Abstract
Human herpesvirus 8 (HHV-8) viral interleukin-6 (vIL-6) mediates signaling through the gp130 signal transducer but unlike human IL-6 (hIL-6) does not require the nonsignaling gp80 α subunit of the IL-6 receptor complex. By utilizing a gp80-refractory vIL-6 variant, vIL-6(R189L), we found that signal transduction, as measured by STAT1 and STAT3 activation and gp130 tyrosine phosphorylation in gp80+/gp130+ HEK293T cells, was modulated by gp80. Furthermore, the signaling and BAF-130 cell growth-promoting activities of vIL-6 and hIL-6 could be distinguished, and exogenous addition of soluble gp80 enhanced cell growth supported by vIL-6. Our findings demonstrate that gp80 can modulate vIL-6 activity and that vIL-6 and hIL-6 signaling are not directly equivalent.
Several cytokines transduce signals via gp130, in association with other signaling or nonsignaling receptor subunits that allow functional complex formation (reviewed in reference 8). Cellular interleukin-6 (IL-6) proteins associate with the nonsignaling gp80 α receptor subunit and gp130 to form hexameric signaling complexes (IL-62:gp802:gp1302), dimerization of gp130 leading to phosphorylation of gp130 cytoplasmic tyrosine residues by gp130-associated Janus kinases (Jaks) (8). This triggers recruitment and activation of signal transducer and activator of transcription 1 (STAT1) and/or STAT3 transcription factors or Src homology protein 2 (SHP2) that initiates mitogen-activated protein kinase signaling. Negative feedback regulation is mediated in part by SHP2 and STAT-activated suppressor of cytokine signaling 3 (SOCS3) recruitment to gp130 phosphotyrosine-759, leading to tyrosine dephosphorylation and Jak inactivation. It remains unclear what the conformational requirements are for inducing Jak phosphorylation of gp130 tyrosines and whether signaling can be modulated as a function of conformational restraints imposed by specific ligands or non-gp130 receptor subunits. Recent electron microscopy studies with extracellular portions of gp80 and gp130 suggested that the gp80 subunits of the IL-6 receptor complex allow the close juxtapositioning of gp130 subunits at the membrane surface (19). However, human herpesvirus 8 (HHV-8) viral IL-6 (vIL-6) can signal in the absence of gp80, via formation of stable tetrameric complexes with gp130 (vIL-62:gp1302), although vIL-6 also can signal via hexameric complexes that incorporate gp80 (2, 14, 16, 22). It is possible, therefore, that conformational differences with respect to gp130 dimers in the presence and absence of gp80 might influence signal transduction.
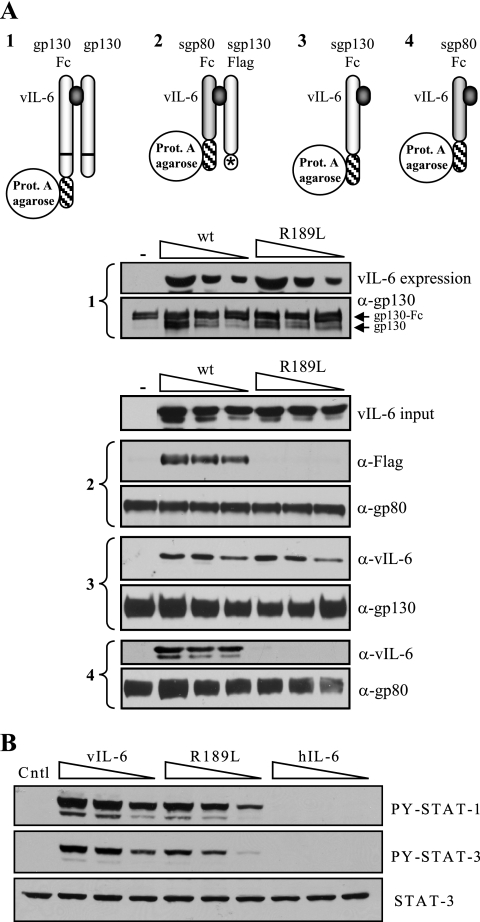
To address this issue, we first sought to dissociate tetrameric signaling by vIL-6 from hexameric signal transduction through the use of an engineered vIL-6 protein. Using coprecipitation-based procedures, we screened several previously reported vIL-6 variants (14) for their abilities to interact with gp130 and gp80 and to induce dimerization of gp130 and heterodimerization of gp130 and gp80; results for one of the vIL-6 proteins, vIL-6(R189L), are shown in Fig. 1A. vIL-6(R189L), containing a substitution for a suspected gp80-interacting “site I” residue, had no detectable interaction with gp80 and could not induce gp80:gp130 complexing while being unaffected with respect to gp130 binding and induced gp130 dimerization. Consistent with these results, vIL-6(R189L) was able to activate STAT1 and STAT3 in gp80−/gp130+ BAF-130 cells (10) (Fig. 1B).
FIG. 1.
Analysis of vIL-6 site I variant R189L for its receptor-binding properties and functional interactions with gp130. (A) Comparison of vIL-6(R189L) with wild-type vIL-6 with respect to its abilities to induce gp130:gp130 (1) and gp80:gp130 (2) complexing and to interact independently with gp130 (3) and gp80 (4). For subpanel 1, coexpression of ligand, gp130, and gp130-Fc was achieved by cotransfections of appropriate expression vectors, and cell lysates were used for protein A-agarose-mediated coprecipitations; for subpanels 2 to 4, soluble receptor components and ligand, derived from conditioned media of separately transfected cells, were mixed in vitro (as described previously [13]). vIL-6(R189L) binding to gp130 (3) and induction of gp130 dimerization (1) were equivalent to that of wild-type vIL-6, but the R189L variant could not bind gp80 independently (4) or induce gp80:gp130 complexing (2). wt, wild type; Prot. A, protein A. (B) STAT1 and STAT3 activation by vIL-6 and vIL-6(R189L) in gp80−/gp130+ BAF-130 cells (20-min cytokine treatment), as determined by Western analysis of cell lysates using phospho-STAT-specific antibodies (Cell Signaling, Beverly, Mass.; catalog no. 9171 and 9131). Total STAT3 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.; catalog no. sc-482) was applied to the stripped membrane to verify equal protein loading. Doses of vIL-6 and vIL-6(R189L) used matched catalog no. sc-482) was applied to the stripped membrane to verify equal protein loading. Doses of vIL-6 and vIL-6(R189L) used matched those indicated in the “vIL-6 input” blot in panel A [note the slightly lower levels of vIL-6(R189L)]. Treatment of cells with pSG5-transfected cell conditioned medium (Cntl) or conditioned medium containing functionally active concentrations of hIL-6 (gp80 dependent) provided negative controls.
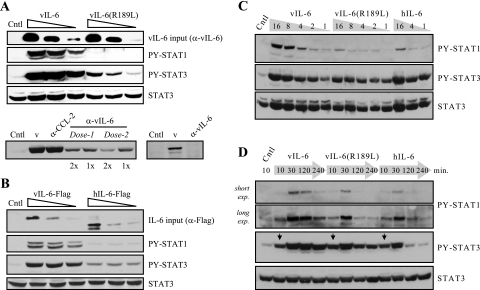
Wild-type vIL-6, vIL-6(R189L), and human IL-6 (hIL-6) were used to treat gp80+/gp130+ HEK293T cells to determine their relative abilities to activate STAT1 and STAT3. Various matched doses of vIL-6 and vIL-6(R189L), in conditioned medium, were applied to HEK293T cells for 15 minutes. Activated, tyrosine-phosphorylated STAT1 and STAT3 in cell extracts were detected by Western blotting utilizing phosphospecific antibodies (Cell Signaling, Beverly, Mass.). The results (Fig. 2A, top) revealed that gp80-refractory vIL-6(R189L) induced much weaker activations of STAT1 and STAT3 than did wild-type vIL-6. vIL-6 antiserum-depleted conditioned media were diminished or deficient (depending on antibody dose) with respect to STAT activation, demonstrating that activity was mediated directly by vIL-6 (Fig. 2A, bottom). A similar experiment was undertaken using protein-normalized Flag-tagged vIL-6 and hIL-6 proteins. Activations of STAT1 and STAT3 induced by hIL-6 were both lower than those induced by vIL-6 (Fig. 2B). Combined, these data demonstrate quantitative differences in signaling between wild-type and R189L versions of vIL-6 and between vIL-6 and hIL-6.
FIG. 2.
Comparisons of STAT1 and STAT3 activation by vIL-6, vIL-6(R189L), and hIL-6 in gp130+/gp80+ HEK293T cells. (A) Transfected cell conditioned media containing the viral cytokines (normalized) were applied, in different doses, to HEK293T cells for 15 min. Cells were then harvested for preparation of cell lysates for Western analysis to determine levels of tyrosine-phosphorylated (activated) STAT1 and STAT3. Probing for total STAT3 provided a protein-loading control. Depletion of vIL-6 by immunoprecipitation with vIL-6 antiserum (22) led to loss of STAT3 activation by vIL-6 conditioned medium (v) in proportion to antibody dose (dose 2 = 3× dose 1) and number (1×, 2×) of cycles of immunoprecipitation (left), with complete loss of activity at a high ratio of antibody to vIL-6 (right). Antiserum to HHV-8 vCCL-2 (vMIP-1B/vMIP-II) was used as a negative control and could not deplete STAT-inducing activity of vIL-6 conditioned medium. These results demonstrate that vIL-6 is the active component in the conditioned medium. (B) STAT induction assays using protein-normalized transfected cell conditioned media containing vIL-6-Flag and hIL-6-Flag. (C) Dose-response experiments to determine the relative activations of STAT1 and STAT3 by vIL-6, vIL-6(R189L), and hIL-6. Dose ranges of the cytokines giving similar STAT3 activation following 20-min treatment were used for this experiment. Wild-type vIL-6 activated higher levels of STAT1 than either vIL-6(R189L) or hIL-6 at doses of the cytokines giving equal levels of STAT3 activation. (D) Time course experiments for STAT activation by vIL-6, vIL-6(R189L), and hIL-6, using doses of the cytokines that gave the same level of STAT3 activation following stimulation for 10 min (arrows) and 20 min (data not shown). exp., exposure; Cntl, control (pSG5-transfected cell conditioned medium).
To investigate whether there were qualitative differences in signaling between the three cytokines, we undertook dose-response experiments using applied concentrations of vIL-6, vIL-6(R189L), and hIL-6 that gave similar levels of STAT3 activation at 20 min. The levels of tyrosine-phosphorylated STAT1 were determined to allow identification of any differences in the relative activations of STAT1 and STAT3. The results of these experiments (Fig. 2C) revealed that there were indeed differences in the ratios of STAT1 and STAT3 activation by vIL-6 and hIL-6 and that gp80-refractory vIL-6(R189L), while less active with respect to STAT3 activation at higher doses, was relatively more impaired for STAT1 activation throughout the dose range. These data suggest that there are functionally important differences between the hexameric (gp80-containing) complexes formed by vIL-6 and hIL-6 and that gp80 can influence vIL-6 signaling in a qualitative manner.
To further compare vIL-6, vIL-6(R189L), and hIL-6 signaling, we followed the kinetics of STAT1 and STAT3 activation by undertaking a time course experiment using STAT3 activation-normalized (20-min stimulation) doses of the three cytokines. The results of these experiments (Fig. 2D) revealed prolonged activations of STAT1 and STAT3 by vIL-6 relative to hIL-6. For vIL-6, STAT signaling was sustained for up to 4 h, at maximal levels for STAT3, while hIL-6 activations of STAT1 and STAT3 returned to basal or near-basal levels by 2 h. STAT1 activation by vIL-6(R189L) was of shorter duration and of lesser amplitude than that induced by vIL-6; STAT3 activation was identical at 10 and 30 min to that induced by wild-type vIL-6 and intermediate between vIL-6- and hIL-6-activated STAT3 at 2 h and 4 h. Together, these data provide further, kinetic evidence for qualitative differences in STAT signaling between vIL-6, vIL-6(R189L), and hIL-6 and suggest that vIL-6 signaling is less susceptible to negative regulation than that induced by hIL-6 and vIL-6(R189L).
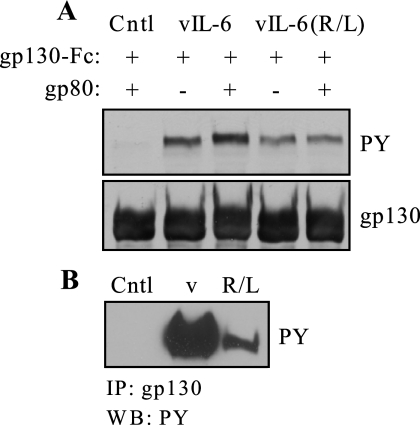
We next examined levels of tyrosine phosphorylation of endogenous gp130 or transfected (overexpressed) gp130-Fc, in the presence or absence of co-overexpressed gp80, in HEK293T cells in response to treatment with vIL-6 and gp80-refractory vIL-6(R189L). For the transfected cells, both vIL-6 and vIL-6(R189L) were able to induce gp130 phosphorylation to equivalent levels in the absence of co-overexpressed gp80, but gp130 phosphorylation was augmented by gp80 only for wild-type vIL-6 (Fig. 3A). This increase, while small, was highly reproducible in repeat experiments. Consistent with this result, relative levels of phosphorylation of endogenously expressed gp130 in HEK293T cells, which naturally express gp80 also, were higher for wild-type vIL-6-treated cells than for those treated with vIL-6(R189L) (Fig. 3B). Thus, gp80 can influence signal transduction via enhancement of the initial event, tyrosine phosphorylation of gp130.
FIG.3.
Tyrosine phosphorylation of gp130 induced by vIL-6 and vIL-6(R189L). (A) HEK293T cells were transfected with a gp130-Fc expression vector with or without cotransfection of a gp80 expression plasmid. Following a 15-min stimulation with vIL-6 or vIL-6(R189L) conditioned medium (vIL-6 protein normalized), cells were lysed, gp130-Fc was precipitated with protein A-agarose, and size-fractionated and membrane-blotted protein was probed with antibody (Cell Signaling; catalog no. 9411) specific for phosphotyrosine (PY) to measure levels of gp130 phosphorylation. Precipitated gp130 (total) was detected by probing the stripped blot with a gp130 antibody (BD Biosciences, San Diego, Calif.; catalog no. 555756). Cotransfection of gp80 with gp130-Fc was able to enhance gp130 phosphorylation induced by vIL-6, but not by vIL-6(R189L), above the high levels obtained with overexpressed gp130-Fc alone. (B) An analogous experiment was undertaken using untransfected HEK293T cells (gp80+/gp130+) to investigate vIL-6- and vIL-6(R189L)-induced tyrosine phosphorylation of endogenous gp130. In this case, gp130 was immunoprecipitated prior to gel fractionation and Western blotting. As before, equal applied doses of vIL-6 and vIL-6(R189L) were used. Cntl, control (pSG5-transfected cell conditioned medium).
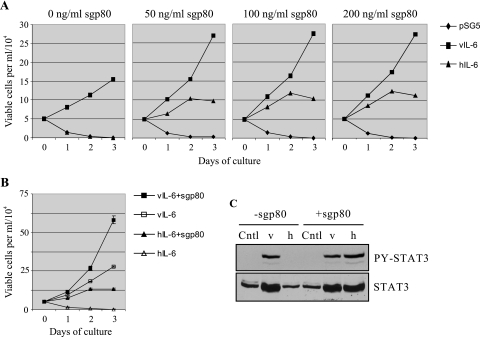
Finally, we examined whether gp80 could influence the biological activity of vIL-6 and whether vIL-6 and hIL-6 exhibited measurable differences in biological effects. To do this, we used IL-6/IL-3-dependent gp80−/gp130+ BAF-130 cells to examine the proliferative effects of the cytokines. Cells were cultured in serum-reduced growth medium containing each of the cytokines, or control medium lacking cytokine, either in the absence or in the presence of recombinant soluble gp80 (sgp80). Three different doses of sgp80 (50 ng, 100 ng, and 200 ng per ml) were used to achieve and verify optimal support of signaling by hIL-6, which is dependent on gp80 for signal transduction, and saturating concentrations of vIL-6 and hIL-6 were utilized to obtain maximal signaling by the cytokines. Numbers of viable cells per unit volume, as established by trypan blue exclusion, were determined at daily intervals up to 3 days. Growth was supported by wild-type vIL-6 either with or without exogenously added sgp80, but gp80 clearly promoted this activity (Fig. 4A). For hIL-6, cell growth was dependent on addition of sgp80, as expected. Cell growth supported by hIL-6 (up to day 2) was lower than that supported by vIL-6, both in the absence and in the presence of gp80. Within the range utilized (50 to 200 ng/ml), different doses of gp80 supported similar levels of BAF-130 cell growth, confirming that applied concentrations of sgp80 were not limiting. A repeat of this experiment, performed in triplicate, using the 50-ng/ml dose of sgp80 confirmed the results of the first experiment and their reproducibility (Fig. 4B). Again, sgp80 was able to augment vIL-6-induced BAF-130 cell proliferation and vIL-6 was more effective than hIL-6 in supporting BAF-130 cell growth. Verification of signaling by the doses of vIL-6 and hIL-6 used in the proliferation experiments was confirmed by STAT3 activation assays in IL-6-treated BAF-130 cells (Fig. 4C).
FIG. 4.
BAF-130 growth assays to determine effects of gp80 on vIL-6 biological activity and differences between vIL-6 and hIL-6. (A) Cytokine-dependent BAF-130 cells were incubated in serum-free medium overnight prior to addition of an equal volume of vIL-6 or hIL-6 conditioned medium (containing 10% fetal bovine serum) either with or without added recombinant sgp80 (50, 100, or 200 ng/ml; R&D Systems, Minneapolis, Minn.; catalog no. 227-SR). Doses of cytokines used were in excess of those that gave maximal STAT3 activation in HEK293T cells. Triplicate samples of cells were extracted daily from each culture for counting of trypan blue-excluding (viable) cells. Values plotted are the averages from three sample counts per culture at each time point. (B) The experiment was repeated, this time performed in triplicate, at 50 ng/ml of sgp80. Error bars indicate the standard deviations from the means calculated from each set of triplicate wells. The pSG5 (empty vector) negative controls, ± sgp80, have been omitted for clarity but mirrored the results for hIL-6 in the absence of sgp80. (C) STAT3 activation assays in BAF-130 cells to confirm activities of the applied doses of vIL-6 and hIL-6 used in the growth assays. Cells were harvested after 24 h of cytokine treatment. Cntl, control (pSG5-transfected cell conditioned medium).
In summary, we have demonstrated for the first time that there are qualitative differences in signal transduction induced by vIL-6 and hIL-6 and that the nonsignaling gp80 subunit of the IL-6 receptor complex can influence signal transduction by the viral cytokine. Furthermore, we have shown that gp80 significantly enhances cell proliferation and survival stimulated by vIL-6, providing biological evidence of the influence of gp80 on vIL-6 signaling. Thus, we have confirmed previous findings from our own and other laboratories (2, 4, 13, 14, 17, 22) that vIL-6 can utilize gp80 and extended these findings to show that gp80 can positively affect gp130 phosphorylation, signal transduction via STAT1 and STAT3, and cell growth.
The mechanistic basis for these gp80-mediated and vIL-6-versus-hIL-6 differences in signal transduction remains to be determined. A reasonable hypothesis is that these effects result from gp80- and ligand-induced conformational differences in the signaling complex that affect relative accessibility of gp130 tyrosine residues to Jaks, SHP2, SOCS3, and/or STATs. As mentioned in the introductory section, biophysical evidence has been published demonstrating the influence of gp80 on the juxtapositioning of regions of gp130 distal to the cytokine-binding domains (19), and models in which gp130 ligands not only mediate gp130 dimerization but also induce specific conformational relationships between the gp130 subunits have been proposed (reviewed in reference 8). Therefore, ligand- and gp80-determined differences in gp130:gp130 complexing could affect signal transduction at the levels of gp130 phosphorylation by Jaks (for which we present evidence here), negative regulation by SOCS3 and/or SHP2 (6, 21), or activation of STAT1 versus STAT3, which are recruited differently to the four distal phosphorylated SH2 motifs of gp130 (7, 9, 18, 20). Notwithstanding our demonstration that gp80 plays a positive role in vIL-6-simulated BAF-130 cell growth (Fig. 4), the actual biological relevance of the effects of gp80 on vIL-6-activated STAT signaling identified in this report is difficult to predict. It is known that STAT3 activation is important for cell survival, and high levels of constitutively active STAT3 have been identified in several lymphoid and other tumors (1, 3, 11). The enhanced and prolonged activation of STAT3 that our results indicate could occur in gp80+/gp130+ cells in response to vIL-6 could conceivably play a direct role in HHV-8-induced neoplasia. STAT1, on the other hand, has been linked classically to interferon responses and induction of cell cycle arrest, apoptosis, and inflammation. However, more recently it has been appreciated that the precise inducer and context of STAT1 activation are crucial determinants of the biological response. For example, gamma interferon and gp130-cytokine oncostatin M both activate STAT1, but only the former can induce STAT1-responsive target genes in human endothelial cells (15), and SOCS3 plays a pivotal role in preventing gamma interferon-like responses following IL-6 stimulation of murine macrophages and liver cells (5, 12). Thus, the biological effects of efficient STAT1 activation by vIL-6, both on cells and on virus replication, will need to be determined empirically.
Acknowledgments
We thank members of the Nicholas laboratory, Daming Chen, Young Bong Choi, Liping Feng, and Gordon Sandford, for advice, comments, and general support.
This work was supported by NIH grant CA76445.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Benekli, M., M. R. Baer, H. Baumann, and M. Wetzler. 2003. Signal transducer and activator of transcription proteins in leukemias. Blood 101:2940-2954. [DOI] [PubMed] [Google Scholar]
- 2.Boulanger, M. J., D. C. Chow, E. Brevnova, M. Martick, G. Sandford, J. Nicholas, and K. C. Garcia. 2004. Molecular mechanisms for viral mimicry of a human cytokine: activation of gp130 by HHV-8 interleukin-6. J. Mol. Biol. 335:641-654. [DOI] [PubMed] [Google Scholar]
- 3.Bowman, T., R. Garcia, J. Turkson, and R. Jove. 2000. STATs in oncogenesis. Oncogene 19:2474-2488. [DOI] [PubMed] [Google Scholar]
- 4.Burger, R., F. Neipel, B. Fleckenstein, R. Savino, G. Ciliberto, J. R. Kalden, and M. Gramatzki. 1998. Human herpesvirus type 8 interleukin-6 homologue is functionally active on human myeloma cells. Blood 91:1858-1863. [PubMed] [Google Scholar]
- 5.Croker, B. A., D. L. Krebs, J. G. Zhang, S. Wormald, T. A. Wilson, E. G. Stanley, L. Robb, C. L. Greenhalgh, I. Forster, B. E. Clausen, N. A. Nicola, D. Metcalf, D. J. Hilton, A. W. Roberts, and W. S. Alexander. 2003. SOCS3 negatively regulates IL-6 signaling in vivo. Nat. Immunol. 4:540-545. [DOI] [PubMed] [Google Scholar]
- 6.Fischer, P., U. Lehmann, R. M. Sobota, J. Schmitz, C. Niemand, S. Linnemann, S. Haan, I. Behrmann, A. Yoshimura, J. A. Johnston, G. Muller-Newen, P. C. Heinrich, and F. Schaper. 2004. The role of the inhibitors of interleukin-6 signal transduction SHP2 and SOCS3 for desensitization of interleukin-6 signalling. Biochem. J. 378:449-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerhartz, C., B. Heesel, J. Sasse, U. Hemmann, C. Landgraf, J. Schneider-Mergener, F. Horn, P. C. Heinrich, and L. Graeve. 1996. Differential activation of acute phase response factor/STAT3 and STAT1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. I. Definition of a novel phosphotyrosine motif mediating STAT1 activation. J. Biol. Chem. 271:12991-12998. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich, P. C., I. Behrmann, S. Haan, H. M. Hermanns, G. Muller-Newen, and F. Schaper. 2003. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374:1-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hemmann, U., C. Gerhartz, B. Heesel, J. Sasse, G. Kurapkat, J. Grotzinger, A. Wollmer, Z. Zhong, J. E. Darnell, L. Graeve, P. C. Heinrich, and F. Horn. 1996. Differential activation of acute phase response factor/Stat3 and Stat1 via the cytoplasmic domain of the interleukin 6 signal transducer gp130. II. Src homology SH2 domains define the specificity of Stat factor activation. J. Biol. Chem. 271:12999-13007. [DOI] [PubMed] [Google Scholar]
- 10.Hibi, M., M. Murakami, M. Saito, T. Hirano, T. Taga, and T. Kishimoto. 1990. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell 63:1149-1157. [DOI] [PubMed] [Google Scholar]
- 11.Hodge, D. R., E. M. Hurt, and W. L. Farrar. 2005. The role of IL-6 and STAT3 in inflammation and cancer. Eur. J. Cancer 41:2502-2512. [DOI] [PubMed] [Google Scholar]
- 12.Lang, R., A. L. Pauleau, E. Parganas, Y. Takahashi, J. Mages, J. N. Ihle, R. Rutschman, and P. J. Murray. 2003. SOCS3 regulates the plasticity of gp130 signaling. Nat. Immunol. 4:546-550. [DOI] [PubMed] [Google Scholar]
- 13.Li, H., and J. Nicholas. 2002. Identification of amino acid residues of gp130 signal transducer and gp80 α receptor subunit that are involved in ligand binding and signaling by human herpesvirus 8-encoded interleukin-6. J. Virol. 76:5627-5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li, H., H. Wang, and J. Nicholas. 2001. Detection of direct binding of human herpesvirus 8-encoded interleukin-6 (vIL-6) to both gp130 and IL-6 receptor (IL-6R) and identification of amino acid residues of vIL-6 important for IL-6R-dependent and -independent signaling. J. Virol. 75:3325-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahboubi, K., and J. S. Pober. 2002. Activation of signal transducer and activator of transcription 1 (STAT1) is not sufficient for the induction of STAT1-dependent genes in endothelial cells. Comparison of interferon-gamma and oncostatin M. J. Biol. Chem. 277:8012-8021. [DOI] [PubMed] [Google Scholar]
- 16.Molden, J., Y. Chang, Y. You, P. S. Moore, and M. A. Goldsmith. 1997. A Kaposi's sarcoma-associated herpesvirus-encoded cytokine homolog (vIL-6) activates signaling through the shared gp130 receptor subunit. J. Biol. Chem. 272:19625-19631. [DOI] [PubMed] [Google Scholar]
- 17.Nicholas, J., V. R. Ruvolo, W. H. Burns, G. Sandford, X. Wan, D. Ciufo, S. B. Hendrickson, H.-G. Guo, G. S. Hayward, and M. S. Reitz. 1997. Kaposi's sarcoma-associated human herpesvirus 8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat. Med. 3:287-292. [DOI] [PubMed] [Google Scholar]
- 18.Schmitz, J., H. Dahmen, C. Grimm, C. Gendo, G. Muller-Newen, P. C. Heinrich, and F. Schaper. 2000. The cytoplasmic tyrosine motifs in full-length glycoprotein 130 have different roles in IL-6 signal transduction. J. Immunol. 164:848-854. [DOI] [PubMed] [Google Scholar]
- 19.Skiniotis, G., M. J. Boulanger, K. C. Garcia, and T. Walz. 2005. Signaling conformations of the tall cytokine receptor gp130 when in complex with IL-6 and IL-6 receptor. Nat. Struct. Mol. Biol. 12:545-551. [DOI] [PubMed] [Google Scholar]
- 20.Stahl, N., T. J. Farruggella, T. G. Boulton, Z. Zhong, J. E. Darnell, and G. D. Yancopoulos. 1995. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science 267:1349-1353. [DOI] [PubMed] [Google Scholar]
- 21.Terstegen, L., P. Gatsios, J. G. Bode, F. Schaper, P. C. Heinrich, and L. Graeve. 2000. The inhibition of interleukin-6-dependent STAT activation by mitogen activated protein kinases depends on tyrosine 759 in the cytoplasmic tail of glycoprotein 130. J. Biol. Chem. 275:18810-18817. [DOI] [PubMed] [Google Scholar]
- 22.Wan, X., H. Wang, and J. Nicholas. 1999. Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J. Virol. 73:8268-8278. [DOI] [PMC free article] [PubMed] [Google Scholar]