Abstract
Early stages of infection by the mouse polyomavirus have been studied using HeLa cells stably expressing small interfering RNA to protein disulfide isomerase (PDI). Infectibility measured by nuclear T antigen expression was reduced commensurately with the degree of PDI downregulation. Infectibility was restored by transfection with a plasmid expressing PDI but not with a control expressing catalytically inactive enzyme. Deconvolution microscopy using fluorescently labeled virus and cellular markers showed that virus reaches the endoplasmic reticulum (ER) normally in cells with reduced PDI but subsequently fails to exit the ER. Simian virus 40 infection was not inhibited in PDI-downregulated cells. The results are discussed in terms of structural differences between the two viruses and current knowledge of virus disassembly in the ER.
Polyomaviruses pass through the endoplasmic reticulum (ER), where they undergo rearrangement before exiting into the cytosol and entry into the nucleus (4, 5, 8, 9, 11, 13). The need for structural rearrangement or partial disassembly is clear from the fact that although nuclear localization sequences are abundant (>500/particle distributed among the major and minor capsid proteins and cellular histones in the viral minichromosome), none are exposed in the fully assembled virus. Structural studies have shown that disulfide bonds and calcium binding sites are important in the assembly and stability of polyoma particles (3, 12); disassembly in vitro is achieved by reduction of disulfide bonds and chelation (1).
Steps of disassembly in vivo and the cellular factors involved are only partially understood. Der-2, a member of the derlin family of proteins, has recently been shown to be essential for infection by the mouse polyomavirus (Py) (7). The derlins normally function by recognizing misfolded proteins in the ER and directing their translocation into the cytosol for proteasomal degradation. This suggests that a partially unfolded intermediate in virus disassembly utilizes the “quality control machinery” in the ER to escape. ERp29, a chaperone-like protein, brings about a conformational change in the virus. This leads in vitro to exposure of the C-terminal arm of the major capsid protein VP1, allowing cleavage by trypsin and also to increased hydrophobicity of the particle. The expression of a dominant-negative form of ERp29 inhibits infection (8). ERp29 is a member of the protein disulfide isomerase (PDI) family that lacks the CXXC motif in its thioredoxin domain (2) and is catalytically inactive. This raises the question of whether PDI itself may also be essential for Py disassembly in the ER and for infectibility.
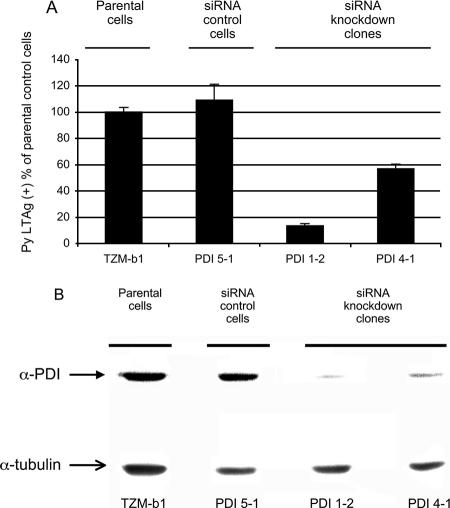
A series of HeLa cell clones stably expressing a small interfering RNA (siRNA) targeting PDI have been constructed and used in studies of infection by human immunodeficiency virus type 1 (10). Though HeLa cells are not fully permissive to Py, they express all the factors necessary for the early steps of infection, from virus attachment and internalization to initiation of early gene expression in the nucleus. To investigate the role of PDI in Py infection, this series of HeLa clones was infected by the RA wild-type strain of Py. Infected cells were examined 32 h later at the single-cell level by large-T-antigen (LTAg) nuclear immunofluorescence. The percentages of cells expressing large T antigen were substantially reduced in two independent clones expressing siRNA to PDI (clones 1-2 and 4-1) compared to those in either the parental HeLa cells (TZM-b1) or cells expressing a control siRNA (clone 5-1) (Fig. 1A). Levels of expression of PDI in the clones were determined by Western blotting (Fig. 1B). PDI 1-2, the clone which reproducibly showed the lowest levels of infection, also expressed the smallest amount of PDI. The reduction in infectibility appears to be roughly commensurate with the degree of downregulation of PDI.
FIG. 1.
Py infection of PDI siRNA HeLa cells. (A) Parental HeLa (TZM-b-1), control siRNA (5-1), and siRNA PDI knockdown clones (1-2 and 4-1) were infected with Py and fixed and stained for LTAg expression 32 h postinfection (4). (B) Western blot of extracts from cells shown in panel A with antibody to PDI or α-tubulin.
Rescue experiments were undertaken to further establish the importance of PDI in infection by Py. PDI 1-2 cells were transfected with plasmids expressing wild-type PDI, a catalytically inactive PDI (in which the CXXC motif was replaced by SXXS) (10), or empty vector. An eGFP plasmid was included as a transfection marker. Twenty-four hours posttransfection, cells on coverslips were infected by Py at a multiplicity of infection of several hundred PFU/cell (determined by a plaque assay of the input virus on NIH 3T3 cells). After an additional 32 h, cells were fixed and assayed for LTAg expression. Cells were analyzed in two groups based on the expression or absence of expression of the eGFP transfection marker. As shown in Table 1, the percentages of eGFP-positive cells that were also LTAg positive were four- to sixfold higher following transfection with wild-type PDI than those for mutant PDI or the vector control. No rescue of infectibility was seen in eGFP-negative cells on the same coverslips, i.e., cells that were not transfected. Restoration of active PDI in PDI-downregulated cells thus restores their ability to be infected by Py.
TABLE 1.
Plasmid rescue
| Py | % (95% CI) for indicated cell groupa
|
|
|---|---|---|
| LTAg +, eGFP + | LTAg +, eGFP − | |
| Empty vector | 6.1 (1.2) | 7.6 (1.8) |
| Wild-type PDI | 38.3 (7.4) | 6.0 (1.6) |
| SXXS PDI | 10.1 (2.5) | 6.8 (1.6) |
+, positive for indicated antigen or marker; −, negative for indicated antigen or marker; CI, confidence interval.
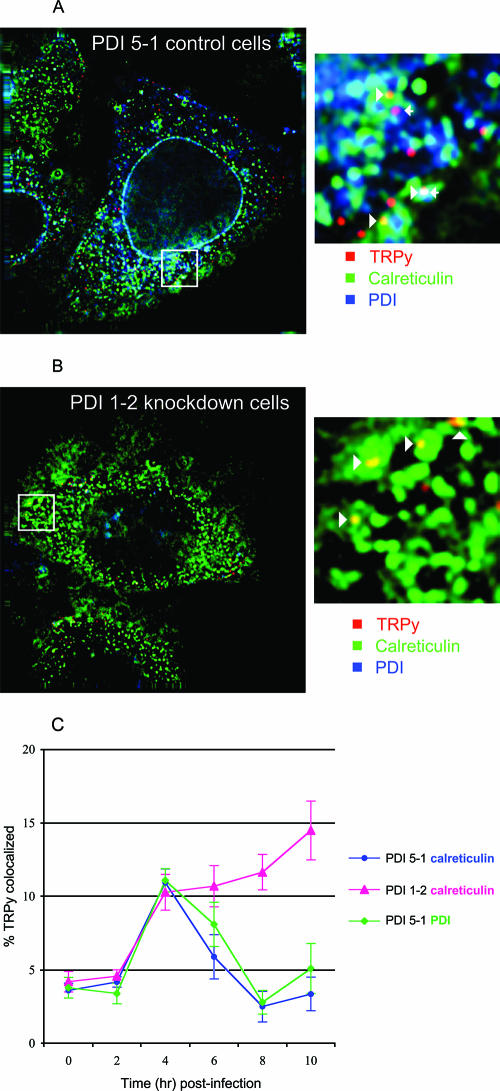
To examine the stage of infection where Py is blocked in PDI-deficient cells, fluorescently labeled virus (TRPy) was used to infect PDI 1-2 and PDI 5-1 cells at roughly 100 TRPy particles/cell. Uptake and colocalization of virus with PDI and calreticulin as ER markers were followed by deconvolution microscopy as previously described (4). At 4 h postinfection, virus was found colocalized with PDI and calreticulin in clone 5-1 (Fig. 2A) and with calreticulin in clone 1-2 (Fig. 2B) (PDI was nearly undetectable by immunofluorescence in clone 1-2, consistent with results obtained by Western blotting [Fig. 1B]). Quantitation and kinetics of colocalization were followed over a 10-h period. The numbers of virus particles internalized and reaching the ER from 2 to 4 h postinfection were the same in both clones, indicating that PDI does not affect virus attachment or endocytosis. In clone 5-1, which expresses normal levels of PDI, the amount of TRPy colocalizing with ER markers declined progressively from 4 to 8 h. The kinetics of virus entry and exit from the ER in these cells are closely similar to those seen in other virus-susceptible cells (4). In contrast, in the PDI-deficient clone 1-2, virus failed to leave the ER over the 10-h period (Fig. 2C). The kinetics of accumulation and retention in the ER in these cells are similar to those observed in cells blocked in infection due to the expression of a dominant-negative form of Der-2 (7). These results indicate that Py undergoes rearrangement in the ER mediated by PDI in order to escape from the ER and to establish an infection.
FIG. 2.
Colocalization of TRPy with the ER. (A) Clone PDI 5-1 (siRNA control cells). (B) Clone PDI 1-2 (PDI knockdown cells). Cells were infected with TRPy (red), fixed at 4 h postinfection, and processed for immunodetection of calreticulin (green) or PDI (blue). Z sections (0.2 mm) were imaged and subjected to deconvolution as previously described (4). The enlarged areas show particles colocalized with calreticulin (arrowheads) and with PDI (arrows). (C) Quantitation and kinetics of colocalization. Cells were infected and processed at the indicated times as described for panels A and B.
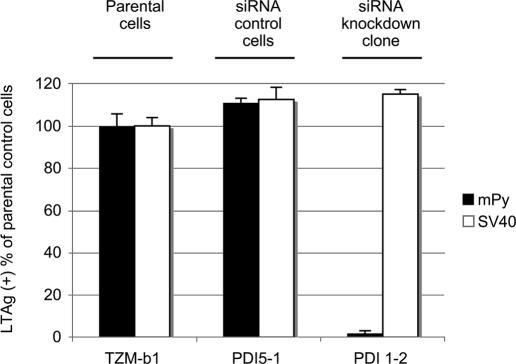
Py and simian virus 40 (SV40) share extensive structural and biological properties yet differ in receptor specificity (13) and other important respects. To determine whether SV40 also requires PDI for infection, the HeLa clones were infected with SV40 or Py and monitored for expression of the respective LTAgs by immunofluorescence. While Py was inhibited by over 95% in clone 1-2 compared to parental or siRNA control cells, infection by SV40 was essentially the same in all three clones (Fig. 3). SV40 thus does not depend on PDI for infection of HeLa cells. The cysteines in SV40 VP1 undergo multiple intra- and intermolecular disulfide bond formation during synthesis and assembly of pentamers in the cytoplasm (6), presumably in a PDI-independent manner. The positions of cysteines in the VP1s of Py and SV40 are only partly conserved, and the dispositions of disulfide bonds in fully assembled particles are different for the two viruses. In Py, the VP1s within each pentamer are linked by disulfide bonds involving Cys-19 and Cys-114 of neighboring VP1 molecules (12). This disulfide ring at the base of the pentamer is not found in SV40 due to the replacement of the N-terminal cysteine with a proline.
FIG. 3.
Py and SV40 infection of PDI siRNA HeLa cells. Parental HeLa cells (TZM-b1), control siRNA cells (PDI 5-1), and the siRNA PDI knockdown cells (PDI 1-2) were infected with Py or SV40 and analyzed at 32 h postinfection by immunofluorescent staining for the viral TAgs.
The results presented here indicate an important role for PDI in infection by Py, possibly in reducing and rearranging disulfide bonds that stabilize the capsomeres. The action of PDI together with that of ERp29 may represent sequential or coordinated steps in the disassembly of Py in the ER. These factors most likely act upstream of the step mediated by Der-2 in the translocation of the rearranged and partially disassembled particle (7). Additional factors may also be involved. The nature of the altered particle that crosses the ER membrane and the portal of exit remain unknown.
Acknowledgments
The expert technical assistance of John You and John Carroll is gratefully acknowledged. We also thank Thilo Stehle for helpful discussions comparing Py and SV40 structures.
This work was supported by grant RO1 CA-082395 from the National Cancer Institute. The TZM-b 1 reagent was obtained from John C. Kappes, Xiaoyun Wu, and Tranzyme Inc. through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Brady, J. N., V. D. Winston, and R. A. Consigli. 1978. Characterization of a DNA-protein complex and capsomere subunits derived from polyoma virus by treatment with ethyleneglycol-bis-N,N′-tetraacetic acid and dithiothreitol. J. Virol. 27:193-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrari, D. M., P. Nguyen Van, H. D. Kratzin, and H. D. Soling. 1998. ERp28, a human endoplasmic-reticulum-lumenal protein, is a member of the protein disulfide isomerase family but lacks a CXXC thioredoxin-box motif. Eur. J. Biochem. 255:570-579. [DOI] [PubMed] [Google Scholar]
- 3.Garcea, R. L., and R. C. Liddington. 1997. Structural biology of polyomaviruses, p. 187-208. In W. Chiu, R. M. Burnett, and R. L. Garcea (ed.), Structural biology of viruses. Oxford Press, New York, N.Y.
- 4.Gilbert, J., and T. L. Benjamin. 2004. The uptake pathway of polyoma virus via ganglioside GD1a. J. Virol. 78:12259-12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kartenbeck, J., H. Stukenbrok, and A. Helenius. 1989. Endocytosis of simian virus 40 into the endoplasmic reticulum. J. Cell Biol. 109:2721-2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, P. P., A. Nakanishi, S. W. Clark, and H. Kasamatsu. 2002. Formation of transitory intrachain and interchain disulfide bonds accompanies the folding and oligomerization of simian virus 40 Vp1 in the cytoplasm. Proc. Natl. Acad. Sci. USA 99:1353-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lilley, B. N., J. M. Gilbert, H. L. Ploegh, and T. L. Benjamin. 2006. Murine polyomavirus requires the endoplasmic reticulum protein Derlin-2 to initiate infection. J. Virol. 80:8739-8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magnuson, B., E. K. Rainey, T. Benjamin, M. Baryshev, S. Mkrtchian, and B. Tsai. 2005. ERp29 triggers a conformational change in polyomavirus to stimulate membrane binding. Mol. Cell 20:289-300. [DOI] [PubMed] [Google Scholar]
- 9.Norkin, L. C., H. A. Anderson, S. A. Wolfrom, and A. Oppenheim. 2002. Caveolar endocytosis of simian virus 40 is followed by brefeldin A-sensitive transport to the endoplasmic reticulum, where the virus disassembles. J. Virol. 76:5156-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ou, W., and J. Silver. 2006. Role of protein disulfide isomerase and other thiol-reactive proteins in HIV-1 envelope protein-mediated fusion. Virology 350:406-417. [DOI] [PubMed] [Google Scholar]
- 11.Pelkmans, L., J. Kartenbeck, and A. Helenius. 2001. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat. Cell Biol. 3:473-483. [DOI] [PubMed] [Google Scholar]
- 12.Stehle, T., and S. C. Harrison. 1997. High-resolution structure of a polyomavirus VP1-oligosacccharide complex: implications for assembly and receptor binding. EMBO J. 16:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai, B., J. M. Gilbert, T. Stehle, W. Lencer, T. L. Benjamin, and T. A. Rapoport. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 22:4346-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]