the dominant and subordinate, respectively.
We estimated dyadic relatedness for a subset of 23 nests using highly polymorphic microsatellite markers. Six microsatellite loci were amplified from DNA of the original dominant and its replacement on 21 experimental nests (loci Pdom7, Pdom20, Pdom122, Pdom127b, Pdom139 and Pdom140; Henshaw 2000). The wasps from approximately half of the nests were genotyped at Pdom20 and Pdom122, whereas all the individuals were scored at the remaining four loci. Population allele frequencies were estimated using the data from these nests and females from two additional nests that were genotyped at the same loci. The PCR products were separated on 6% polyacrylamide gels using standard molecular protocols (Strassmann et al. 1996). The loci had 9 (Pdom7), 14 (Pdom20), 20 (Pdom122), 19 (Pdom127b), 11 (Pdom139) and 9 (Pdom140) alleles represented in our samples. The alleles were scored twice independently, and all of the samples were typed twice at each locus to minimize the chance of errors. The relatedness was estimated using the program Relatedness v. 5.08 (www.gsoftnet.us/GSoft.html: Queller & Goodnight 1989). Colonies were weighted equally and standard errors were obtained by jackknifing over loci. We also used the program Kinship v. 1.3.1 (www.gsoftnet.us/GSoft.html) to test whether each dominant–replacement pair was significantly more likely to comprise full sisters (r=0.75) than cousins (r=0.1875), the next nearest likely relationship. The Kinship analysis had a power of 99.7% to detect full sisters at the α=0.05 level.
Statistical analyses were performed using generalized linear model (GLM) in the Genstat v. 6.0 package (Lawes Agricultural Trust; www.vsn-intl.com). For the analysis of the factors affecting the probability of an escalated contest, we used binomial errors and a logit link function. Data on contest duration were log transformed before analysis to permit the specification of a normal error structure. For both analyses, we fitted nine terms (temperature, date, nest size, number of days that the dominant was away, group size, relative body size, dominant aggression rate, subordinate ovarian development and dominant ovarian development) in a maximal model and then dropped terms by backward elimination until further removals led to a significant (p<0.05) decrease in the explanatory power of the model, as assessed from tabulated values of F (when using normal errors) or χ2 (when using binomial errors). We report significance levels when dropping each term individually from the minimum model containing only significant terms. For conservatism, the degrees of freedom used in significance tests reflect the number of terms fitted in the maximal, rather than the minimal, model.
5. Results
The return of the original dominant led to an escalated contest with the rank 2 individual in 17 out of 28 trials. In the other 11 trials, the rank 2 wasp immediately submitted to the returning dominant, lowering its antennae and allowing itself to be mounted. The dominants appeared specifically to seek out rank 2 individuals, and individuals of lower rank were ignored by the returning dominant even if they happened to be encountered first, suggesting either that the identity of the next individual to inherit was known to the dominant prior to its removal, or that the new dominant behaves in a recognizable way. In all the seven control trials involving the removal and release of subordinate wasps, the returning individual landed on the nest and immediately submitted to the dominant foundress (rate of escalation in experimental versus control trials: χ12=8.26, p=0.01).
In a GLM, two terms had significant effects on the probability of an escalated contest upon the return of the dominant: group size, and the level of ovarian development in the rank 2 individual. Rank 2 wasps with a lower ovarian development score, and those in larger groups, were more likely to fight the returning dominant (ovarian development: χ1,132=7.7, p=0.006; group size: χ1,132=5.66, p=0.017; see figure 1b). All other terms in the GLM (temperature, date, days away, relative body size, nest size, dominant aggression rate and dominant ovarian development) were non-significant when dropped from the model containing the two significant terms (all p>0.2, except dominant aggression rate, p=0.1). In contrast to the pattern for rank 2 individuals, the level of ovarian development in dominants had no effect on the probability of an escalated contest (χ1,132=0.05, p=0.83). There was no relationship between dominant ovarian development and group size (F1,16=0.62, p=0.43; figure 1b), but there was a significant positive relationship between group size and ovarian development in rank 2 wasps (F1,18=4.48, p=0.04; figure 1b). Subordinate ovarian development did not vary with either relative or absolute body size (relative body size: F1,16=1.42, p=0.27; absolute body size F1,16=0.142, p=0.25).
Ovarian development in dominants was significantly greater than that of rank 2s (paired t-test: t17=2.36, p=0.03). Importantly, there was no relationship between the number of days that the dominant was away from the nest and the level of ovarian development in rank 2 individuals (F1,17=0.04, p=0.84), among dominant individuals (F1,17=0.1, p=0.75) or among all individuals combined (F1,34=0.13, p=0.72), suggesting that observed levels of ovarian development largely reflect pre-removal levels. This is plausible, given the relatively short period of the dominant's absence (5 days or less in 90% of trials).
Estimates of the dyadic relatedness between dominant and rank 2 wasps were obtained for 21 experimental nests, 14 of which involved an escalated contest. In 19 out of 21 nests, mean relatedness between rank 1 and rank 2 individuals was high (mean±s.e.=0.75±0.03), and was not significantly different from the values expected from sampling of full sisters. On two nests, both involving an escalated contest, relatedness between dominant and subordinate was significantly lower than that expected for full sisters (dyadic relatedness values 0.07 and 0.16). There was no indication that relatedness had any influence on the probability of an escalated contest (escalated contests: n=14, mean relatedness±s.e.=0.67±0.07; non-escalated contests: n=7; mean relatedness =0.72±0.06; unpaired t-test: t19=0.59, p=0.56). Neither relatedness nor a binary sister/non-sister term (assigned using the Kinship program) had significant effects on the probability of escalation when included as terms in a GLM with group size and subordinate ovarian development (relatedness: χ1,142=1.96, p=0.16; sister/non-sister: χ1,142=1.9, p=0.17).
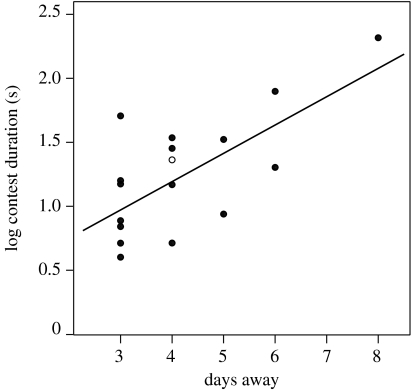
Among the 17 trials that led to an escalated contest, median contest duration was 15.9 s (inter-quartile range 7.5–33.5 s; range 4.0–207.5 s). The contest duration increased exponentially with the number of days that the dominant had been away from the nest (linear regression using log-transformed data: F1,15=13.75, p=0.002; figure 2). This relationship remained significant when a high-leverage data point at 8 days was excluded (F1,14=4.33, p=0.04). All other terms in the GLM, including relative body size, had non-significant effects and were dropped from the model. Across all trials, the original dominant was successful in regaining its position in 27 out of 28 trials. An exception occurred when a returning dominant (from a group of five) submitted after an escalated contest lasting 20 s. This ex-dominant then immediately mounted the rank 3 individual, which submitted without a struggle and had no further interaction with the ex-dominant in the 10 min following the end of fight. This behaviour suggests that the ex-dominant, having lost an escalated conflict over the rank 1 position, was demoted to the rank 2 position in the group, in line with the assumptions of our model.
Figure 2.
Contest duration versus number of days for which the dominant was removed in the 17 out of 28 trials that involved an escalated contest. Note the log scale on the y-axis. The significant least-squares regression is shown (p=0.002). Open circle, the single trial in which the original dominant lost the contest.
6. Discussion
Our experiment shows that a newly promoted subordinate's decision of whether to fight to retain control of the nest depends on its degree of reproductive development and on the size of the group. Specifically, subordinates with lower levels of ovarian development, and those in larger, more productive groups, were more likely to enter into the escalated conflict with the returning dominant. Genetic relatedness between dominant and subordinate was typically high (greater than 0.5 on 90% of nests), suggesting that most pairs of females tested were full sisters. Only 2 out of 21 dominant–subordinate dyads were distantly related, compared to 35% of foundresses in an Italian population of P. dominulus (Queller et al. 2000). Our attempt to detect a relatedness effect, therefore, may have been limited by low variation at our site. However, the results accord with previous evidence that paper wasps do not distinguish degrees of relatedness among nestmates (Queller et al. 1990). The probability of escalation did not depend on relative body size, indicating that factors other than size or RHP play a key role in the decision to fight.
The result that subordinates with relatively high levels of ovarian development were less likely to fight the returning dominant concurs with the logic of our simple model. We predicted that subordinates which obtain a larger fraction of current reproduction will have less to gain from a reversal in the dominance roles than those which obtain little or no share of reproduction, and so will be less likely to enter into a potentially costly fight for control of the nest. Ovarian development reflects reproductive partitioning in paper wasps and other social insects (Reeve 1991; Field et al. 1998; Seppa et al. 2002; Sumner et al. 2002; Bolton et al. in press), so it is probably reasonable to assume that our measure of a subordinate's reproductive development is positively correlated with its relative reproductive output. The second main result, that subordinates in larger, more productive groups were more likely to escalate, also fits the prediction from our analysis of the costs and benefits of swapping rank. Group size is tightly correlated with nest size in our population (F1,23=10.39, p=0.004), and the differences in nest size at worker emergence are amplified thereafter as workers join in the task of nest construction. Group size during the founding phase is therefore an excellent index of the parameter k in our model that measures instantaneous group productivity.
These results offer the first evidence that reproductive suppression of subordinates can lead to an increased likelihood of escalated conflict. The results therefore lend support for the key assumption of the peace incentive model of reproductive skew (Reeve & Ratnieks 1993). Selection should favour dominants which respond to the threat of escalated conflict by adjusting the degree to which they suppress subordinates, but we have not tested directly whether dominants do in fact respond in this way. The positive relationship between group size and subordinate ovarian development is consistent with the idea that dominants do use peace incentives, but it is also the pattern expected if dominants lose control over reproduction in large groups (Clutton-Brock 1998; Reeve et al. 1998; Beekman et al. 2003). Distinguishing whether the threat of fighting is a determinant of, or a response to, the level of skew will require an experimental change in one factor (e.g. the level of subordinate reproduction) to look for an observed effect on the other factor (e.g. probability of escalated fighting). Note that the threat of aggression as a means of obtaining a share of reproduction is much more relevant in our system than the threat of departure (as assumed by concession models of skew; Vehrencamp 1983; Reeve 1991; Reeve & Emlen 2000). This is because in our system if the rank 4 or rank 5 subordinate favours staying over leaving, even though they receive little or no current reproduction (Cant & English 2006) and have a low chance of inheriting (Cant & Field 2001), then staying will certainly be favoured by the rank 2 subordinate on which we focus.
The duration for which dominants and subordinates fought increased exponentially with the number of days that the dominant had been away. The most likely explanation for this result is that the dominant became progressively weaker during the period spent in storage. Interestingly, however, the period of absence had no effect on whether the subordinate entered into a fight or not. This suggests that the subordinate's decision to fight may be driven by factors that do not change much over the few days of the dominant's absence—in particular, group size, previous estimates of fighting ability, and the level of subordinate reproductive suppression. However, once the decision to fight is taken, contest duration reflects the time taken for the dominant to reassert its superiority, perhaps through a process of sequential or cumulative assessment (Enquist & Leimar 1987; Payne 1998). One could try to distinguish whether it is relative body condition or the period of absence per se that drives the result shown in figure 2 by providing food for the dominant, or starving the subordinate, for the period that the dominant is away from the nest.
The original dominant emerged as the winner in 16 out of 17 escalated contests. This agrees with the common finding in studies of animal contests that the owner of a resource has an advantage in the escalated conflict (Grafen 1987). Dominants were smaller than subordinates in ca 20% of cases, so relative size does not account for the extreme bias in the outcome of the contests. As discussed by Parker (1974; see also Enquist & Leimar 1987), however, there may be substantial payoff asymmetries between a holder and a challenger, which have nothing to do with relative strength. In our experiment, a dominant individual is trying to regain its position on a nest in which the majority of young are its own, and are close to reaching maturity. A subordinate, on the other hand, would have to invest considerable time and energy in producing and rearing its own offspring to reach an equivalent reproductive output. In terms of our model, the payoff D is not equal for dominants and subordinates, since in the subordinate's case, the payoff of winning must be devalued by the time and the effort expended in raising reproductive output from pk to (1−p)k. These reinvestment costs are especially relevant in P. dominulus, in which the development time of offspring can be long (approx. two months) and the breeding season is restricted, so that any new eggs laid at the time of a takeover will take a considerable fraction of the season to mature. We suggest that it is this asymmetry in the payoffs of winning, rather than in strength, that underlies the overwhelming success of dominants in escalated conflict in this species.
7. Conclusion: reproductive suppression and escalated conflict
The idea that subordinates might use the threat of fighting to evade reproductive suppression is plausible, intuitive, and likely to be broadly applicable to a wide range of social species (Clutton-Brock 1998; Reeve et al. 1998). Apart from a few early analyses (Reeve & Keller 1997; Reeve & Ratnieks 1993), there has been relatively little theoretical work on how direct aggression might influence the degree of reproductive skew between the breeders. Skew models and attempts to test them have focused almost exclusively on the threat of departure or eviction from the group, while the simple threat of force from within the group has been neglected. Group-stability constraints, determined by expected fitnesses outside the group, may sometimes define the ‘battleground’ in which conflict over reproductive sharing takes place, but within that battleground conflicts over current reproduction will typically be resolved according to physical power (Cant 2006). The resolution of this conflict will depend on the credibility of a subordinate's threat to fight, which in turn depends on the net payoffs of winning, the costs of fighting and the information each party has about the state and motivation of the other. Our experiment confirms that a subordinate's current reproductive status is a key determinant of the economic balance of fighting versus waiting. Since reproductively suppressed subordinates are more likely to fight for dominant status, the results provide the first evidence that the attempts by dominants to reproductively suppress subordinates will increase the threat of escalated conflict. Definitive evidence that dominants respond to this threat by altering the level of suppression requires further work.
Acknowledgments
Thanks to J. Llop for help in the field, and to E. Almond, C. Bridge and K. Nunn for assistance with labwork. The manuscript was improved by comments from T. Clutton-Brock, N. Davies, R. Johnstone, A. Young and two anonymous referees. The work was supported by a Royal Society University Research Fellowship (to M.A.C.) and Summer Studentship (to S.E.), and by grants from the Natural Environment Research Council (to J.F.) and the National Science Foundation (to H.K.R.).
Supplementary Material
References
- Alberts S.C, Watts H.E, Altmann J. Queuing and queue-jumping: long term patterns of reproductive skew in male savannah baboons. Anim. Behav. 2003;65:821–840. doi:10.1006/anbe.2003.2106 [Google Scholar]
- Beekman M, Komdeur J, Ratnieks F.L.W. Reproductive conflicts in animal societies: who has power? Trends Ecol. Evol. 2003;18:277–282. doi:10.1016/S0169-5347(03)00068-5 [Google Scholar]
- Bolton, A., Sumner, S., Shreeves, G., Casiraghi, M. & Field, J. In press. Colony genetic structure in a facultatively eusocial hover wasp. Behav. Ecol (doi:10.1093/beheco/arl020)
- Bridge A.P, Elwood R.W, Dick J.T. Imperfect assessment and limited information preclude optimal strategies in male–male fights in the orb-weaving spider Metellina mengei. Proc. R. Soc. B. 2000;267:273–279. doi: 10.1098/rspb.2000.0997. doi:10.1098/rspb.2000.0997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant M.A. A tale of two theories: parent–offspring conflict and reproductive skew. Anim. Behav. 2006;71:255–263. doi:10.1016/j.anbehav.2005.03.040 [Google Scholar]
- Cant M.A, English S. Stable group size in cooperative breeders: the role of inheritance and reproductive skew. Behav. Ecol. 2006;17:560–568. doi:10.1093/beheco/arj065 [Google Scholar]
- Cant M.A, Field J. Helping effort and future fitness in animal societies. Proc. R. Soc. B. 2001;268:1959–1964. doi: 10.1098/rspb.2001.1754. doi:10.1098/rspb.2001.1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant M.A, Llop J.B, Field J. Individual variation in social aggression and the probability of inheritance: theory and a field test. Am. Nat. 2006;167:837–852. doi: 10.1086/503445. doi:10.1086/503445 [DOI] [PubMed] [Google Scholar]
- Clutton-Brock T.H. Reproductive skew, concessions and limited control. Trends Ecol. Evol. 1998;13:288–292. doi: 10.1016/s0169-5347(98)01402-5. doi:10.1016/S0169-5347(98)01402-5 [DOI] [PubMed] [Google Scholar]
- Davies N.B. Territorial defence in the Speckled Wood Butterfly Pararge aegeria: the resident always wins. Anim. Behav. 1978;26:138–147. doi:10.1016/0003-3472(78)90013-1 [Google Scholar]
- Davies N.B. Oxford University Press; Oxford, UK: 1992. Dunnock behaviour and social evolution. [Google Scholar]
- Emlen S.T. An evolutionary theory of the family. Proc. Natl Acad. Sci. USA. 1995;92:8092–8099. doi: 10.1073/pnas.92.18.8092. doi:10.1073/pnas.92.18.8092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: decision rules and assessment of relative strength. J. Theor. Biol. 1983;102:387–410. doi:10.1016/0022-5193(83)90376-4 [Google Scholar]
- Enquist M, Leimar O. Evolution of fighting behaviour: the effect of variation in resource value. J Theor. Biol. 1987;127:187–205. doi:10.1016/S0022-5193(87)80130-3 [Google Scholar]
- Field J, Solis C.R, Queller D.C, Strassmann J.E. Social and genetic structure of paper wasp cofoundress associations: tests of reproductive skew models. Am. Nat. 1998;151:545–563. doi: 10.1086/286140. doi:10.1086/286140 [DOI] [PubMed] [Google Scholar]
- Field J.P, Shreeves G, Sumner S. Group size, queuing and helping decisions in facultatively eusocial hover wasps. Behav. Ecol. Sociobiol. 1999;45:378–385. doi:10.1007/s002650050574 [Google Scholar]
- Field J, Cronin A, Bridge C. Future fitness and helping in social queues. Nature. 2006;441:214–217. doi: 10.1038/nature04560. doi:10.1038/nature04560 [DOI] [PubMed] [Google Scholar]
- Grafen A. The logic of divisively asymmetric contests: respect for ownership and the desperado effect. Anim. Behav. 1987;35:462–467. doi:10.1016/S0003-3472(87)80271-3 [Google Scholar]
- Gust D.A. Moving up the dominance hierarchy in young sooty mangabeys. Anim. Behav. 1995;50:15–21. doi:10.1006/anbe.1995.0216 [Google Scholar]
- Haley M.P. Resource-holding power asymmetries, the prior residence effect, and reproductive pay-offs in male northern elephant seal fights. Behav. Ecol. Sociobiol. 1994;34:427–434. [Google Scholar]
- Henshaw M.T. Microsatellite loci for the social wasp Polistes dominulus and their application in other polistine wasps. Mol. Ecol. 2000;9:2155–2234. doi: 10.1046/j.1365-294x.2000.01053.x. doi:10.1046/j.1365-294X.2000.01053.x [DOI] [PubMed] [Google Scholar]
- Johnson J.I, Nobbelin F, Bohlin T. Territorial competition among wild brown trout fry: effects of ownership and body size. J. Fish Biol. 1999;54:469–472. doi:10.1111/j.1095-8649.1999.tb00846.x [Google Scholar]
- Kokko H, Johnstone R.A. Social queuing in animal societies: a dynamic model of reproductive skew. Proc. R. Soc. B. 1999;266:571–578. doi:10.1098/rspb.1999.0674 [Google Scholar]
- Maynard Smith J, Price G.R. The logic of animal conflicts. Nature. 1973;246:15–18. doi:10.1038/246015a0 [Google Scholar]
- Monnin T, Peeters C. Dominance hierarchy and reproductive conflicts among subordinates in a monogynous queenless ant. Behav. Ecol. 1999;10:323–332. doi:10.1093/beheco/10.3.323 [Google Scholar]
- Monnin T, Ratnieks F.L.W. Reproduction versus work in queenless ants: when to join a hierarchy of hopeful reproductives? Behav. Ecol. Sociobiol. 1999;46:413–422. doi:10.1007/s002650050637 [Google Scholar]
- Parker G.A. Assessment strategy and the evolution of animal conflicts. J. Theor Biol. 1974;47:223–243. doi: 10.1016/0022-5193(74)90111-8. doi:10.1016/0022-5193(74)90111-8 [DOI] [PubMed] [Google Scholar]
- Payne R.J.H. Gradually escalating fights and displays: the cumulative assessment model. Anim. Behav. 1998;56:651–662. doi: 10.1006/anbe.1998.0835. doi:10.1006/anbe.1998.0835 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K.F. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. doi:10.2307/2409206 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Hughes C.R, Strassmann J.E. Wasps fail to make distinctions. Nature. 1990;344:388. doi:10.1038/344388a0 [Google Scholar]
- Queller D.C, Zacchi F, Cervo R, Turillazzi S, Henshaw M.T, Santorelli L.A, Strassmann J.E. Unrelated helpers in a social insect. Nature. 2000;405:784–787. doi: 10.1038/35015552. doi:10.1038/35015552 [DOI] [PubMed] [Google Scholar]
- Ratnieks F.L.W, Foster K.R, Wenseleers T. Conflict resolution in insect societies. Annu. Rev. Ent. 2006;51:581–608. doi: 10.1146/annurev.ento.51.110104.151003. doi:10.1146/annurev.ento.51.110104.151003 [DOI] [PubMed] [Google Scholar]
- Reeve H.K. Polistes. In: Ross K.G, Matthews R.W, editors. The social biology of wasps. Cornell University Press; Ithaca, NY: 1991. pp. 99–148. [Google Scholar]
- Reeve H.K, Emlen S.T. Reproductive skew and group size: an N-person staying incentive model. Behav. Ecol. 2000;11:640–647. doi:10.1093/beheco/11.6.640 [Google Scholar]
- Reeve H.K, Keller L. Reproductive bribing and policing as evolutionary mechanisms for the suppression of within-group selfishness. Am. Nat. 1997;150:S42–S58. doi: 10.1086/286049. doi:10.1086/286049 [DOI] [PubMed] [Google Scholar]
- Reeve H.K, Ratnieks F.L.W. Queen–queen conflicts in polygynous societies: mutual tolerance and reproductive skew. In: Keller L, editor. Queen number and sociality in insects. Oxford University Press; Oxford, UK: 1993. pp. 45–85. [Google Scholar]
- Reeve H.K, Emlen S.T, Keller L. Reproductive sharing in animal societies: reproductive incentives or incomplete control by dominant breeders? Behav. Ecol. 1998;9:267–278. [Google Scholar]
- Seppa P, Queller D.C, Strassmann J.E. Reproduction in foundress associations of the social wasp, Polistes carolina: conventions, competition, and skew. Behav. Ecol. 2002;13:531–542. doi:10.1093/beheco/13.4.531 [Google Scholar]
- Shreeves G, Cant M.A, Bolton A, Field J. Insurance-based advantages for subordinate co-foundresses in a temperate paper wasp. Proc. R. Soc. B. 2003;27:1617–1622. doi: 10.1098/rspb.2003.2409. doi:10.1098/rspb.2003.2409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurjonsdottir H, Parker G.A. Dung fly struggles: evidence for assessment strategy. Behav. Ecol. Sociobiol. 1981;8:219–230. doi:10.1007/BF00299834 [Google Scholar]
- Stacey P.B, Koenig W.D. Cambridge University Press; Cambridge, UK: 1990. Cooperative breeding in birds. [Google Scholar]
- Strassmann J.E, Meyer D.C. Gerontocracy in the social wasp, Polistes exlamans. Anim. Behav. 1983;31:431–438. doi:10.1016/S0003-3472(83)80063-3 [Google Scholar]
- Strassmann J.E, Solis C.R, Barefield K, Queller D.C. Strategies for finding and using highly polymorphic DNA microsatellite loci for studies of genetic relatedness and pedigree. In: Ferraris J, Palumbi S, editors. Molecular methods in zoology and evolution. Wiley-Liss; New York NY: 1996. pp. 528–549. [Google Scholar]
- Sumner S, Casiraghi M, Foster W, Field J. High reproductive skew in tropical hover wasps. Proc. R. Soc. B. 2002;269:179–186. doi: 10.1098/rspb.2001.1884. doi:10.1098/rspb.2001.1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehrencamp S.L. A model for the evolution of despotic versus egalitarian societies. Anim. Behav. 1983;31:667–682. doi:10.1016/S0003-3472(83)80222-X [Google Scholar]
- Wiley R.H, Rabenold K.N. The evolution of cooperative breeding by delayed reciprocity and queuing for favorable social positions. Evolution. 1984;38:609–621. doi: 10.1111/j.1558-5646.1984.tb00326.x. doi:10.2307/2408710 [DOI] [PubMed] [Google Scholar]
- Wilson E.O. Harvard University Press; Cambridge, MA: 1971. The insect societies. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.