Abstract
A combination of structural and pigmentary components is responsible for many of the colour displays of animals. Despite the ubiquity of this type of coloration, neither the relative contribution of structures and pigments to variation in such colour displays nor the relative effects of extrinsic factors on the structural and pigment-based components of such colour has been determined. Understanding the sources of colour variation is important because structures and pigments may convey different information to conspecifics. In an experiment on captive American goldfinches Carduelis tristis, we manipulated two parameters, carotenoid availability and food availability, known to affect the expression of carotenoid pigments in a full-factorial design. Yellow feathers from these birds were then analysed in two ways. First, we used full-spectrum spectrometry and high-performance liquid chromatography to examine the extent to which variation in white structural colour and total carotenoid content was associated with variation in colour properties of feathers. The carotenoid content of yellow feathers predicted two colour parameters (principal component 1—representing high values of ultraviolet and yellow chroma and low values of violet–blue chroma—and hue). Two different colour parameters (violet–blue and yellow chroma) from white de-pigmented feathers, as well as carotenoid content, predicted reflectance measurements from yellow feathers. Second, we determined the relative effects of our experimental manipulations on white structural colour and yellow colour. Carotenoid availability directly affected yellow colour, while food availability affected it only in combination with carotenoid availability. None of our manipulations had significant effects on the expression of white structural colour. Our results suggest that the contribution of microstructures to variation in the expression of yellow coloration is less than the contribution of carotenoid content, and that carotenoid deposition is more dependent on extrinsic variability than is the production of white structural colour.
Keywords: American goldfinch, Carduelis tristis, carotenoid pigmentation, diet, honest signalling, sexual selection
1. Introduction
Coloration in animals can arise through the deposition of carotenoids, melanins or other pigments (i.e. pigmentary colours) and through the precise arrangement of pigments and tissues at a nanostructural scale (i.e. structural colours) (Fox & Vevers 1960; Gill 1995; Hill & McGraw 2006). Structural and pigmentary coloration of bird feathers have been traditionally treated as distinct modes of colour display (Fitzpatrick 1998), but many colour displays involve a combination of structures and pigments (Dyck 1978; Rutowski et al. 2005; Prum 2006). In fact, pigments can create colour only by absorbing light from a background of (usually white) reflective tissue in which they are deposited (Mason 1923; Shawkey & Hill 2005; Andersson & Prager 2006).
Recent evidence indicates that carotenoid pigments combine with the microstructure of feathers to create brilliant yellow plumage colour in birds (Shawkey & Hill 2005). When these colour-generating mechanisms coexist, nothing is known about the relative importance of pigment versus structural features of the coloured tissue to the generation of variability in coloration. In addition, little is known about the relative condition dependency of structural versus carotenoid components of the same colour display. Carotenoid-based colours commonly respond to factors such as dietary pigment access and food intake (reviewed in Hill 2002); avian structural colours, although studied less extensively, have been shown to respond to similar perturbations (McGraw et al. 2002; Hill et al. 2005).
Here, we performed manipulations of carotenoid availability and food access in a full-factorial design experiment on captive, moulting American goldfinches (Carduelis tristis). We aimed to determine: (i) the relative contributions of structural colour and carotenoids to intraspecific variation in colour and (ii) the relative effects of these two important environmental variables on the structural and pigmentary aspects of feather coloration.
2. Material and methods
We captured goldfinches from large winter flocks in January and February 2005 in Lee County, AL, USA (32°35′ N, 82°28′ W) by trapping them at established feeding stations. As birds were captured, we sorted them by sex and age (first-year or older) following Pyle et al. (1987). We retained only first-year males for this study, releasing females and older males. In this way, we removed the effect of sex and age on coloration.
Within a week after capture, we placed birds in small cages (0.5 m3) in rooms with large windows and abundant natural light, allowing them to moult on a natural light regime. We randomly assigned two birds to each cage, and we systematically assigned each cage to a high- or low-carotenoid and food treatment. A total of four treatment combinations were possible and our design called for 12 replicates for each treatment combination, so that we maintained 24 cages of birds housing 48 individuals.
Intensity of carotenoid pigmentation is sensitive to diet in American goldfinches (McGraw & Gregory 2004). Carotenoids were provided as a 70 : 30 mixture of lutein and zeaxanthin following Navara & Hill (2003). Males in half of the cages were provided with high-carotenoid supplementation in the form of starch gel beadlets mixed with water at a concentration of 50 mg l−1, and the remaining half were provided with low-carotenoid supplementation at a concentration of 0.5 mg l−1 of drinking water. High- and low-carotenoid supplementations were chosen based on the response of male goldfinches to various doses of supplemental carotenoids in Navara & Hill (2003).
Food intake, independent of carotenoid intake, influences plumage colour in male goldfinches (McGraw et al. 2005). We manipulated food access as in the prior study; males either were given unlimited access to food or had all food removed from their cages during mornings or afternoons. On the mornings when birds had no food, food dishes were removed just before dark on the evening before and returned at the mid-point of daylight the following day. Alternatively, food was removed at the midpoint of daylight and returned to cages just before sunrise the following morning. We staggered food removal between mornings and evenings following Hill (2000), such that birds in the food-restricted treatment group had no access to food for 38% of daylight hours during moult.
On 30 April 2005, when all the birds had completed pre-alternate moult, we removed all males from cages and pulled 20 feathers from the upper breast of each male. We taped feathers from each individual directly on top of each other on gloss-free black construction paper and measured full-spectrum colour following the methods described by Shawkey & Hill (2005).
Following this initial analysis, we removed the feathers from the construction paper and extracted carotenoids from them following the thermochemical method (heated, acidified pyridine) described by McGraw & Gregory (2004). This procedure breaks bonds between carotenoids and keratin in the feather, leaving the underlying white structural colour exposed (Shawkey & Hill 2005). After removing feathers from the acidified pyridine and allowing them to dry, we again taped them to black construction paper and recorded spectral data as mentioned earlier.
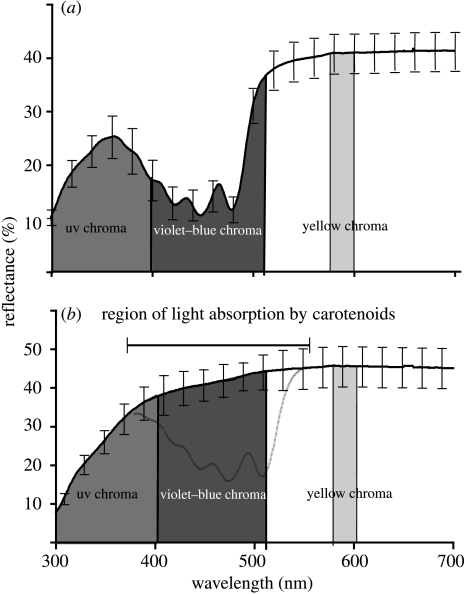
We calculated several colour variables from spectral reflectance data. These variables were chosen based on their ability to describe different aspects of the spectral curve and on precedent in the literature. We restricted these indices to wavelengths between 320 and 700 nm, as evidence suggests that passerine birds are sensitive to ultraviolet (UV) wavelengths (Cuthill et al. 2000) and that 700 nm is the upper limit of the vertebrate visual system (Jacobs 1981). Hue was calculated as the point of maximum inflection of the curve. Brightness, the mean of reflectances from 320 to 700 nm, is a measure of the total amount of light reflected by the feathers (Jacobs 1981; Endler 1990; Andersson 1999). UV chroma, violet–blue chroma and yellow chroma are the percentages of total light reflected in the ranges of 320–400 (Andersson et al. 1998), 400–512 and 575–600 nm, respectively. Violet–blue chroma was used here because carotenoids absorb light in these wavelengths (Bauernfiend 1981); hence, higher concentrations of carotenoids should create lower violet–blue chroma values (MacDougall & Montgomerie 2003). Figure 1 shows a graphical explanation of these variables. Finally, following MacDougall & Montgomerie (2003), we calculated UV and yellow saturation as the difference between the minimum reflectance in the blue wavelengths and the maximum reflectance in the UV and yellow wavelengths, respectively. We then used values from these two variables and violet–blue chroma in a principal components analysis and used PC1 as our output variable. For comparison, we measured the same variables from the spectra of the same feathers after removal of carotenoids.
Figure 1.
Average reflectance spectra (±1 s.e.) of (a) intact yellow and (b) de-pigmented white feathers of American goldfinches C. tristis used in this study with regions used in the calculation of colour variables highlighted. The theoretical region of carotenoid absorption from the white structural colour is highlighted in (b).
Carotenoids extracted from feathers were analysed following the high-performance liquid chromatography (HPLC) methods of McGraw & Gregory (2004), with a few modifications. We injected 50 μl of each sample into a Waters Alliance 2695 HPLC system fitted with a Waters YMC RP-30 Carotenoid column (250×4.6 mm) and a built-in column heater set at 30°C. We used an isocratic system at a constant flow rate of 1 ml min−1 for 20 min to elute all xanthophylls present in feathers. The data were collected from 250 to 600 nm using a Waters 2996 photodiode array detector. Carotenoid concentrations were determined using Empower v. 5.0 software by comparing peak areas at lambda-max to external standard curves.
To determine the relative effects of variation in the underlying white structural colour and carotenoid concentration on variation in yellow colour, we used backward stepwise regressions. In each regression, a colour variable (hue, UV chroma, violet–blue chroma, yellow chroma and PC1) from intact yellow colour feathers was used as the dependent and the same variable calculated from de-pigmented feathers and carotenoid concentration were used as independents.
To identify the influence of carotenoid access and food access on the expression of both the yellow colour of intact barbs and the underlying white structural colour, we used MANOVAs for a full-factorial design. Total carotenoid content of the feathers and the colour variables, as measured for both intact yellow barbs and de-pigmented white barbs, were the response variables, while carotenoid access and food access were the treatment variables.
3. Results
Spectra of yellow feathers before carotenoid extraction exhibited the peak/trough/plateau shape, which is typical of yellow feathers that are pigmented with carotenoids (figure 1a; MacDougall & Montgomerie 2003; Shawkey & Hill 2005). Following extraction, feathers appeared white to the human eye, and their spectra showed a fairly uniform reflectance, following a sharp increase in the short wavelengths typical of white plumage (figure 1b; MacDougall & Montgomerie 2003; Mennill et al. 2003). Total carotenoid concentration was not correlated with the measurements of any colour variable taken from de-pigmented feathers, suggesting that our treatment affected all feathers equally.
As in MacDougall & Montgomerie (2003), PC1 explained a large proportion of variation in the colour variables (for yellow colour: 71.6%, eigenvalue=2.2; for white colour: 65.8%, eigenvalue=2.0). UV and yellow saturation loaded positively (for yellow colour: 0.62 and 0.65, respectively; for white colour: 0.45 and 0.58, respectively), while violet–blue chroma loaded negatively (for yellow colour: −0.44; for white colour: −0.68) on PC1. Thus, high PC1 values represent high values of UV and yellow saturation and low values of violet–blue chroma. Means and standard deviations of all colour variables are listed in table 1.
Table 1.
Values of colour variables measured from intact yellow and de-pigmented white American goldfinch (Carduelis tristis) feathers. (Values are presented ±1 s.e.)
| feather state | brightness (%) | UV chroma (%) | violet–blue chroma (%) | yellow chroma (%) | hue (nm) | PC1 |
|---|---|---|---|---|---|---|
| intact | 23.92±1.08 | 19.94±0.37 | 16.79±0.49 | 12.20±0.14 | 492.12±0.27 | −4.17×10−8±0.21×10−8 |
| de-pigmented | 35.86±1.41 | 15.31±0.43 | 27.99±0.15 | 7.70±0.06 | 543.14±6.62 | −2.71×10−7±0.20×10−7 |
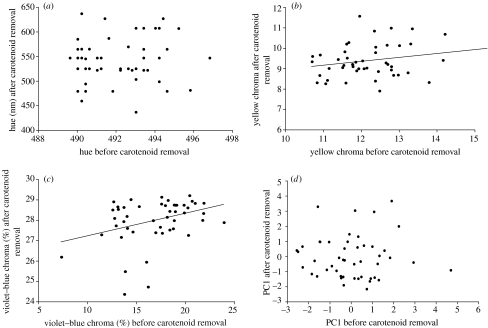
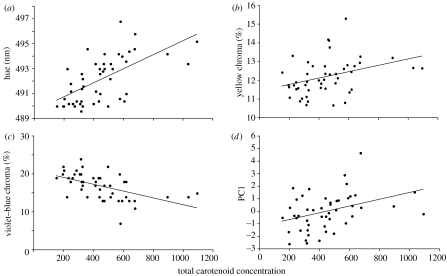
Overall feather brightness (pre-carotenoid extraction) was not related to either carotenoid concentration or structural brightness (post-carotenoid extraction; table 2). UV chroma was not related to either carotenoid concentration or structural UV chroma (table 2). Violet–blue chroma increased as structural violet–blue chroma increased and carotenoid concentration decreased (table 2, figures 2 and 3). Yellow chroma increased as structural yellow chroma and carotenoid concentration increased (table 2, figures 2 and 3). Hue and PC1 increased as carotenoid concentration increased (table 2, figures 2 and 3).
Table 2.
Backward linear regression models predicting colour variables measured from intact yellow American goldfinch (C. tristis) feathers using the same variables measured from the same feathers following total carotenoid removal and total concentration of carotenoids. (Only predictors that significantly contribute to the model are presented. A table showing all predictors is available in the electronic supplementary material.)
| dependent variable | predictors | β | p |
|---|---|---|---|
| brightness | none | 0.187 | |
| UV chroma | none | 0.090 | |
| violet–blue chroma | carotenoid concentration | −0.546 | 0.000 |
| violet–blue chroma of white feathers | 0.342 | 0.004 | |
| yellow chroma | carotenoid concentration | 0.385 | 0.006 |
| yellow chroma of white feathers | 0.274 | 0.047 | |
| hue | carotenoid concentration | 0.618 | 0.000 |
| PC1 | carotenoid concentration | 0.376 | 0.008 |
Figure 2.
Scatterplots of colour variables measured from intact yellow feathers of American goldfinches C. tristis and the same colour variables measured from the same feathers following carotenoid removal.
Figure 3.
Scatterplots of colour variables measured from intact yellow feathers of American goldfinches C. tristis and total carotenoid concentration of those feathers.
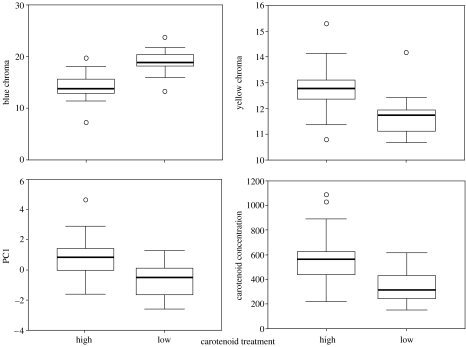
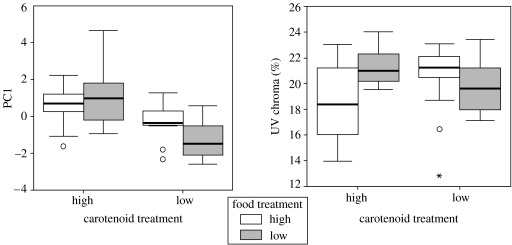
As expected, birds in the high carotenoid availability treatment group deposited more carotenoids into their feathers (table 3, figure 4). Carotenoid availability significantly influenced the expression of several colour variables in intact yellow feathers; birds in the low-carotenoid treatment group exhibited higher blue chroma, lower yellow chroma and lower PC1 scores than those in the high-carotenoid treatment group (table 3, figure 4). We found no main effects of food access on carotenoid deposition or colour. The interaction term between carotenoids and food was significant for PC1 (table 3, figure 5) and UV chroma, and post hoc ANOVA revealed that PC1 scores and UV chroma values for birds in the low food treatment group were lower than those in the high food treatment group only when they were also in the low-carotenoid treatment group (PC 1: F1,23=6.83, p=0.016; UV chroma: F1,23=5.76, p=0.026, figure 5).
Table 3.
Fixed factorial MANOVA describing the effects of experimental carotenoid and diet manipulation on the expression of yellow plumage colour in American goldfinches C. tristis. (Only significant effects are shown. A table showing all the effects is available in the electronic supplementary material.)
| source | dependent variable | mean squared | F | p |
|---|---|---|---|---|
| carotenoid treatment | carotenoid concentration | 536063.11 | 19.279 | 0.000 |
| PC1 | 26.072 | 19.767 | 0.000 | |
| yellow chroma | 0.001 | 16.300 | 0.000 | |
| blue chroma | 0.024 | 40.338 | 0.000 | |
| carotenoid×food | PC1 | 6.860 | 5.201 | 0.028 |
| UV chroma | 0.003 | 5.736 | 0.021 |
Figure 4.
Boxplots of colour variables measured from intact yellow feathers of American goldfinches C. tristis grown in captivity under either a high- or low-carotenoid treatment. The line within each box represents the median colour score, the upper and lower borders of each box are the 25th and 75th percentiles and the lower and upper bars are the 10th and 90th percentiles.
Figure 5.
Boxplots of colour variables measured from intact yellow feathers of American goldfinches C. tristis grown in captivity under either a high- or low-carotenoid treatment and either a high or low food treatment. The line within each box represents the median colour score, the upper and lower borders of each box are the 25th and 75th percentiles and the lower and upper bars are the 10th and 90th percentiles.
None of the treatment groups or interaction terms had any significant effect on the expression of underlying white structural colour, although a few had almost significant effects (see table 4 in the electronic supplementary material).
4. Discussion
As far as we are aware, this study is the first to examine the relative contributions of white structural colour and carotenoid pigments to variation in yellow plumage colour and is the first to assess the effects of multiple environmental factors on the expression of both white structural colour and intact carotenoid-/structure-based colour. We show that variation in carotenoid content of feathers largely determines variation in yellow plumage colour, but that variation in structural colour may play a minor role. Similarly, our experiment revealed that carotenoid access was more important to variation in yellow colour, although food access played an indirect role, and that environmental challenges during moult had little or no effect on the expression of white structural colour.
Violet–blue and yellow chroma were significantly related to both structural coloration and carotenoid concentration. Thus, the relative amount of violet–blue and yellow chroma reflected by the underlying white colour might affect the amount of light reflected in these same wavelengths by the intact yellow feather. Reflection in the violet–blue range was also negatively related to carotenoid concentration, an expected result given how strongly carotenoids absorb light in these wavelengths (Bauernfiend 1981). Thus, both structural and pigmentary elements may contribute to variation in this short-wave reflectance trough, as well as in reflectance in the long (yellow) wavelengths.
The reason why variation in other colour parameters was unaffected by variation in white structural coloration is not clear. Perhaps bonds between keratin and carotenoids create unexpected optical phenomena that affect variables such as brightness and UV chroma. Examining the potentially complex interactions between structures and pigments will be an interesting avenue for further research.
While it is well established that carotenoid deposition in feathers is dependent on food intake and carotenoid access (Hill 2002), it is not known whether the same holds true for white structural colour (Prum 2006). The unordered arrangements of keratin and air that create much white plumage colour do not seem likely to be sensitive to environmental perturbation during development (Shawkey & Hill 2005; Prum 2006; Shawkey & Hill 2006). If structural white colour and the carotenoid concentration both indicate condition, then individuals in good condition during moult should grow white barbs with low violet–blue chroma values. However, our experimental tests showed weak, non-significant effects of two well-known environmental variables on the expression of white structural colour. Thus, any variation in white colour must be created randomly either by hormonal profiles, genetic factors or extrinsic factors during or after feather growth (e.g. soiling, feather-degrading bacteria). If variation in white colour is linked to some genetic aspects of quality, then single-colour patches could serve as multicomponent signals, with carotenoid content indicating condition during moult and white colour indicating genetic quality (Grether et al. 2004).
In this study, carotenoid access had a strong effect on variation in the hue and chroma of yellow feathers. Food access appeared to affect colour only in conjunction with carotenoid access. However, these results must be viewed cautiously. With our forced experimental manipulations, we attempted to recreate physiological endpoints for our high- and low-diet treatments, but these factors may interact differently in nature. In future studies, we hope that our simplified design can be made more tractable for experimentally testing these ideas in a wild bird population.
In conclusion, we have shown that variation in most aspects of yellow plumage colour is caused primarily by variation in carotenoid content, with a relatively weaker contribution by the underlying white structural colour. Our results also suggest a role for white colour in the signalling properties of this type of colour, but this role is smaller than that of carotenoids and is probably not condition-dependent. Finally, carotenoid availability appears to be a more important environmental determinant of variation in yellow plumage colour than food access. These results are an important synthesis of previous research (reviewed in Hill 2002) and a critical step forward in our understanding of the roles that combinations of structures and pigments play in animal signalling.
Acknowledgments
All the works with captive animals were performed in accordance with Auburn University IACUC protocols. We thank R. Montgomerie for allowing us to use his spectral processing program, S. Beissinger's lab group and N. Morehouse for helpful comments on the manuscript, and R. Rutowski for useful discussions. This work was partially supported by NSF grants IBN0235778 and DEB0218313 to G.E.H. M.D.S. was supported during the writing of this manuscript by NSF grant IOB-0517549.
Supplementary Material
Tables showing significant and non-significant factors in analyses of yellow plumage coloration.
References
- Andersson S. Morphology of UV reflectance in a whistling-thrush: implications for the study of structural colour signalling in birds. J. Avian Biol. 1999;30:193–204. [Google Scholar]
- Andersson S, Ornbörg J, Andersson M. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc. R. Soc. B. 1998;265:445–450. doi:10.1098/rspb.1998.0315 [Google Scholar]
- Andersson S, Prager M. Quantification of coloration. In: Hill G.E, McGraw K.J, editors. Bird coloration. Mechanisms and measurements. vol. 1. Harvard University Press; Cambridge, MA: 2006. [Google Scholar]
- Bauernfiend J.C. Academic Press; London, UK: 1981. Carotenoids as colorants and vitamin A precursors. [Google Scholar]
- Cuthill I.C, Partridge J.C, Bennett A.T.D, Church S.C, Hart N.S, Hunt S. Ultraviolet vision in birds. Adv. Study Behav. 2000;29:159–214. [Google Scholar]
- Dyck J. Olive green feathers: reflection of light from the rami and their structure. Anser. 1978;(Suppl. 3):57–75. [Google Scholar]
- Endler J.A. On the measurement and classification of color in studies of animal color patterns. Biol. J. Linn. Soc. 1990;41:315–352. [Google Scholar]
- Fitzpatrick S. Colour schemes for birds: structural coloration and signals of quality in feathers. Ann. Zool. Fennici. 1998;35:67–77. [Google Scholar]
- Fox H.M, Vevers G. Macmillan; New York, NY: 1960. The nature of animal colors. [Google Scholar]
- Gill F. W. H. Freeman and Co.; New York, NY: 1995. Ornithology. [Google Scholar]
- Grether G.F, Kolluru G.R, Nersissian K. Individual color patches as multicomponent signals. Biol. Rev. 2004;79:583–610. doi: 10.1017/s1464793103006390. doi:10.1017/S1464793103006390 [DOI] [PubMed] [Google Scholar]
- Hill G.E. Energetic constraints on expression of carotenoid-based plumage coloration. J. Avian Biol. 2000;31:559–566. doi:10.1034/j.1600-048X.2000.310415.x [Google Scholar]
- Hill G.E. Oxford University Press; New York, NY: 2002. A red bird in a brown bag: the function and evolution of ornamental plumage coloration in the House Finch. [Google Scholar]
- Hill G.E, McGraw K.J, editors. Bird coloration. Mechanisms and measurements. vol. I. Harvard University Press; Boston, MA: 2006. [Google Scholar]
- Hill G.E, Doucet S.M, Buchholz R. The effect of coccidial infection on iridescent plumage coloration in wild turkeys. Anim. Behav. 2005;69:387–394. doi:10.1016/j.anbehav.2004.03.013 [Google Scholar]
- Jacobs G.H. Academic press series in cognition and perception. Academic Press; New York, NY: 1981. Comparative color vision. [Google Scholar]
- MacDougall A.K, Montgomerie R. Assortative mating by carotenoid-based plumage colour: a quality indicator in American goldfinches, Carduelis tristis. Naturwissenschaften. 2003;90:464–467. doi: 10.1007/s00114-003-0459-7. doi:10.1007/s00114-003-0459-7 [DOI] [PubMed] [Google Scholar]
- Mason C.W. Structural colors in feathers. J. Phys. Chem. 1923;27:201–251. doi:10.1021/j150228a001 [Google Scholar]
- McGraw K.J, Gregory A.J. Carotenoid pigments in male American goldfinches: what is the optimal biochemical strategy for becoming colourful? Biol. J. Linn. Soc. 2004;83:273–280. doi:10.1111/j.1095-8312.2004.00388.x [Google Scholar]
- McGraw K.J, Mackillop E.A, Dale J, Hauber M.E. Different colors reveal different information: how nutritional stress affects the expression of melanin- and structurally based ornamental plumage. J. Exp. Biol. 2002;205:3747–3755. doi: 10.1242/jeb.205.23.3747. [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Hill G.E, Parker R.S. The physiological costs of being colourful: nutritional control of carotenoid utilization in the American goldfinch (Carduelis tristis) Anim. Behav. 2005;69:653–660. doi:10.1016/j.anbehav.2004.05.018 [Google Scholar]
- Mennill D.J, Doucet S.M, Montgomerie R, Ratcliffe L.M. Achromatic color variation in black-capped chickadees, Poecile atricapilla: black and white signals of sex and rank. Behav. Ecol. Sociobiol. 2003;53:350–357. [Google Scholar]
- Navara K.J, Hill G.E. Dietary carotenoid pigments and immune function in a songbird with extensive carotenoid-based plumage coloration. Behav. Ecol. 2003;14:909–916. doi:10.1093/beheco/arg085 [Google Scholar]
- Prum R.O. Anatomy, physics and evolution of avian structural colors. In: Hill G.E, McGraw K.J, editors. Bird coloration: mechanisms. Mechanisms and measurements. vol. I. Harvard University Press; Boston, MA: 2006. [Google Scholar]
- Pyle P, Howell S.N.G, Yunick R.P, DeSante D.F. Slate Creek Press; Bolinas, CA: 1987. Identification guide to North American Passerines. [Google Scholar]
- Rutowski R.L, Macedonia J.M, Morehouse N, Taylor-Taft L. Pterin pigments amplify iridescent ultraviolet signal in males of the orange sulphur butterfly, Colias eurythme. Proc. R. Soc. B. 2005;272:2329–2335. doi: 10.1098/rspb.2005.3216. doi:10.1098/rspb.2005.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D, Hill G.E. Carotenoids need structural colours to shine. Biol. Lett. 2005;1:121–125. doi: 10.1098/rsbl.2004.0289. doi:10.1098/rsbl.2004.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawkey M.D, Hill G.E. Significance of a basal melanin layer to production of non-iridescent structural plumage colour: evidence from an amelanotic Steller's jay (Cyanocitta stelleri) J. Exp. Biol. 2006;209:1245–1250. doi: 10.1242/jeb.02115. doi:10.1242/jeb.02115 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables showing significant and non-significant factors in analyses of yellow plumage coloration.