Abstract
In a study of genes expressed differentially in the freshwater crayfish Pacifastacus leniusculus infected experimentally with the white spot syndrome virus (WSSV), one protein, known as antilipopolysaccharide factor (ALF), was chosen, among those whose transcript levels increased upon viral infection, for further studies. ALF RNA interference (RNAi) experiments in whole animals and in cell cultures indicated that ALF can protect against WSSV infection, since knockdown of ALF by RNAi specifically resulted in higher rates of viral propagation. In a cell culture of hematopoietic tissue (Hpt) from P. leniusculus, quantitative PCR showed that knockdown of ALF by RNAi resulted into WSSV levels that were about 10-fold higher than those treated with control double-stranded RNA (dsRNA). In addition, RNAi experiments with other crayfish genes that had been found to be up-regulated by a WSSV infection did not result in any changes of viral loads. Thus, the cell culture does not respond to dsRNA in a similar manner, as shown earlier for dsRNA injected into shrimp, which gave a higher degree of resistance to WSSV infection. If ALF transcription in whole animals was stimulated by the administration of UV-treated WSSV, a partial protection against a subsequent challenge with the active virus was conferred to the host. This is the first crustacean gene product identified with the capacity to interfere with replication of this important pathogen.
White spot syndrome virus (WSSV), which is spread all around the world, is one of the most common and most disastrous diseases for shrimp (22) and also is infecting many different species of crustaceans, including freshwater crayfish (11, 14, 16, 31). Although during recent years knowledge at the molecular level has been gathered on the ways in which invertebrates defend themselves against bacteria and fungi, still little is known about the defense mechanisms elicited by virus.
However, recently some data from the insect Drosophila melanogaster provided some more insight on antiviral mechanisms. For instance, the Jak-STAT signaling pathway is required but not sufficient for the antiviral response against Drosophila C virus (5). The Toll pathway is crucial for the antiviral activity against Drosophila X virus (32), and very recently, Dicer-2 was proved to play an essential role in host defense against flock house virus, Drosophila C virus, and Sindbis virus in vivo (6).
Using suppression subtractive hybridization (SSH), expression sequence tag, cDNA microarrays, or mRNA differential display approaches to enable the identification of genes differentially expressed in the shrimp yielded a number of genes which were considered to be potentially involved in the viral defense mechanism (4, 7, 17, 18, 21). Some of these were proven to be related to an antiviral process or the immune defense mechanism of viral infection, such as the antiviral gene PmAV (17), the interferon-like protein (IntlP) and (2′-5′) oligo(A) synthetase-like protein (9), and a synthetin-like protein (2, 28). Moreover, RNA interference (RNAi) has been demonstrated to be implicated in antiviral response, since both double-stranded RNA (dsRNA) and small interfering RNA can trigger the antiviral process against WSSV in shrimp (19, 20, 30).
To isolate up-regulated genes in the freshwater crayfish Pacifastacus leniusculus, an alternative animal model for WSSV, SSH was used to compare genes expressed in the hemocytes of crayfish before and after WSSV challenge, since hemocytes are important as immune cells in crustaceans. In the present study, among a number of differentially expressed genes, antilipopolysaccharide factor (ALF) was identified and characterized, and it was shown that ALF interferes with WSSV propagation using RNA interference both in vivo and in vitro, which is the first reported RNAi study in vitro with a crustacean. It is likely that ALF plays an important role in the immune defense against viral infection of crayfish.
MATERIALS AND METHODS
Construction of subtracted cDNA libraries using SSH.
Intermolt healthy crayfish and WSSV stock suspension were provided as mentioned in the supplementary materials available at http://www.fu.uu.se/jamfys/pub3.html. SSH of pre-WSSV-infected and post-WSSV-infected crayfish was performed with the Clontech PCR select cDNA subtraction kit (Clontech, Palo Alto, CA) by following the manufacturer's instructions.
WSSV propagation in crayfish in which ALF expression was increased and cumulative mortality assay with WSSV infection.
UV-inactivated WSSV was prepared as described in reference 12, and crayfish saline buffer (CFS; 0.2 M NaCl, 5.4 mM KCl, 10 mM CaCl2, 2.6 mM MgCl2, 2 mM NaHCO3, pH 6.8) was used for virus dilution. Two hundred microliters of UV-inactivated WSSV (20 μl of UV-inactivated WSSV stock suspension and 180 μl of CFS) was injected via the base of the fourth walking leg, and 200 μl of UV-treated crayfish plasma (20 μl of UV-inactivated control plasma and 180 μl of CFS) was used as a control treatment, since the WSSV stock suspension was prepared from plasma of WSSV-infected crayfish. After 24 h, hemocytes total RNA was isolated for conventional reverse transcription (RT)-PCR and quantitative RT-PCR. Quantitative PCR data were analyzed by comparative quantitation. To test the cumulative mortality caused by WSSV infection of crayfish in which ALF was up-regulated by UV-inactivated WSSV, crayfish were injected with 200 μl of live WSSV stock suspension mixed with UV-inactivated WSSV stock suspension (20 μl of WSSV stock suspension mixed with 20 μl of UV-inactivated WSSV stock suspension and 160 μl of CFS) as an ALF up-regulated treatment and 200 μl of live WSSV stock suspension mixed with UV-treated crayfish plasma (20 μl of WSSV stock suspension mixed with 20 μl of UV-treated control plasma and 160 μl of CFS) as a control treatment. The cumulative mortality was recorded daily, and the data were analyzed by Student's t test.
Generation of dsRNA.
Oligonucleotide primers were designed to amplify a 541-bp region of the P. leniusculus ALF gene from the forward subtracted library, and they were incorporated with T7 promoter sequences (italic) at the 5′ ends: 107+, 5′-TAATACGACTCACTATAGGGATGCGGACGTGGGTACTAGTGA-3′; 647−, 5′-TAATACGACTCACTATAGGGTCCAGGAAGATGCGACTACCA-3′. Control 657-bp templates were generated by PCR using primers specific for portions of the green fluorescent protein (GFP) gene from the pd2EGFP-1 vector (Clontech, Palo Alto, CA, USA), and the primers incorporated with T7 promoter sequences (italic) were as follows: 63+, 5′-TAATACGACTCACTATAGGGCGACGTAAACGGCCA CAAGT; 719−, 5′-TAATACGACTCACTATAGGGTTCTTGTACAGCTCGTCCATG C-3′. To generate dsRNA, PCR products purified by gel extraction (QIAGEN, Hilden, Germany) were used as templates for in vitro transcription using the MegaScript kit (Ambion, Austin, TX), and dsRNA was purified with the Trizol LS reagent (Invitrogen, Carlsbad, CA) method.
dsRNAi in vivo.
Small intermolt crayfish (20 ± 2 g, fresh weight) were used for in vivo RNAi experiments. Briefly, 150 μg of ALF and GFP control dsRNA dissolved in CFS (200 μl) was injected via the base of the fourth walking leg. The injection was repeated 24 h after the first dsRNA injection. WSSV infection was done 12 h later, following the second dsRNA injection. Total RNA from hemocytes was extracted after 36 h of WSSV infection. Crayfish ALF and WSSV VP28 transcripts were determined by RT-PCR and quantitative PCR using comparative quantitation.
Crayfish Hpt cell culture and maintenance.
The hematopoietic tissue (Hpt) cells were isolated from freshwater crayfish, P. leniusculus, as described by Söderhäll et al. (24). Briefly, the hematopoietic tissue was dissected from the dorsal side of the stomach, and washed with CPBS (crayfish phosphate-buffered saline: 10 mM Na2HPO4, 10 mM KH2PO4, 150 mM NaCl, 10 μM CaCl2, and 10 μM MnCl2, pH 6.8), and then incubated in 500 μl of 0.1% collagenase (type I and IV) (Sigma, Steinheim, Germany) in CPBS at room temperature for 45 min to dissociate the Hpt cells. The separated cells were washed twice with CPBS by spinning down at 2,500 × g for 5 min at room temperature. The cell pellet was then resuspended in modified L-15m81 medium (25) and subsequently seeded at a density of 5 × 104 cells/150 μl in 96-well plates. Hpt cells were supplemented with a crude astakine preparation (25) after about 30 min of attachment at room temperature, and one-third of the medium was changed every second day.
dsRNAi in vitro and WSSV infection.
To avoid the cytotoxicity of a cationic liposome-based gene delivery system, we used a modified histone H2A (histone from calf thymus, type II-A; Sigma, Steinheim, Germany) protocol (more details about histone H2A experiments are in the supplementary material Fig. 3 at http://www.fu.uu.se/jamfys/pub3.html) (8) for dsRNA transfection into crayfish Hpt cell cultures. Shortly thereafter, 4 μl of dsRNA (250 ng/μl) was mixed with 3 μl of histone H2A (1 mg/ml) for one well of Hpt cell culture and incubated for 5 to 10 min at room temperature, followed by mixture with 20 μl of modified L-15m81 medium (25), and added to the 3-day-old Hpt cell cultures. The cells were incubated for 12 h at 16°C. After 12 h of incubation at 16°C, the medium was replaced with 150 μl of L-15m81 medium together with 5 μl of WSSV stock suspension (11) and 5 μl of crude astakine preparation and incubated for another 12 h. Thereafter, the cells were washed twice with L-15m81 medium and supplied with fresh medium containing a crude astakine preparation. The Hpt cells were incubated at 20°C for 36 h with a WSSV inoculation followed by total RNA preparation.
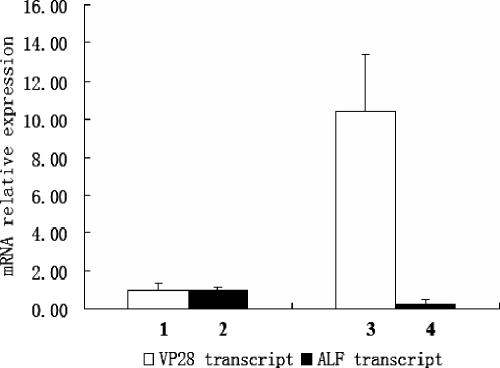
FIG. 3.
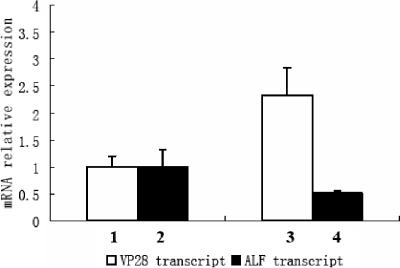
RNA interference of ALF and WSSV replication in vivo. RNA interference was performed with an injection of 150 μg of ALF dsRNA or GFP dsRNA into crayfish (20 ± 2 g [fresh weight]). The same injection was repeated 24 h after the first dsRNA injection. These crayfish were infected with WSSV by injection of 200 μl of live WSSV stock suspension 12 h after the second dsRNA injection. Hemocyte total RNA was prepared 36 h post-WSSV infection for quantitative RT-PCR. The crayfish ribosomal protein 40s gene was used as an internal control for WSSV VP28 gene and crayfish ALF gene quantitation by quantitative RT-PCR. The experiment has been repeated three times, the data represent means of triplicates, and the error bars indicate standard deviations. Columns 1 and 2 show VP28 and ALF transcript levels, respectively, after injection with GFP dsRNA. Columns 3 and 4 show VP28 and ALF transcript levels, respectively, after injection with ALF dsRNA.
Total RNA preparation and RT-PCR.
Total RNA was isolated as described in the supplementary materials at http://www.fu.uu.se/jamfys/pub3.html and followed by RNase-free DNase I (Ambion, Austin, TX) treatment. cDNA was synthesized with ThermoScript (Invitrogen, Carlsbad, CA), and PCRs were done with WSSV VP28 (GenBank accession no. AF502435)-specific primers. Crayfish ribosomal protein 40s gene (R40s) primers were used in all PCR experiments as controls. The presence of ALF gene transcripts was examined by RT-PCR with the same template to compare the gene silencing efficiency. The PCR program was as follows: 94°C for 3 min, followed by 32 cycles of 94°C for 30 s, 60°C for 30 s, and 72°C for 40 s for the VP28 gene and 29 cycles for the ribosomal 40s gene and the ALF gene. The PCR products were analyzed on a 1.5% agarose gel stained with ethidium bromide.
SYBR green quantitative RT-PCR.
The detection and comparative quantification of WSSV replication in crayfish Hpt cell cultures and animals were done by quantitative RT-PCR using the QuantiTect SYBR green PCR kit (QIAGEN, Hilden, Germany). The expression of the ALF or WSSV VP28 gene was normalized to the expression of the mRNA encoding the crayfish ribosomal protein gene (R40s) for each sample. The primers used were as follows: ALF forward, 5′-CAGAGACCAGCCGATGTTCTTA-3′; reverse, 5′-CGGCTTTGCTAACTAAATGCCC-3′; VP28 (GenBank accession no. AF502435) forward, 5′-GGGAACATTCAAGGTGTGGA-3′; reverse, 5′-GGTGAAGGAGGAGGTGTTGG-3′; crayfish ribosomal protein 40s gene forward, 5′-GACGAATGGCATACACCTGAGAGG-3′; reverse, 5′-CAGGACTCTGCAGTTCAAGCTGATG-3′. SYBR green quantitative RT-PCR amplification was done in a Rotor-Gene 3000 (Corbett Robotics, Australia). The cDNA was synthesized using oligo(dT) as described in the RT-PCR section. The cDNA samples were diluted 1:10 with RNase-free sterilized water. The amplification was carried out in a 25-μl reaction volume which contained 12.5 μl of 2× QuantiTect SYBR green PCR master mix, 0.4 μM concentrations of each primer, and 5 μl of diluted cDNA template. RNase-free distilled water was added to reach a total volume of 25 μl per reaction. All runs employed a negative control without target DNA. Thermal cycling conditions were as follows: 95°C for 15 min, followed by 45 cycles of 94°C for 15 s, 62°C for 30 s, and 72°C for 30 s. All PCRs were performed in triplicates.
In situ hybridization.
The gene silencing efficiency was determined by mRNA-cDNA in situ hybridization as well, and the WSSV replication in both ALF dsRNA-silenced and GFP dsRNA-treated Hpt cell cultures was analyzed by RT-PCR. The cDNA probe for ALF (48+ to 498−) was labeled with digoxigenin using Klenow enzyme (Roche, Mannheim, Germany). The Hpt cells were cultured as described above but on coverslips in six-well plates. For study of ALF expression, the cells were fixed with 95% ethanol for 5 min at room temperature and stored in 70% ethanol at −20°C until used in hybridization experiments according to the method described by Söderhäll et al. (24). Both RNase H-treated cells and cells without any probe were used as negative controls in the hybridization. Coverslips were mounted in VECTASHIELD medium (Vector Laboratories), and the fluorescent signal was detected by confocal microscopy (Leica).
RESULTS
The suppression subtractive hybridization technique was used to identify transcripts enriched following a WSSV infection in hemocytes of a single animal. The WSSV-up-regulated genes encoded proteins including a wide range of functional categories (see the supplementary materials at http://www.fu.uu.se/jamfys/pub3.html). Among the transcripts enriched after the viral infection were several clones with sequence homology to the Limulus ALF. A full-length clone encoding a 120-amino-acid protein was isolated, and its sequence exhibited 28% to 32% sequence identities to ALFs from other crustaceans (Fig. 1). Furthermore, the crayfish ALF sequence contains conserved features, such as the positions of two conserved cysteine residues and the putative lipopolysaccharide (LPS) binding site located between these two cysteines (10).
FIG. 1.
Amino acid sequence alignment of Pacifastacus ALF with ALFs from Tachypleus, Limulus, Litopenaeus, and Penaeus. Sequences are shown for the ALF protein from Tachypleus tridentatus (GenBank accession no. P07087), Limulus polyphemus (GenBank accession no. P07086), Litopenaeus stylirostris (GenBank accession no. AAY33769), Penaeus monodon, and Pacifastacus leniusculus. The black boxes enclose the conserved amino acid residues, and a putative LPS binding site is located between two conserved cysteines indicated with asterisks. The numbers show the positions of amino acid residues of each protein.
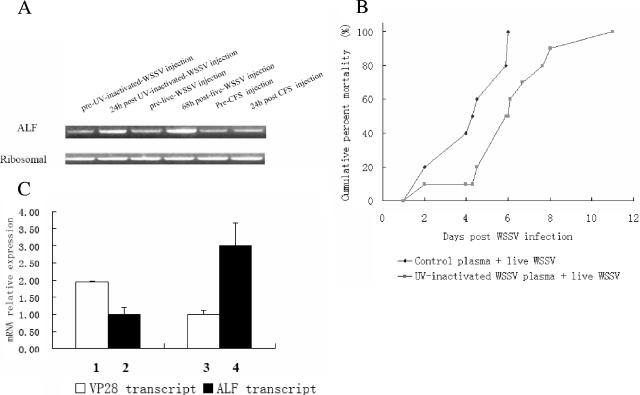
Since ALFs have been implicated in immune reactions in horseshoe crabs as well as in shrimps, we decided to study it further. Naive animals were injected with active or UV-inactivated virus, and the ALF expression in the hemocytes was monitored by RT-PCR. In both cases, viral injections resulted in enhanced ALF expression, whereas a saline injection had no effect on ALF levels (Fig. 2A). Thus, these data and the results from the SSH approach seem to indicate that elevated ALF levels may be a consequence of WSSV presence. To determine whether these enhanced ALF levels could influence the outcome of the viral infection, we triggered elevated ALF levels with UV-inactivated virus injected together with an infectious WSSV preparation. Treating the animals with inactivated virus resulted in a slower disease progress, as manifested by the longer time taken to reach 100% mortality than for animals receiving active virus only (Fig. 2B). A comparison between animals receiving active virus only and those receiving both inactivated and active virus showed >2-times-higher transcripts level of the viral VP28 gene in the former but 3-times-higher ALF transcripts level in the animals receiving both inactivated and active virus (Fig. 2C). In summary, pretreating animals with UV-inactivated virus resulted in higher ALF levels, slower viral replication, and a slower increase in cumulative mortality values upon injection of the active virus than those in animals receiving active virus only.
FIG. 2.
(A) ALF up-regulation in vivo by UV-inactivated WSSV and live WSSV. Crayfish were first bled for hemocyte RNA isolation before the experiments started and then kept 1 week, followed by injection with 200 μl of UV-inactivated WSSV suspension (20 μl of UV-inactivated WSSV and 180 μl of CFS) or 200 μl of live WSSV suspension (20 μl of WSSV stock suspension and 180 μl of CFS) as described in Materials and Methods. CFS (200 μl) injection was also done as a control treatment. RT-PCR for the crayfish ALF gene and ribosomal protein 40s gene were performed on hemocyte total RNA isolated from crayfish 24 h postinjection with UV-inactivated WSSV or 68 h postinjection with live WSSV. For the CFS control, RNA isolation was performed 24 h postinjection. (B) Cumulative mortality of crayfish infected with WSSV. Crayfish ALF was found to be up-regulated in vivo by an injection of UV-inactivated WSSV. Thus, 200 μl of WSSV mixed suspension (20 μl of WSSV stock suspension mixed with 20 μl of UV-inactivated WSSV stock suspension and 160 μl of CFS) was injected via the base of fourth walking leg. UV-treated control crayfish plasma was used as a control treatment, since the WSSV stock suspension was prepared from plasma of WSSV-infected crayfish. Ten crayfish were used for each group. The mortality was recorded daily. The experiment was repeated three times. The cumulative mortality of crayfish in which up-regulated ALF was compared with that of control treated crayfish, and the data were analyzed by Student's t test (P = 0.0015). (C) Quantitative RT-PCR on WSSV replication in crayfish in which ALF was up-regulated by UV-inactivated WSSV. Crayfish were injected with either 200 μl of WSSV mixed suspension (20 μl of WSSV stock suspension mixed with 20 μl of UV-treated control plasma and 160 μl of CFS) or 200 μl of WSSV mixed suspension (20 μl of WSSV stock suspension mixed with 20 μl of UV-inactivated WSSV stock suspension and 160 μl of CFS) as described above. Total RNA was isolated from hemocytes 36 h postinjection for detection of the WSSV VP28 gene and the crayfish ALF gene by quantitative RT-PCR. The crayfish ribosomal protein 40s gene was used as an internal control. Quantitative RT-PCR data were analyzed by comparative quantitation. The experiment has been repeated three times, the data represent means of triplicates, and the error bars indicate standard deviations. Columns 1 and 2 show VP28 and ALF transcript levels, respectively, after injection with 20 μl of WSSV stock suspension mixed with 20 μl of UV-treated control plasma and 160 μl of CFS. Columns 3 and 4 show VP28 and ALF transcript levels, respectively, after injection with 20 μl of WSSV stock suspension mixed with 20 μl of UV-inactivated WSSV stock suspension and 160 μl of CFS.
To assess further the role of ALF in protecting against WSSV, RNAi was employed. Injection of GFP dsRNA did not affect VP28 (Fig. 3, column 1) nor ALF transcription (Fig. 3, column 2). In contrast, ALF dsRNA injection resulted in a partial reduction of ALF transcription levels (Fig. 3, column 4), which was accompanied by an increase of VP28 mRNA levels (Fig. 3, column 3). These data suggest that ALF in some way either directly or indirectly affects the replication of WSSV and thus has an antiviral effect toward this virus. Injecting ALF dsRNA into animals with WSSV virus resulted in a faster disease progress than that of the animals that had received control dsRNA (data not shown). These results, however, were highly variable, probably due to individual differences among animals and different sensitivities to the multiple injections required. In addition, the need for large quantities of RNA for whole-animal experiments and complexity of assessing several tissues prompted us to instead use cell culture experiments to further explore the effects of ALF RNAi on the virus.
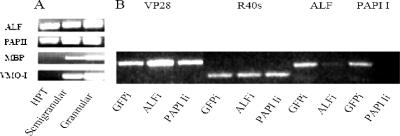
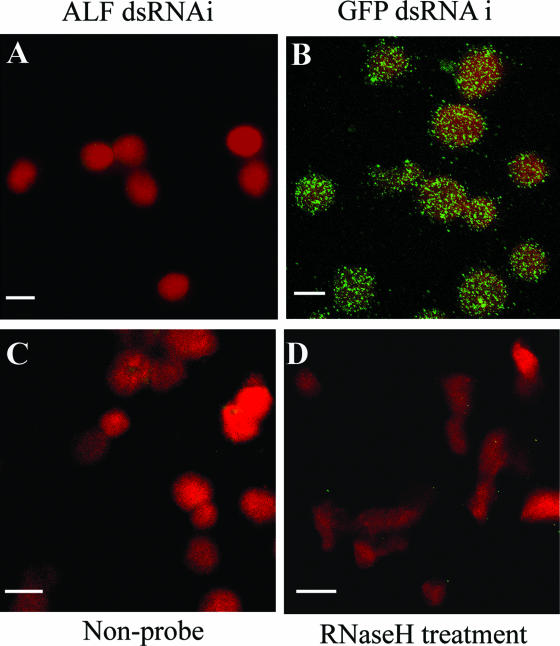
First, levels of four different transcripts earlier found to be up-regulated during WSSV infection were compared in the Hpt cell cultures and in the two major mature hemocyte types, granular and semigranular cells. ALF and the serine proteinase inhibitor PAPI I (13) were transcribed in Hpt cells as well as in mature hemocytes (Fig. 4A). Two other known immune factors (1, 15, 29), the mannose binding protein (MBP) and a major constituent of hemocyte granules, the vitelline membrane outer layer protein I (VMO-I) (27), were transcribed in mature hemocytes only (Fig. 4A). Thus, an attempt was made to knockdown by RNAi ALF and PAPI I in the cell culture to investigate if that would influence the outcome of a WSSV infection. As shown in Fig. 4B, introduction of ALF dsRNA and PAPI I dsRNA, respectively, resulted in an almost complete disappearance of the corresponding cognate transcripts, thus demonstrating that sequence-specific RNAi is possible in this system. The disappearance of PAPI I transcript had no effect on the virus, whereas knocking down ALF resulted in enhanced levels of viral VP28 transcript indicative of enhanced viral replication (Fig. 4). A successful RNAi experiment on ALF in the cell culture is also shown by another technique, in situ hybridization, in Fig. 5. The addition of the ALF dsRNA resulted in a consistent disappearance of the ALF transcript in all cells (Fig. 5A), whereas dsRNA for GFP did not affect ALF transcription (Fig. 5B). Both RNase-treated cells and cells without a probe were used as controls and were negative (Fig. 5C and D).
FIG. 4.
(A) ALF, PAPI I, MBP, and VMO-I transcripts in crayfish Hpt cell cultures and hemocytes. ALF, PAPI I, MBP, and VMO-I transcripts in crayfish Hpt cell cultures, semigranular cells, and granular cells were determined by RT-PCR. Total RNA was isolated from semigranular and granular cells which had been separated by Percoll gradient centrifugation (23). Hpt cells were cultured for 7 days, followed by total RNA preparation for RT-PCR as described in Materials and Methods. (B) WSSV replication in dsRNA-silenced crayfish Hpt cell cultures detected by RT-PCR. For ALF or PAPI I gene silencing, 3-day-old crayfish Hpt cell cultures were transfected with 1 μg of ALF dsRNA or PAPI I dsRNA/well, respectively, using histone H2A as a transfection reagent. One microgram of GFP dsRNA/well was used for control transfections. WSSV infection was done with inoculation of 5 μl of WSSV stock suspension/well into the Hpt cell cultures 12 h post-dsRNA transfection. The total RNA of Hpt cell cultures was prepared 36 h post-viral infection for RT-PCR of WSSV VP28, crayfish ribosomal protein 40s, ALF, or PAPI I.
FIG. 5.
RNAi of ALF in Hpt cell cultures detected by in situ hybridization. One microgram of ALF or GFP dsRNA/well was transfected into the Hpt cell cultures on coverslips using histone H2A as transfection reagent. In situ hybridization was performed 3 days post-dsRNA transfection using a digoxigenin-labeled ALF cDNA fragment as a probe. Hpt cells without probe or RNase H-treated Hpt cells were also used as negative controls. The fluorescent signal was detected by confocal microscopy. (A) ALF dsRNA-silenced cells; (B) GFP dsRNA-treated cells; (C) control cells without any probe; (D) RNase H treatment of control cells. Bars, 10 μm.
We have earlier shown that the crayfish Hpt cell culture can propagate the WSSV (12). The silencing effects of ALF and PAPI I on viral propagation were therefore tested. An assessment with quantitative PCR showed that silencing ALF expression with RNAi resulted in a 10-fold increase of VP28 levels compared to control dsRNA (Fig. 6). Thus, the silencing effects of ALF seem to be specific and not a result of a general stimulation of an antiviral response by any dsRNA.
FIG. 6.
RNAi of ALF and effect on WSSV replication in vitro. Crayfish Hpt cell cultures were transfected with 1 μg of ALF or GFP dsRNA/well using histone H2A which was followed by a WSSV infection via inoculation of 5 μl of WSSV stock suspension/well 12 h post-dsRNA transfection. Total RNA was isolated from the cell cultures 36 h post-WSSV infection for quantitative RT-PCR. The quantitative RT-PCR was performed for WSSV VP28 and crayfish ALF by using crayfish ribosomal protein 40s gene as an internal control, and the data were analyzed by comparative quantitation. The experiment has been carried out four times, the data represent means of four replicates, and the error bars indicate standard deviations. Columns 1 and 2 show VP28 and ALF transcript levels, respectively, after transfection with GFP dsRNA. Columns 3 and 4 show VP28 and ALF transcript levels, respectively, after transfection with ALF dsRNA.
DISCUSSION
By the use of suppression hybridization, a number of genes potentially involved in viral defense and/or genes whose transcription is stimulated by the viral presence were identified. Some of these genes code for proteins known to be involved in defense reactions, such as VMO-I, MBP, and proteinase inhibitors (1, 3, 13, 15, 29), although there is little information available about to which extent, if any, they are effective in an antiviral response. Our experiments have, so far, not indicated that these proteins, with the exception of ALF, have a specific role in reducing viral replication. The role for ALF in viral propagation is intriguing, as its removal by RNAi results in a significant enhancement of viral replication. The reverse experiment, to constitutively overexpress ALF in crayfish hemocytes, is not yet technically possible. However, the injection of noninfective UV-inactivated WSSV resulted in both higher ALF transcript levels and a slower progress in viral multiplication. Although the injection of inactive virus may have multiple effects, the results are in line with those of the RNAi experiments in suggesting a protective role for ALF against WSSV. It is worth emphasizing that, in our hands, injection of other dsRNA molecules except ALF, although effective in reducing their respective cognate transcript levels, has no apparent effect on viral levels. In Litopenaeus vannamei, using whole animals, a more general induction of antiviral immunity by unspecific dsRNA has been reported (20). It is possible that such an unspecific antiviral effect, if operating in the crayfish, is too weak to be discernible in the cell culture assays used here. Also, in L. vannamei, the antiviral protection provided by this general mechanism induced by any dsRNA is fairly limited, with respect to the amount of virus that can be dealt with, compared to the effects of targeted specific dsRNA molecules (19). The possibility that tissues other than hemocytes could be able to mount a general antiviral response induced by any dsRNA should, however, not be excluded.
The apparent ability of ALF to so strongly interfere with viral replication warrants further exploration. This is the first example of an identified endogenous factor interfering with WSSV propagation in any crustacean and thus an important step in elucidating the immune response against this devastating pathogen. So far, our attempts to determine whether the protein interacts with the virus extracellularly or intracellularly have failed to provide a conclusive result. The presence of a signal sequence for endoplasmic reticulum localization in the ALF open reading frame may though suggest that it acts outside the cell. Horseshoe crab ALF, when tested in vitro, binds lipid A, interferes with LPS-induced plasma coagulation, and reduces bacterial growth. The physiological function of this protein is not yet fully clear, although participation by ALF in regulation of the coagulation cascade is an obvious possibility. In shrimp, several ALFs are transcriptionally induced upon bacterial challenge, and at least one of them has been shown to reduce the growth of fungi and bacteria in vitro (26). To our knowledge, any effects on viruses by ALF have not been previously reported, and the contribution of ALF to crustacean immunity, when its antiviral properties are also considered, could thus be substantial.
Acknowledgments
This work has been financed by the Swedish Science Research Council and Formas (K.S.) and by the Carl Trygger Foundation (I.S.).
REFERENCES
- 1.Back, J. F., J. M. Bain, D. V. Vadehra, and R. W. Burley. 1982. Proteins of the outer layer of the vitelline membrane of hen's eggs. Biochim. Biophys. Acta 705:12-19. [DOI] [PubMed] [Google Scholar]
- 2.Bangrak, P., P. Graidist, W. Chotigeat, K. Supamattaya, and A. Phongdara. 2002. A syntenin-like protein with postsynaptic density protein (PDZ) domains produced by black tiger shrimp Penaeus monodon in response to white spot syndrome virus infection. Dis. Aquat. Org. 49:19-25. [DOI] [PubMed] [Google Scholar]
- 3.Cerenius, L., and K. Söderhäll. 2004. The prophenoloxidase-activating system in invertebrates. Immunol. Rev. 198:116-126. [DOI] [PubMed] [Google Scholar]
- 4.Dhar, A. K., A. Dettori, M. M. Roux, K. R. Klimpel, and B. Read. 2003. Identification of differentially expressed genes in shrimp (Penaeus stylirostris) infected with white spot syndrome virus by cDNA microarrays. Arch. Virol. 148:2381-2396. [DOI] [PubMed] [Google Scholar]
- 5.Dostert, C., E. Jouanguy, P. Irving, L. Troxler, D. Galiana-Arnoux, C. Hetru, J. A. Hoffmann, and J. L. Imler. 2005. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of drosophila. Nat. Immunol. 6:946-953. [DOI] [PubMed] [Google Scholar]
- 6.Galiana-Arnoux, D., C. Dostert, A. Schneemann, J. A. Hoffmann, and J. L. Imler. 2006. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat. Immunol. 7:590-597. [DOI] [PubMed] [Google Scholar]
- 7.Gross, P. S., T. C. Bartlett, C. L. Browdy, R. W. Chapman, and G. W. Warr. 2001. Immune gene discovery by expressed sequence tag analysis of hemocytes and hepatopancreas in the Pacific White Shrimp, Litopenaeus vannamei, and the Atlantic White Shrimp, L. setiferus. Dev. Comp. Immunol. 25:565-577. [DOI] [PubMed] [Google Scholar]
- 8.Hariton-Gazal, E., J. Rosenbluh, A. Graessmann, C. Gilon, and A. Loyter. 2003. Direct translocation of histone molecules across cell membranes. J. Cell Sci. 116:4577-4586. [DOI] [PubMed] [Google Scholar]
- 9.He, N., Q. Qin, and X. Xu. 2005. Differential profile of genes expressed in hemocytes of White Spot Syndrome Virus-resistant shrimp (Penaeus japonicus) by combining suppression subtractive hybridization and differential hybridization. Antivir. Res. 66:39-45. [DOI] [PubMed] [Google Scholar]
- 10.Hoess, A., S. Watson, G. R. Siber, and R. Liddington. 1993. Crystal structure of an endotoxin-neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5 A resolution. EMBO J. 12:3351-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiravanichpaisal, P., E. Bangyeekhun, K. Söderhäll, and I. Söderhäll. 2001. Experimental infection of white spot syndrome virus in freshwater crayfish Pacifastacus leniusculus. Dis. Aquat. Org. 47:151-157. [DOI] [PubMed] [Google Scholar]
- 12.Jiravanichpaisal, P., K. Söderhäll, and I. Söderhäll. 2006. Characterization of white spot syndrome virus replication in in vitro-cultured haematopoietic stem cells of freshwater crayfish, Pacifastacus leniusculus. J. Gen. Virol. 87:847-854. [DOI] [PubMed] [Google Scholar]
- 13.Johansson, M. W., P. Keyser, and K. Söderhäll. 1994. Purification and cDNA cloning of a four-domain Kazal proteinase inhibitor from crayfish blood cells. Eur. J. Biochem. 223:389-394. [DOI] [PubMed] [Google Scholar]
- 14.Jory, D. E., and H. M. Dixon. 1999. Shrimp white spot virus in the Western Hemisphere. Aquac. Mag. 25:83-91. [Google Scholar]
- 15.Klein, N. J. 2005. Mannose-binding lectin: do we need it? Mol. Immunol. 42:919-924. [DOI] [PubMed] [Google Scholar]
- 16.Krishna, R. R., R. K. Rao, and P. H. Badu. 1997. White spot diease. World Aquac. 12:14-19. [Google Scholar]
- 17.Luo, T., X. Zhang, Z. Shao, and X. Xu. 2003. PmAV, a novel gene involved in virus resistance of shrimp Penaeus monodon. FEBS Lett. 551:53-57. [DOI] [PubMed] [Google Scholar]
- 18.Pan, D., N. He, Z. Yang, H. Liu, and X. Xu. 2005. Differential gene expression profile in hepatopancreas of WSSV-resistant shrimp (Penaeus japonicus) by suppression subtractive hybridization. Dev. Comp. Immunol. 29:103-112. [DOI] [PubMed] [Google Scholar]
- 19.Robalino, J., T. Bartlett, E. Shepard, S. Prior, G. Jaramillo, E. Scura, R. W. Chapman, P. S. Gross, C. L. Browdy, and G. W. Warr. 2005. Double-stranded RNA induces sequence-specific antiviral silencing in addition to nonspecific immunity in a marine shrimp: convergence of RNA interference and innate immunity in the invertebrate antiviral response? J. Virol. 79:13561-13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robalino, J., C. L. Browdy, S. Prior, A. Metz, P. Parnell, P. Gross, and G. Warr. 2004. Induction of antiviral immunity by double-stranded RNA in a marine invertebrate. J. Virol. 78:10442-10448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojtinnakorn, J., I. Hirono, T. Itami, Y. Takahashi, and T. Aoki. 2002. Gene expression in haemocytes of kuruma prawn, Penaeus japonicus, in response to infection with WSSV by EST approach. Fish Shellfish Immunol. 13:69-83. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberry, B. (ed.). 2004. World shrimp farming. Shrimp News International, San Diego, Calif.
- 23.Smith, V. J., and K. Söderhäll. 1983. Induction of degranulation and lysis of haemocytes in the freshwater crayfish, Astacus astacus by components of the prophenoloxidase activating system in vitro. Cell Tissue Res. 233:295-303. [DOI] [PubMed] [Google Scholar]
- 24.Söderhäll, I., E. Bangyeekhun, S. Mayo, and K. Söderhäll. 2003. Hemocyte production and maturation in an invertebrate animal; proliferation and gene expression in hematopoietic stem cells of Pacifastacus leniusculus. Dev. Comp. Immunol. 27:661-672. [DOI] [PubMed] [Google Scholar]
- 25.Söderhäll, I., Y. A. Kim, P. Jiravanichpaisal, S. Y. Lee, and K. Söderhäll. 2005. An ancient role for a prokineticin domain in invertebrate hematopoiesis. J. Immunol. 174:6153-6160. [DOI] [PubMed] [Google Scholar]
- 26.Somboonwiwat, K., M. Marcos, A. Tassanakajon, S. Klinbunga, A. Aumelas, B. Romestand, Y. Gueguen, H. Boze, G. Moulin, and E. Bachere. 2005. Recombinant expression and anti-microbial activity of anti-lipopolysaccharide factor (ALF) from the black tiger shrimp Penaeus monodon. Dev. Comp. Immunol. 29:841-851. [DOI] [PubMed] [Google Scholar]
- 27.Sricharoen, S., J. J. Kim, S. Tunkijjanukij, and I. Söderhäll. 2005. Exocytosis and proteomic analysis of the vesicle content of granular hemocytes from a crayfish. Dev. Comp. Immunol. 29:1017-1031. [DOI] [PubMed] [Google Scholar]
- 28.Tonganunt, M., A. Phongdara, W. Chotigeat, and K. Fujise. 2005. Identification and characterization of syntenin binding protein in the black tiger shrimp Penaeus monodon. J. Biotechnol. 120:135-145. [DOI] [PubMed] [Google Scholar]
- 29.Uyeda, A., C. Inuzuka, Y. Doi, S. Kido, and M. Kikuchi. 1994. Cloning and sequencing of hen magnum cDNAs encoding vitelline membrane outer layer protein I (VMO-I). Gene 144:311-312. [DOI] [PubMed] [Google Scholar]
- 30.Westenberg, M., B. Heinhuis, D. Zuidema, and J. M. Vlak. 2005. siRNA injection induces sequence-independent protection in Penaeus monodon against white spot syndrome virus. Virus Res. 114:133-139. [DOI] [PubMed] [Google Scholar]
- 31.Yang, F., W. Wang, R. Z. Chen, and X. Xu. 1997. A simple and efficient method for purification of prawn baculovirus DNA. J. Virol. Methods 67:1-4. [DOI] [PubMed] [Google Scholar]
- 32.Zambon, R. A., M. Nandakumar, V. N. Vakharia, and L. P. Wu. 2005. The Toll pathway is important for an antiviral response in Drosophila. Proc. Natl. Acad. Sci. USA 102:7257-7262. [DOI] [PMC free article] [PubMed] [Google Scholar]