Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV or HHV-8) is the etiological agent of Kaposi's sarcoma, a highly vascularized, endothelial-derived tumor. A direct role for KSHV-mediated induction of angiogenesis has been proposed based upon the nature of the neoplasia and various KSHV gene overexpression and infection model systems. We have found that KSHV infection of endothelial cells induces mRNA of hypoxia-induced factor 1α (HIF1α) and HIF2α, two homologous alpha subunits of the heterodimeric transcription factor HIF. HIF is a master regulator of both developmental and pathological angiogenesis, composed of an oxygen-sensitive alpha subunit and a constitutively expressed beta subunit. HIF is classically activated posttranscriptionally with hypoxia, leading to increased protein stability of HIF1α and/or HIF2α. However, we demonstrate that both alpha subunits are up-regulated at the transcript level by KSHV infection. The transcriptional activation of HIF leads to a functional increase in HIF activity under normoxic conditions, as demonstrated by both luciferase reporter assay and the increased expression of vascular endothelial growth factor receptor 1 (VEGFR1), an HIF-responsive gene. KSHV infection synergizes with hypoxia mimics and induces higher expression levels of HIF1α and HIF2α protein, and HIF1α is increased in a significant proportion of the latently infected endothelial cells. Src family kinases are required for the activation of HIF and the downstream gene VEGFR1 by KSHV. We also show that KS lesions, in vivo, express elevated levels of HIF1α and HIF2α proteins. Thus, KSHV stimulates the HIF pathway via transcriptional up-regulation of both HIF alphas, and this activation may play a role in KS formation, localization, and progression.
Kaposi's sarcoma (KS) is a highly vascularized tumor predominately made up of cells of endothelial origin. Classical KS is an indolent cancer mostly affecting the dermal lower extremities of elderly men of Mediterranean and Eastern European descent and is histologically similar to the more aggressive African endemic KS, and the very aggressive AIDS-associated KS. All forms exhibit striking degrees of pathological neoangiogenesis (16). Even early patch or plaque stages of the disease contain extremely high levels of vascularization, which is indicated by the red or purple appearance of the lesion. The etiological agent of KS, Kaposi's sarcoma-associated herpesvirus (KSHV), is a gammaherpesvirus, further subclassified as a rhadinovirus (9). KSHV is also associated with two lymphoproliferative disorders, primary effusion lymphoma (PEL) and multicentric Castleman's disease, indicating the oncogenic potential of the virus (7, 52). In KS tumors, KSHV is found in the major cell type, an endothelial-derived spindle cell, where it is predominantly latent with a small number, 1 to 5%, of cells supporting lytic infection (53). A primary role for latent KSHV infection of spindle cells has been proposed as a driving force in sarcomagenesis, as the virus is required but likely not sufficient for disease.
Our lab and others have shown that KSHV infection of dermal microvasculature-derived blood endothelial cells induces differentiation to lymphatic endothelial cells (6, 25, 61). Following the infection of blood endothelial cells, the virus induces the expression of many markers specific to lymphatic endothelium, including vascular endothelial growth factor receptor 3 (VEGFR3). VEGFR3 is a receptor for VEGF-C and -D (35). Normally following lymphatic differentiation, the expression of VEGFR1, a receptor for VEGF-A, VEGF-B, and placental growth factor (15, 41, 42), is decreased (43), which makes lymphatic endothelial cells responsive to a different set of vascular growth factors. Interestingly, our previous analysis also demonstrated that VEGFR1 mRNA is increased for 24 to 96 h following KSHV infection of blood endothelial cells (6), which is counter to the lymphatic differentiation pathway. Other studies utilizing microarray indicated that VEGFR1 mRNA is induced 4 h postinfection upon viral binding and entry of KSHV in primary human dermal microvascular endothelial (HMVEC-d) cells (40). If functional VEGFR1 protein is expressed, it could make the KSHV-infected endothelial cells responsive to mitogens that activate both lymphangiogenesis and vascular angiogenesis.
VEGFR1 expression is often controlled by the hypoxia-induced factor (HIF) family of transcription factors (20, 55). HIF is a heterodimeric transcription factor composed of an oxygen-sensitive alpha protein, the prototypical HIF1α (59, 60) or the vascular-enriched HIF2α (57), and a constitutively expressed beta subunit, HIF1β, also known as the aryl hydrocarbon receptor nuclear translocator (24). HIF is a master regulator of both developmental and pathological angiogenesis associated with the vascularization of the embryo and tumors. During normoxia, HIF1α and -2α are modified by oxygen-dependent prolyl hydroxylases (17, 26, 46). This facilitates recognition by the E3 ligase von Hippel-Lindau (VHL) factor, which rapidly leads to polyubiquitination and proteosomal degradation (36). However, under hypoxic conditions, the HIF alpha subunits are stabilized, translocated to the nucleus, heterodimerized, and activated to induce genes containing hypoxia response elements (HREs), which include VEGF and its receptor VEGFR1 as well as genes involved in erythropoeisis, iron uptake, glucose transport, and glycolysis (49). This allows the hypoxic cells to survive under low oxygen and initiates neoangiogenesis by recruiting new blood vessel formation from existing vasculature. HIF is also inducible under normoxic conditions by a variety of growth factors, cytokines, and vasomodulatory hormones (14). Previous work by Poole et al. indicated that HIF1α was increased by microarray analysis with long-term infection of HMVEC-d cells (44), and our microarray results indicated that both HIF1α and -2α expression levels may be increased following infection (6). In addition, two hypoxia-inducible genes, VEGFR1 and the carcinoembryonic antigen-related cell adhesion molecule 1 (10), were also increased following infection, while a decrease in the hypoxia-repressible secretion of monocyte chemoattractant protein 1 (5) was noted, indicating a potential normoxic induction of the HIF pathway (6).
HIF1α has previously been proposed to play a role in KSHV lytic infection. A KSHV lytic gene, ORF74, which encodes a viral G-protein-coupled receptor (vGPCR), can lead to the activation of HIF1α when ectopically expressed, though this is not at the transcriptional level (51). Interestingly, HIF1α mRNA escapes lytic KSHV-induced host shutoff in endothelial cells and this was shown to be separable from the activity of the vGPCR (21). Also, work in a PEL cell line demonstrated an increase in lytic reactivation mediated by hypoxia (13) and the same group identified functional HREs within the KSHV genome (23). However, our array data showing an increase in the abundance of both HIF alpha transcripts were based upon a predominantly latent infection system.
Here we demonstrate that latent KSHV infection of primary HMVEC-d cells and human telomerase-immortalized microvascular endothelial (TIME) cells leads to increased expression of HIF1α and HIF2α. HIF is functionally activated in infected cells, and the hypoxia-responsive VEGFR1 is induced upon infection under normoxic conditions. Hypoxic mimic stimulation of infected endothelial cells leads to a synergistic increase in both HIF activity and expression of VEGFR1. This increased protein expression of HIF1α, HIF2α, and VEGFR1 upon KSHV infection is blocked by the Src family kinase inhibitor, SU6656, implicating this pathway in the viral induction of normoxic HIF. Finally, we also show that these effects are a result of KSHV latent gene expression in endothelial cells and demonstrate increased HIF1α and -2α expression in KS lesions in vivo.
MATERIALS AND METHODS
Cell lines and reagents.
Both primary dermal human microvascular endothelial cells, HMVEC-d (Clonetics) and hTERT-TIME cells (58), were cultured in endothelial basal medium 2, with endothelial growth medium 2 supplements added (Cambrex), under standard culture conditions. The following chemical stocks and concentrations, from the listed companies, were stored at −20°C: deferoxamine mesylate (DFO), 50 mM stock in water; SU6656 “in solution,” 10 mM stock in dimethyl sulfoxide (Calbiochem); MG132, 2 mM stock in methanol (Sigma).
KSHV infection of endothelial cells.
KSHV infections of TIME and HMVEC-d cells were performed as described previously (32), and UV irradiation of KSHV was carried out on concentrated viral stocks with five auto-cross-links of 12,000 μJoules each with a UV Stratalinker 1800 (Stratagene), and virus stocks were then added similarly to the infectious inoculum. All cells were infected in complete medium with serum and overlaid with complete medium after 2 h. Cells infected with the UV-irradiated virus were negative for latency-associated nuclear antigen (LANA) by immunofluorescence assay at 48 h postinfection. KSHV lytic infection was induced using a KSHV ORF50 adenovirus vector, Ad50 (1) (a kind gift from D. Ganem), as described previously (22).
Northern blotting and TaqMan assays.
Total RNA was extracted from trypsinized cells using the RNA-Bee reagent (Tel-Test, Inc.), and mRNA was purified using the Oligotex mRNA midi extraction kit (QIAGEN). Two hundred fifty nanograms of mRNA was used for Northern blot analysis. One hundred nanograms of PCR products was used to generate specific probes to HIF1A, HIF2A, and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) by using the Rediprime random prime labeling system (Amersham Biosciences). Real-time reverse transcription-PCR (RT-PCR) was carried out on cDNA made from 100 ng of purified mRNA using SuperScript first-strand synthesis for RT-PCR (Invitrogen) utilizing commercially available probes to HIF1A, HIF2A, and β2-microglobulin (B2M) (Assays-on-Demand; ABI). The severalfold change upon infection was normalized by the delta threshold cycle method to B2M from UV-irradiated KSHV-treated cells. Error bars were generated using the standard error of the mean (n = 3).
Western blot analysis.
NE-PER nuclear and cytopasmic extraction kit (Pierce) and whole-cell radioimmunoprecipitation assay extracts were supplemented with Complete EDTA-free (Roche), 1 mM sodium orthovanadate, and 1 mM sodium fluoride. Fifty micrograms of nuclear extracts or 10 μg of whole-cell lysates were resolved on a 4 to 15% gradient gel (Bio-Rad), transferred to Immobilon (Millipore), blocked with 5% milk in phosphate-buffered saline with 0.05% Tween 20, and probed with the following specific antibodies in the same solution: anti-HIF1α, anti-HIF1β, and anti-transcription factor IIβ (TFIIβ) (BD Biosciences); anti-HIF2α (U.S. Biologicals); anti-VEGFR1 (Abcam); full-length specific anti-VEGFR1 (Santa Cruz); and anti-β-actin (Sigma). Horseradish peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.) of the appropriate species were used, and the blots were visualized using the ECL Plus reagent (Amersham) and exposed to blue X-ray film (Phenix Research Products).
Immunofluorescence assays.
Infection rates were monitored using antibodies against the latent KSHV protein LANA (a kind gift from A. Polson and D. Ganem) and the lytic protein ORF59 (Advanced Biotechnologies Incorporated) as described previously (32). Immunofluorescence for HIF1α and ORF50 was performed under the same conditions using a 1:50 dilution of the mouse monoclonal antibody anti-HIF1α (BD Biosciences), a 1:1,000 dilution of anti-ORF50 antibody (a kind gift from D. Lukac and D. Ganem), and the appropriate secondary antibodies (Molecular Probes). Fluorescence was monitored with a Nikon Eclipse E400 microscope with a MicroPublisher 3.3 RTV camera using QCapture Suite (Qimaging). All relative images were generated under identical exposure times and camera settings.
HRE-luciferase assays.
TIME cells were transfected in a six-well dish using the TransIt Jurkat reagent (Mirus) with 2 μg of a firefly luciferase expression vector containing either a wild-type or a mutant triple repeat of the erythropoietin 3′ HRE in the enhancer region (31) as well as 1 ng of control Renilla luciferase expression vector pRL-SV40 (Promega) for transfection efficiency normalization. The cells were infected with KSHV after 4 h of transfection, harvested 24 h postinfection, and then subjected to the dual-luciferase assay (Promega) and read on a luminometer following the manufacturer's recommendations. Transfection efficiencies were normalized for Renilla activity, and specific HIF-dependent activity was calculated by subtracting HRE mutant activity from the wild-type HRE-containing construct to remove constitutive activity. All experiments were measured in duplicate and repeated in triplicate. Initial transfection efficiency of TIME cells was observed with a green fluorescent protein construct, and 5 to 10% of the cell population was green fluorescent protein positive with 2 μg of construct transfected.
Immunohistochemistry.
Slides mounted with sections of paraffin-embedded Kaposi's sarcoma were obtained from the AIDS Cancer Specimen Resource (ACSR). Slides were deparaffinized, rehydrated, and washed with phosphate-buffered saline. Following antigen retrieval in 0.1 mM sodium citrate (pH 6.0) and the quenching of endogenous peroxidase activity with 3% H2O2, samples were blocked with 5% normal horse serum prior to incubation with primary antibodies overnight at 4°C. Signals were processed according to the supplied protocol (Elite ABC kit). Slides were counterstained with hematoxylin QS, dehydrated, and mounted using Permount (Fischer Scientific). HIF1α and HIF2α antibodies were purchased from Novus Biologicals (catalog no. NB100-131 and NB100-132). An antibody against KSHV LANA was obtained from Advanced Biotechnologies Incorporated. The Elite ABC kits, DAB (3,3′diaminobenzidine), and hematoxylin QS were purchased from Vector Laboratories.
RESULTS
KSHV induces HIF alpha transcripts upon infection of endothelial cells.
Our previous microarray analysis of latent KSHV-infected endothelial cells indicated an increase in both HIF1α and HIF2α mRNA, sustained from 24 to 96 h postinfection. To confirm and expand these results, Northern blot analyses were performed on mock- and KSHV-infected TIME and primary HMVEC-d cells. KSHV infection of either the immortalized or the primary endothelial cells led to an increase of both HIF1α and HIF2α transcripts at 48 h postinfection compared to that for the GAPDH loading control (Fig. 1A). This increase was then quantified using commercially available TaqMan real-time RT-PCR probes, which revealed about a fivefold and two- to threefold inductions upon infection, respectively (Fig. 1B). The real-time analysis was normalized to cells treated with UV-irradiated KSHV, which allows viral binding and entry but blocks viral gene expression. As such, the increase in the abundance of HIF alpha transcripts upon KSHV infection was a result of viral gene expression. In addition, we see no significant change when we use real-time RT-PCR to compare HIF1α mRNA in cells infected with UV-irradiated virus to HIF1α mRNA in cells that were mock infected (the change in cycle threshold was −0.8 [n = 7]; all numbers are normalized to β2-microglobulin). This indicates that, in our experiments, binding and entry alone do not significantly change the levels of HIF1α mRNA at 48 h postinfection. Furthermore, using Northern blot analysis of polyadenylated RNA at 48 h postinfection, we see no significant difference between HIF1α mRNA levels in cells that were infected with UV-irradiated virus and that for cells that were mock infected (data not shown).
FIG. 1.
KSHV infection increases HIF alpha mRNA in human dermal microvascular endothelial cells. (A) Northern blot analysis of 250 ng of mRNA from mock- and KSHV-infected TIME (lanes 1 and 2) and primary HMVEC-d cells (lanes 3 and 4) probed for HIF1α, HIF2α, and the load control, GAPDH, at 48 h postinfection. −, absence of; +, presence of. (B) Quantitative real-time RT-PCR analysis of the severalfold change in the expression of HIF1α and HIF2α with KSHV infection, normalized to B2M, at 48 h postinfection compared to that with UV-irradiated KSHV treatment of TIME cells (n = 3). Error bars indicate standard deviations.
HIF alpha proteins are increased in KSHV-infected TIME cells.
Since HIF alpha proteins are usually rapidly degraded under normoxic conditions, rendering them undetectable, mock- and KSHV-infected TIME cells were treated with the hypoxia mimic, DFO (150 μM), to unmask any potential transient increase in protein abundance. Western blot analysis was performed on nuclear extracts from normoxic and DFO-treated cells. As shown in Fig. 2, HIF1α and HIF2α are detectable in the presence of the hypoxia mimic and there was a large increase of both proteins in KSHV-infected lysates at 48 h postinfection, when the hypoxia mimic was present for the last 8 h. The upper band, above 120 kDa in the HIF2α immunoblot, is a cross-reactive band, which is unchanged under all conditions. The HIF1β subunit is constitutively expressed, and the nuclear abundance was increased in both mock- and KSHV-infected nuclear extracts exposed to DFO to similar degrees, as expected, since KSHV did not increase its transcript abundance. This is indicative of a functional HIF heterodimer, which leads to a larger nuclear pool of both the alpha and the beta subunits (11). The nuclear load control, TFIIβ, was unchanged under all conditions, demonstrating the specificity of the stabilization of HIF alpha subunits by KSHV. The increase in stabilized HIF alpha proteins is consistent with KSHV increasing the transient expression of the proteins via increased transcript abundance.
FIG. 2.
KSHV-infected TIME cells stabilize more HIF alpha subunits when exposed to the hypoxia mimic. Western blot analysis of mock (lanes 1 and 3)- and KSHV-infected (lanes 2 and 4) TIME cell nuclear extracts at 48 h postinfection in the absence (−) (lanes 1 and 2) or presence (+) (lanes 3 and 4) of an HIF-stabilizing hypoxia mimic (150 μM DFO) for the last 8 h. The extracts were probed with antibodies against HIF1α, HIF2α, HIF1β, and the load control, TFIIβ.
HIF alpha proteins are degraded by oxygen-dependent ubiquitination and proteosomal processing, and proteosome inhibitors will lead to an increase in normoxic protein abundance with sufficient time (46). A time course of treatment with the proteosome inhibitor MG132 was carried out, and the resulting nuclear extracts were subjected to Western blot analysis to alternatively verify the aforementioned hypoxia mimic results. As shown in Fig. 3, with KSHV, a large increase of both nuclear HIF1α and -2α protein was seen with 2 h of treatment with 20 μM MG132, indicating that, upon stabilization, KSHV-infected cells express more HIF alpha subunits than do uninfected cells. There is also an increase in HIF1β with MG132 treatment, and this increase is more apparent with KSHV infection, once again indicating potentially functional nuclear HIF heterodimer.
FIG. 3.
KSHV-infected TIME cells stabilize more HIF alpha subunits in the presence (+) of a proteosome inhibitor. Western blot analysis of mock (lanes 1 to 3)- and KSHV-infected nuclear extracts (lanes 4 to 6) at 24 h postinfection. The extracts were treated with a proteosome inhibitor (20 μM MG132) for either 1 h (lanes 2 and 5) or 2 h (lane 3 and 6) to stabilize HIF and probed with antibodies against HIF1α, HIF2α, HIF1β, and the load control, TFIIβ. −, absence of KSHV infection; +, presence of KSHV infection.
The increase in HIF1α is due to latent gene expression.
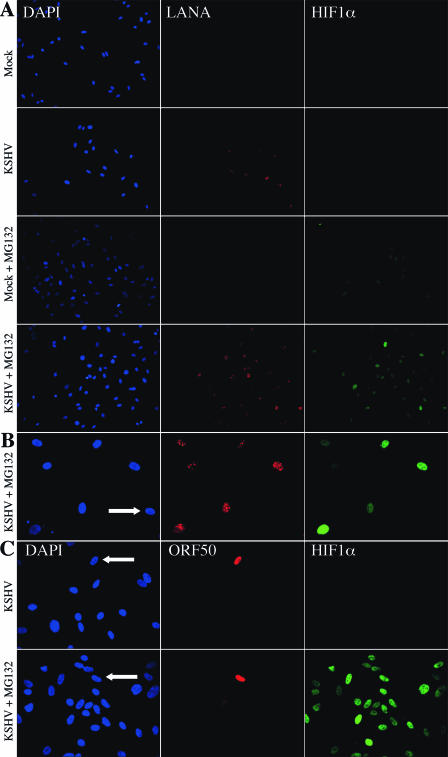
The infection of endothelial cells with KSHV leads to the establishment of latency in most cells; however, there is a very low percentage of cells expressing markers of lytic replication. As such, it is hard to gauge the relative contribution of lytic or latent gene expression to changes induced, on average, in a mixed cell culture population. To examine HIF1α expression on a single-cell basis, HIF1α protein was monitored by immunofluorescence during a time course of MG132 treatment of mock- and KSHV-infected TIME cells. At numerous time points between 48 to 96 h postinfection, the effect of KSHV plus MG132 was monitored. HIF1α was stabilized to very high levels in a greater proportion of the infected cells compared to that in mock-infected cells at all time points tested from 0.5 h to 3 h of MG132 treatment. In the control vehicle-treated cells, neither mock- nor KSHV-infected cells expressed detectable HIF1α under normoxic conditions, as expected (Fig. 4A). As shown in Fig. 4A, HIF1α protein stained much brighter in LANA-positive (KSHV latently infected) cells treated with MG132 for 1.5 h compared to that in uninfected cells. Also of note, many MG132-treated mock-infected cells were completely negative for HIF1α, while some are weakly positive, but most, if not all, of the LANA-expressing cells, about 80% of the total cell population, were positive to strongly positive for HIF1α. Similar results were seen with 2 h of treatment with another proteosome inhibitor, lactacystin (data not shown). This staining pattern is further demonstrated at a higher magnification from a separate experiment (Fig. 4B) with an indicated uninfected cell, which is LANA and HIF1α negative with 3 h of MG132, while the KSHV-infected LANA-positive cells are a mixed population of positive and strong positive HIF1α expressers. The role of lytic gene expression in the stabilization of HIF1α was investigated by costaining infected cells for HIF1α and ORF50, the KSHV lytic switch protein. As shown in Fig. 4C, there is no stabilization of HIF1α in the absence of MG132, even in an ORF50-positive cell, and the stabilization of HIF1α with 2 h of MG132 does not occur solely in the ORF50-positive cells. Importantly, MG132 (or lactacystin) treatment does not affect the percentage of cells expressing LANA (Fig. 4A), ORF59 (see below), or ORF50 (Fig. 4C and see below). Overall, uninfected TIME cells stabilized with MG132 (or lactacystin) were either negative or very weakly positive for HIF1α staining, while KSHV-infected cells were all positive to strongly positive. Thus, with MG132 stabilization, KSHV latently infected endothelial cells exhibit a net increase in the heterogeneous staining of HIF1α.
FIG. 4.
Latent KSHV-infected TIME cells stabilize more HIF1α protein than do uninfected cells. Results show the immunofluorescent detection of nucleic acid (DAPI, blue), KSHV latent nuclear antigen (LANA, red), and HIF1α (green) in mock- and KSHV-infected TIME cells 48 h postinfection in the absence or presence of the proteosome inhibitor, 20 μM MG132, for 1.5 h (magnification, ×200) (A) and 3 h (magnification, ×400) (B). The white arrow indicates an uninfected cell within the KSHV-infected population. (C) Immunofluorescent detection of nucleic acid (DAPI, blue), KSHV lytic switch protein, ORF50 or Rta (red), and HIF1α (green) in KSHV-infected TIME cells 48 h postinfection in the absence or presence of 20 μM MG132 for 2 h. The white arrows indicate the ORF50-positive cells (magnification, ×400). Panels A, B, and C are from separate experiments with similar infection rates (∼80% latent, <0.6% lytic).
To determine whether either the hypoxia mimic or proteosome inhibitor altered KSHV lytic replication, we used immunofluorescence to examine the effect of DFO and MG132 on the percentage of cells staining positive for a viral latent (LANA) or two lytic markers (ORF50 and ORF59). There is no significant increase in the percentage of cells expressing LANA, ORF50, or ORF59, with 24 h of 150 μM DFO treatment at 48 h postinfection (control cells were 87% LANA positive, 0.2% ORF50 positive, and <0.2% ORF59 positive. DFO-treated cells were 88% LANA positive, 0.2% ORF50 positive, and 0.6% ORF59 positive), indicating that the hypoxia mimic fails to induce lytic reactivation in microvascular endothelial cells. Also, neither 2 h of MG132 treatment (both were 0.2% ORF50 positive) nor 2 h of treatment followed by 22 h without the proteosome inhibitor led to lytic induction (also 0.2% ORF50 positive). This indicates that our treatment conditions do not significantly alter the percentage of lytic or latently infected cells, supporting the hypothesis that the up-regulation of HIF is occurring predominantly in latently infected cells.
KSHV-induced HIF is active under normoxia and synergizes with a hypoxia mimic to increase activity.
HIF activity can be monitored by luciferase reporter constructs containing HRE consensus sites. Here we employed a reporter of HIF activity, utilizing a dual-luciferase assay for HRE-specific activation of a firefly luciferase gene containing a triple repeat of the erythropoietin gene HRE in the 3′ enhancer region, driven by the simian virus 40 promoter. This reporter can be activated by either HIF1α or HIF2α containing functional heterodimers. Because KSHV infection activates many promoters, the activation of the HIF-responsive construct was compared to that of an identical construct, except that the HRE sites were mutated such that they are insufficient for HIF binding (31). All experiments were normalized to the Renilla luciferase cotransfection control, driven by the simian virus 40 promoter without a 3′ enhancer. The exploitation of these constructs allowed for the quantification of the activity resulting from the transient increase in HIF alpha subunit proteins induced by KSHV latent infection. As shown in Fig. 5A, the acute hypoxia mimic (8 h with DFO) induces a twofold increase in HRE-luciferase activity, KSHV infection induces a threefold increase in this activity at 24 h postinfection, and DFO, for the last 8 h of infection, synergizes to increase HRE activity about sixfold over the control, mock-infected cells. This indicates that the KSHV-induced transient HIF is more active than DFO alone and that there is a synergistic increase when infected cells are treated with DFO.
FIG. 5.
KSHV infection and hypoxia mimic synergize to increase transcription from an HRE reporter construct and expression of the HRE-driven VEGFR1. (A) HIF-specific DNA-binding luciferase reporter assay in TIME cells (as described in Materials and Methods) shown as relative activity for the control, the mimic (TIME cells treated with 150 μM DFO for 8 h), KSHV at 24 h postinfection, and KSHV with DFO (KSHV+DFO). Error bars indicate standard deviations. (B) Western blot analysis of whole-cell lysates from mock (lanes 1 to 3)- and KSHV-infected TIME cells (lanes 4 to 6) at 48 h postinfection in the absence (lanes 1 and 4) or presence of 150 μM DFO for the last 4 h (lanes 2 and 5) or 8 h (lanes 3 and 6). Lysates were probed for full-length VEGFR1 (upper blot), pan-VEGFR1 antibody, and the load control β-actin. (C) Western blot analysis of HMVEC-d whole-cell lysates from mock-infected (lanes 1), mimic (KSHV-infected TIME cells treated with 150 μM DFO for 8 h) (lane 2), KSHV-infected (lane 3) and KSHV+DFO lysates (lane 4) from 24 h postinfection probed for VEGFR1 and the load control β-actin. −, absence of KSHV infection; +, presence of KSHV infection.
Expression of VEGFR1 is increased by KSHV activation of HIF.
Our previous array data and real-time RT-PCR confirmation showed that FLT1, an HRE-inducible gene encoding VEGFR1, is induced from 24 to 96 h postinfection during KSHV latent infection as a result of viral gene expression (6). To investigate the expression levels of this protein, we compared mock- and KSHV-infected cells treated with DFO. Consistent with the activity data from Fig. 5A, whole-cell lysates from 48 h of KSHV infection expressed significantly more of both the soluble and full-length isoforms of VEGFR1 and the increase in full-length VEGFR1 was synergistic in the presence of DFO in a time-dependent fashion from 4 to 8 h (Fig. 5B). This result was further verified by KSHV infection of primary HMVEC-d. As shown in Fig. 5C, 8 h of 150 μM DFO increases soluble VEGFR1, KSHV increases both soluble and full-length VEGFR1 and the increase with KSHV and DFO is synergistic at 24 h postinfection. This indicates that KSHV infection can induce both measurable HIF transcriptional activity and HRE-responsive genes, even in the absence of detectable HIF alpha proteins, suggesting a functional role for a transient increase in HIF alpha protein.
To verify that VEGFR1 is indeed downstream of HIF activation, we utilized an Src family kinase inhibitor, SU6656 (4), which blocks one of the few pathways known to induce transcriptional up-regulation of both HIF1α and HIF2α (29, 56). Treatment with SU6656 for the last 20 of 48 h of infection was sufficient to mitigate the viral-induced up-regulation of both HIF1α and HIF2α in a dose-dependent fashion (Fig. 6A), with a concomitant decrease in mRNA (P. A. Carroll and M. Lagunoff, unpublished data). A total of 20 μM SU6656 also decreased nuclear HIF1β, indicating the loss of nuclear HIF activity (Fig. 6A). This treatment also blocks the downstream gene, VEGFR1, in a dose-dependent fashion, with a maximal decrease at 20 μM, when nuclear HIF1β is decreased to mock levels (Fig. 6B). Similar results were seen at 24 h postinfection (data not shown). This demonstrates that HIF1α, HIF2α, and VEGFR1 are all coregulated and indicates that the HRE activity resulting from KSHV infection has functional ramifications on downstream gene activation, even under normoxic conditions.
FIG. 6.
KSHV-induced HIF and VEGFR1 can be blocked in a dose-dependent manner by the Src family kinase inhibitor SU6656. (A) Western blot analysis of nuclear extracts from mock (lanes 1 and 3)- or KSHV-infected TIME cells (lanes 2 and 4 to 7) at 48 h postinfection and treated with vehicle (lanes 1 and 2) or 20 μM MG132 (lanes 3 to 7) for the last 2 h and the effect of pretreatment with 5, 10 and 20 μM SU6656 (lanes 5, 6, and 7, respectively) for the last 20 h on MG132 stabilization of HIF by KSHV. The extracts were probed for HIF1α, HIF2α, HIF1β, and the load control TFIIβ. (B) Whole-cell lysates from mock (lane 1)- or KSHV-infected TIME cells (lanes 2 to 5) at 48 h postinfection and the effect of treatment with 5, 10, and 20 μM SU6656 (lanes 3, 4, and 5, respectively) for the last 20 h on KSHV-induced VEGFR1 expression. The lysates were probed for β-actin as a load control. −, absence of indicated virus or drug; +, presence of indicated virus or drug.
VEGFR1 is not induced by KSHV lytic gene expression.
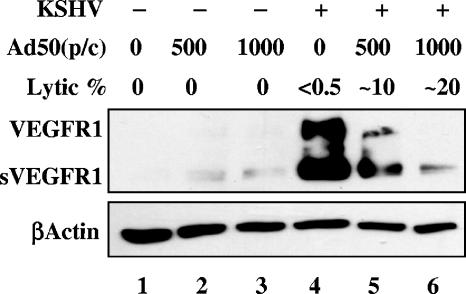
HIF1α mRNA can survive host shutoff during lytic KSHV infection (21). The effects of lytic reactivation on TIME cell VEGFR1 expression was monitored by Western blot analysis of cells induced to go lytic. KSHV-infected TIME cells were induced by superinfection with an adenoviral vector encoding KSHV ORF50, the lytic switch protein, which is sufficient to initiate the lytic cycle (1). The induction was carried out under conditions sufficient to induce host shutoff (22) and was monitored by immunofluorescence for the lytic marker ORF59. Interestingly, by 24 h postinduction, the increase of VEGFR1 protein by KSHV infection of TIME cells was mitigated by lytic gene expression in a dose-dependent manner (<0.5% to ∼20% lytic), whereas the expression of β-actin was unchanged, as monitored by whole-cell lysate Western blot analysis (Fig. 7). This indicates that FLT1 mRNA does not escape host shutoff or that HIF1α, though stabilized, is not active on the FLT1 promoter during KSHV lytic infection. This is consistent with earlier microarray results which did not demonstrate widespread lytic induction of hypoxia-responsive genes (21). This also supports the role of KSHV latency in functional HIF activation.
FIG. 7.
Lytic induction decreases VEGR1 expression with KSHV infection in a dose-dependent manner. Western blot analysis of whole-cell lysates from mock (lanes 1 to 3)- or KSHV-infected cells (lanes 4 to 6) superinfected with either 500 (lanes 2 and 5) or 1,000 particles per cell (p/c) (lanes 3 and 6) of an adenovirus expressing ORF50 (Ad50) to induce lytic reactivation at 24 h postinfection. The lysates were probed for VEGFR1 and the load control β-actin. The percentage of cells expressing ORF59, a marker of lytic replication, is also listed for each condition. −, absence of KSHV infection; +, presence of KSHV infection.
KS lesions express elevated levels of HIF1α and HIF2α proteins in vivo.
Since KS lesions are potentially subjected to proinflammatory cytokines and growth factors that can induce normoxic HIF expression (54) and the architectural changes to the vasculature could result in perturbations to the microcirculation leading to true hypoxia, we examined the expression of HIF proteins in vivo by immunohistochemistry. We stained slides of paraffin-embedded samples (obtained from the ACSR) containing nonconsecutive serial sections from the same blocks of AIDS-associated dermal KS tumor tissue for HIF1α, HIF2α, and the viral antigen LANA to indicate KSHV-infected tissues within the lesion. Figure 8 shows results in which we aligned sections from the same regions (though not from serial sections) on these slides. As expected, KSHV is present in the spindle cells throughout the lesion, as monitored by the brown LANA nuclear staining (Fig. 8). HIF1α and HIF2α were highly expressed in a subpopulation of the spindle-like cells (Fig. 8) that appeared to also be positive for KSHV LANA. Though dual-label immunohistochemistry was not employed, we believe these are overlapping populations and numerous studies have demonstrated the expression of LANA is in most if not all of the spindle cells of a KS lesion. Also, as shown in the ×400 magnification (right column of Fig. 8), the uninfected cells adjacent to the vascular space at the bottom left of each panel are also negative for nuclear HIF1α and HIF2α expression, whereas the spindle cells internal to the open space are a mixed population of KSHV-positive and uninfected cells, a subset of which express nuclear, stable HIF1α and HIF2α. The HIF1α antibody exhibits a higher background than does the HIF2α antibody, but there is detectable nuclear HIF for both. Also of note, a number of the HIF2α-positive cells appear to potentially be infiltrating dendritic cells. Similar HIF2α and HIF1α staining was seen with tissue from two different KS tumors from different donors (data not shown). Thus, KSHV-infected cells within a KS lesion express elevated levels of HIF1α and HIF2α and this does not appear to be a result of general hypoxia since the adjacent tissue is negative for LANA and the two HIF proteins.
FIG. 8.
KS lesions express elevated levels of HIF1α and HIF2α. Slides of AIDS-associated dermal KS tissue were obtained from the ACRS, hematoxylin QS counterstained, and stained brown by immunohistochemistry for LANA, HIF1β, and HIF2β as indicated. Shown for each are photos at ×100 (left) and ×400 (right) magnifications from the dashed box of the photo at ×100 magnification.
DISCUSSION
The data presented here demonstrate a novel viral regulatory mechanism for the induction of functionally active HIF transcription factors. The hypoxic response is generally regulated by the protein stability of HIF alpha subunits, mediated by irreversible, oxygen-dependent prolyl-hydroxylases (17). These enzymes modify key residues within the HIF1α or HIF2α oxygen-dependent degradation domain (26), allowing recognition by the E3 ligase VHL (36). VHL then induces polyubiquitination and proteosomal degradation of HIF1α or HIF2α (27, 28). A number of viral gene products, including the KSHV vGPCR protein, have been demonstrated to stabilize HIF1α protein via posttranslational modification (51). In contrast, we found that, while KSHV latent infection functionally activates HIF, it does not significantly stabilize the protein. Rather, KSHV up-regulates the transcription of HIF1α and HIF2α, thereby increasing the transient levels of HIF alpha proteins. Importantly, we found that these transient levels are enough to induce HIF transcriptional activity and the hypoxia-responsive gene VEGFR1. This increase in activity requires the activity of at least one Src family kinase (SFK) member since SU6656, a SFK inhibitor (4), had a dose-dependent inhibitory effect on the increase in expression of both HIF alpha subunits, the increased nuclear localization of the beta subunit, and the increased expression of VEGFR1 in infected cells. Interestingly, oncogenically active viral Src has been shown to induce HIF1α by increasing transcript abundance, which leads to normoxic activity (29). The expression of viral Src synergizes with hypoxia to lead to even more HIF-1 stabilization, activity, and expression of the downstream gene VEGF (29). We found similar synergy of KSHV infection and DFO, a hypoxia mimic, in both TIME cells and primary HMVEC-d. Src family kinases were also implicated in the normoxic induction of HIF2α via increased transcription by interleukin 1β (IL1β) (56) as well as a hypoxia-induced positive feedback loop involving increased HIF2α mRNA (48). SU6656 is a potent and specific inhibitor of SFKs and has previously been used in endothelial cells at doses of 2 to 10 μM, which are similar to doses used here (12, 39, 63). Thus, it is likely that KSHV activates HIF-1 and/or -2 through activated SFK(s), possibly Src itself.
Our data show that the activation of HIF is due to latent gene expression. However, HIF1α mRNA is increased during lytic replication as well and is one of the few host mRNAs that escape viral-induced host cell shutoff (21). Importantly, latent gene products are also expressed during lytic replication. The previous study compared KSHV lytic-infected cells to uninfected cells, not to predominantly latently infected TIME cells, and therefore did not directly address events occurring during KSHV latent infection. There is no concomitant increase in lytic replication in endothelial cells upon HIF activation, even in the presence of the hypoxia mimic DFO. In addition, we examined HIF on a single-cell basis and found that a much higher percentage of cells expressed elevated levels of HIF1α than the lytic markers ORF50 or ORF59, about 0.5% of the total population. Thus, the phenomenon described here is due to latent infection.
Acute or chronic hypoxia as well as two mimetic agents has been shown to increase lytic replication in PEL cell lines (13). Of note, we used a significantly higher dose of DFO and still failed to detect any induction of the lytic markers ORF50 or ORF59 by immunofluorescence in our endothelial system, though we did see an induction of HIF activity. However, different stimuli have been demonstrated to have an opposite effect on KSHV lytic gene expression in different cell types. Gamma interferon induces lytic reactivation in PEL cells (3, 8, 37), whereas treatment of infected microvascular endothelial cells with gamma interferon led to a decrease in lytic gene expression, specifically of the switch protein ORF50 (38). Therefore, cell-autonomous effects seem to play roles in the responses of KSHV-infected cells to different stimuli regarding the induction of lytic replication. This sets the interesting precedent that hypoxia may have a similar effect. A circulating KSHV-positive B cell could travel to hypoxic tissue, reactivate in response to the lower oxygen, and produce infectious virus to seed the surrounding endothelium, which then responds to hypoxia by stabilizing the increased levels of HIF and inducing growth and angiogenesis. Interestingly, KS tissue has been shown to express high levels of cytokines which have been shown to stabilize normoxic HIF, such as IL1β (54), and these same cytokines do not induce reactivation in either PEL cell lines (3, 8, 37) or KSHV-infected endothelial cells (38). This could further lead to stabilized normoxic HIF in the KS spindle cells, altering its activity, but not inducing reactivation, thus, providing a perfect microenvironment for latent KSHV-induced HIF.
HIF-1 and -2 are critical for the induction of angiogenesis in endothelial cells (34, 55). We show here that there are high levels of HIF1α and HIF2α expressed in KS tumors, which are endothelial derived. Many tumors experience fluctuations in oxygen levels due to tumor vasculature-associated changes in the microcirculation (45). Dermal KS resides in the skin, a naturally hypoxic tissue (2). In addition, the unique cytokine milieu of KS could provide paracrine factors that would stabilize normoxic HIF, especially if transcriptional up-regulation potentiated the infected cells to respond more robustly to these other factors. This would be similar to the correlation of increased VEGF secretion with higher steady-state abundance of HIF1α mRNA upon only hypoxic stimulation of a number of similar cancer cell lines (30).
KS tumors are highly vascular and contain extremely high levels of neoangiogenesis. Angiogenesis involves cell division, motility, and tubule formation. The functional consequences of KSHV-induced HIF activity and VEGFR1 up-regulation may act on a number of levels. The activation of the HIF pathway could be involved in the expansion of KS tumor cells. Since the predominant tumor cell is endothelial in nature, the activation of the HIF pathway could result in infected cells being able to respond to mitogens in the tumor milieu. KS lesions contain high levels of VEGF and VEGF-C (47, 50). VEGFR1 responds to VEGF, while VEGFR3 responds to VEGF-C. Here we have shown that KSHV increases the expression of VEGFR1 through HIF, and our previous work demonstrated an increase in VEGFR3 expression at the same time postinfection (6). Thus, the activation of HIF and subsequent VEGFR1 up-regulation could ultimately lead to a growth advantage in vivo for latently infected spindle cells. The induction of pathways involved in angiogenesis in the infected cells could also lead to the activation of the surrounding vasculature by the secretion of paracrine factors, thus contributing to the large amounts of angiogenesis seen in the KS tumor. Interestingly, KSHV latent infection and hypoxia mimics act synergistically on HIF activity and VEGFR1 up-regulation. IL1β synergizes with hypoxia to increase VEGF secretion from cells in a similar fashion (18). Synergy is expected if the virus induces higher transient levels of the protein and hypoxia itself stabilizes the protein. The question remains whether the transient levels of HIF play a major role in KS pathogenesis and whether the synergy between KSHV and hypoxia is also important. Classic KS, which appears in the apparent absence of immunosuppression, occurs predominantly on the lower extremities of elderly men, a common site of poor circulation in the elderly. There is also an association of KS with diabetes, which is known to cause circulatory problems (19, 33). In addition, KS occurs on the skin, another site where hypoxic conditions have been measured (2), and KS has been described at the site of a surgical wound, yet another hypoxic tissue (62). Thus, it is possible that KSHV activates the transcription of HIF1α and/or -2α, inducing some activity, but full activation occurs either in a hypoxic microenvironment or in one which provides another required HIF-stabilizing/activating factor. This would indicate that KSHV latently infected endothelial cells are primed for the induction of angiogenesis and, ultimately, sarcomagenesis.
Acknowledgments
We thank the AIDS Cancer Specimen Resource for providing the KS tumor samples, David Lukac and Don Ganem for the LANA and ORF50 antibodies, Don Ganem for the adenovirus expressing ORF50, and Max Gassmann for providing both the wild-type and mutant HRE-luciferase constructs.
M.L. is supported by a research scholar grant from the American Cancer Society, a grant (R01 CA097934-01A1) from the National Cancer Institute, the PEW scholars program in biological sciences sponsored by the PEW Charitable Trust, and a pilot grant from the Puget Sound Partners in Global Health. R.S.Y. is supported by a National Cancer Institute grant (RO1 CA102662).
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Bechtel, J. T., Y. Liang, J. Hvidding, and D. Ganem. 2003. Host range of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 77:6474-6481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedogni, B., S. M. Welford, D. S. Cassarino, B. J. Nickoloff, A. J. Giaccia, and M. B. Powell. 2005. The hypoxic microenvironment of the skin contributes to Akt-mediated melanocyte transformation. Cancer Cells 8:443-454. [DOI] [PubMed] [Google Scholar]
- 3.Blackbourn, D. J., S. Fujimura, T. Kutzkey, and J. A. Levy. 2000. Induction of human herpesvirus-8 gene expression by recombinant interferon gamma. AIDS 14:98-99. [DOI] [PubMed] [Google Scholar]
- 4.Blake, R. A., M. A. Broome, X. Liu, J. Wu, M. Gishizky, L. Sun, and S. A. Courtneidge. 2000. SU6656, a selective Src family kinase inhibitor, used to probe growth factor signaling. Mol. Cell. Biol. 20:9018-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosco, M. C., M. Puppo, S. Pastorino, Z. Mi, G. Melillo, S. Massazza, A. Rapisarda, and L. Varesio. 2004. Hypoxia selectively inhibits monocyte chemoattractant protein-1 production by macrophages. J. Immunol. 172:1681-1690. [DOI] [PubMed] [Google Scholar]
- 6.Carroll, P. A., E. Brazeau, and M. Lagunoff. 2004. Kaposi's sarcoma-associated herpesvirus infection of blood endothelial cells induces lymphatic differentiation. Virology 328:7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cesarman, E., Y. Chang, P. S. Moore, J. W. Said, and D. M. Knowles. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS- related body-cavity-based lymphomas. N. Engl. J. Med. 332:1186-1191. [DOI] [PubMed] [Google Scholar]
- 8.Chang, J., R. Renne, D. Dittmer, and D. Ganem. 2000. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology 266:17-25. [DOI] [PubMed] [Google Scholar]
- 9.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W. J., H. W. Chen, S. L. Yu, C. H. Huang, T. D. Wang, J. J. Chen, C. T. Chien, H. Y. Chen, P. C. Yang, and Y. T. Lee. 2005. Gene expression profiles in hypoxic preconditioning using cDNA microarray analysis: altered expression of an angiogenic factor, carcinoembryonic antigen-related cell adhesion molecule 1. Shock 24:124-131. [DOI] [PubMed] [Google Scholar]
- 11.Chilov, D., G. Camenisch, I. Kvietikova, U. Ziegler, M. Gassmann, and R. H. Wenger. 1999. Induction and nuclear translocation of hypoxia-inducible factor-1 (HIF-1): heterodimerization with ARNT is not necessary for nuclear accumulation of HIF-1α. J. Cell Sci. 112:1203-1212. [DOI] [PubMed] [Google Scholar]
- 12.Cuneo, K. C., L. Geng, J. Tan, J. Brousal, E. T. Shinohara, K. Osusky, A. Fu, Y. Shyr, H. Wu, and D. E. Hallahan. 2006. SRC family kinase inhibitor SU6656 enhances antiangiogenic effect of irradiation. Int. J. Radiat. Oncol. Biol. Phys. 64:1197-1203. [DOI] [PubMed] [Google Scholar]
- 13.Davis, D. A., A. S. Rinderknecht, J. P. Zoeteweij, Y. Aoki, E. L. Read-Connole, G. Tosato, A. Blauvelt, and R. Yarchoan. 2001. Hypoxia induces lytic replication of Kaposi sarcoma-associated herpesvirus. Blood 97:3244-3250. [DOI] [PubMed] [Google Scholar]
- 14.Dery, M. A., M. D. Michaud, and D. E. Richard. 2005. Hypoxia-inducible factor 1: regulation by hypoxic and non-hypoxic activators. Int. J. Biochem. Cell. Biol. 37:535-540. [DOI] [PubMed] [Google Scholar]
- 15.de Vries, C., J. A. Escobedo, H. Ueno, K. Houck, N. Ferrara, and L. T. Williams. 1992. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255:989-991. [DOI] [PubMed] [Google Scholar]
- 16.Ensoli, B., C. Sgadari, G. Barillari, M. C. Sirianni, M. Sturzl, and P. Monini. 2001. Biology of Kaposi's sarcoma. Eur. J. Cancer 37:1251-1269. [DOI] [PubMed] [Google Scholar]
- 17.Epstein, A. C., J. M. Gleadle, L. A. McNeill, K. S. Hewitson, J. O'Rourke, D. R. Mole, M. Mukherji, E. Metzen, M. I. Wilson, A. Dhanda, Y. M. Tian, N. Masson, D. L. Hamilton, P. Jaakkola, R. Barstead, J. Hodgkin, P. H. Maxwell, C. W. Pugh, C. J. Schofield, and P. J. Ratcliffe. 2001. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107:43-54. [DOI] [PubMed] [Google Scholar]
- 18.Frede, S., P. Freitag, T. Otto, C. Heilmaier, and J. Fandrey. 2005. The proinflammatory cytokine interleukin 1β and hypoxia cooperatively induce the expression of adrenomedullin in ovarian carcinoma cells through hypoxia inducible factor 1 activation. Cancer Res. 65:4690-4697. [DOI] [PubMed] [Google Scholar]
- 19.Friedman-Birnbaum, R., S. Weltfriend, and I. Katz. 1990. Kaposi's sarcoma: retrospective study of 67 cases with the classical form. Dermatologica 180:13-17. [DOI] [PubMed] [Google Scholar]
- 20.Gerber, H. P., F. Condorelli, J. Park, and N. Ferrara. 1997. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J. Biol. Chem. 272:23659-23667. [DOI] [PubMed] [Google Scholar]
- 21.Glaunsinger, B., and D. Ganem. 2004. Highly selective escape from KSHV-mediated host mRNA shutoff and its implications for viral pathogenesis. J. Exp. Med. 200:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glaunsinger, B., and D. Ganem. 2004. Lytic KSHV infection inhibits host gene expression by accelerating global mRNA turnover. Mol. Cell 13:713-723. [DOI] [PubMed] [Google Scholar]
- 23.Haque, M., D. A. Davis, V. Wang, I. Widmer, and R. Yarchoan. 2003. Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) contains hypoxia response elements: relevance to lytic induction by hypoxia. J. Virol. 77:6761-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffman, E. C., H. Reyes, F. F. Chu, F. Sander, L. H. Conley, B. A. Brooks, and O. Hankinson. 1991. Cloning of a factor required for activity of the Ah (dioxin) receptor. Science 252:954-958. [DOI] [PubMed] [Google Scholar]
- 25.Hong, Y. K., K. Foreman, J. W. Shin, S. Hirakawa, C. L. Curry, D. R. Sage, T. Libermann, B. J. Dezube, J. D. Fingeroth, and M. Detmar. 2004. Lymphatic reprogramming of blood vascular endothelium by Kaposi sarcoma-associated herpesvirus. Nat. Genet. 36:683-685. [DOI] [PubMed] [Google Scholar]
- 26.Huang, L. E., J. Gu, M. Schau, and H. F. Bunn. 1998. Regulation of hypoxia-inducible factor 1α is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc. Natl. Acad. Sci. USA 95:7987-7992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ivan, M., K. Kondo, H. Yang, W. Kim, J. Valiando, M. Ohh, A. Salic, J. M. Asara, W. S. Lane, and W. G. Kaelin, Jr. 2001. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science 292:464-468. [DOI] [PubMed] [Google Scholar]
- 28.Jaakkola, P., D. R. Mole, Y. M. Tian, M. I. Wilson, J. Gielbert, S. J. Gaskell, A. Kriegsheim, H. F. Hebestreit, M. Mukherji, C. J. Schofield, P. H. Maxwell, C. W. Pugh, and P. J. Ratcliffe. 2001. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292:468-472. [DOI] [PubMed] [Google Scholar]
- 29.Jiang, B. H., F. Agani, A. Passaniti, and G. L. Semenza. 1997. V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res. 57:5328-5335. [PubMed] [Google Scholar]
- 30.Koshikawa, N., A. Iyozumi, M. Gassmann, and K. Takenaga. 2003. Constitutive upregulation of hypoxia-inducible factor-1α mRNA occurring in highly metastatic lung carcinoma cells leads to vascular endothelial growth factor overexpression upon hypoxic exposure. Oncogene 22:6717-6724. [DOI] [PubMed] [Google Scholar]
- 31.Kvietikova, I., R. H. Wenger, H. H. Marti, and M. Gassmann. 1995. The transcription factors ATF-1 and CREB-1 bind constitutively to the hypoxia-inducible factor-1 (HIF-1) DNA recognition site. Nucleic Acids Res. 23:4542-4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lagunoff, M., J. Bechtel, E. Venetsanakos, A. M. Roy, N. Abbey, B. Herndier, M. McMahon, and D. Ganem. 2002. De novo infection and serial transmission of Kaposi's sarcoma-associated herpesvirus in cultured endothelial cells. J. Virol. 76:2440-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laor, Y., and R. A. Schwartz. 1979. Epidemiologic aspects of American Kaposi's sarcoma. J. Surg. Oncol. 12:299-303. [DOI] [PubMed] [Google Scholar]
- 34.Licht, A. H., F. Muller-Holtkamp, I. Flamme, and G. Breier. 2006. Inhibition of hypoxia-inducible factor activity in endothelial cells disrupts embryonic cardiovascular development. Blood 107:584-590. [DOI] [PubMed] [Google Scholar]
- 35.Makinen, T., T. Veikkola, S. Mustjoki, T. Karpanen, B. Catimel, E. C. Nice, L. Wise, A. Mercer, H. Kowalski, D. Kerjaschki, S. A. Stacker, M. G. Achen, and K. Alitalo. 2001. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 20:4762-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maxwell, P. H., M. S. Wiesener, G. W. Chang, S. C. Clifford, E. C. Vaux, M. E. Cockman, C. C. Wykoff, C. W. Pugh, E. R. Maher, and P. J. Ratcliffe. 1999. The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399:271-275. [DOI] [PubMed] [Google Scholar]
- 37.Mercader, M., B. Taddeo, J. R. Panella, B. Chandran, B. J. Nickoloff, and K. E. Foreman. 2000. Induction of HHV-8 lytic cycle replication by inflammatory cytokines produced by HIV-1-infected T cells. Am. J. Pathol. 156:1961-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milligan, S., M. Robinson, E. O'Donnell, and D. J. Blackbourn. 2004. Inflammatory cytokines inhibit Kaposi's sarcoma-associated herpesvirus lytic gene transcription in in vitro-infected endothelial cells. J. Virol. 78:2591-2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakagawa, Y., N. Yamada, H. Shimizu, M. Shiota, M. Tamura, S. Kim-Mitsuyama, and H. Miyazaki. 2004. Tyrosine phosphatase epsilonM stimulates migration and survival of porcine aortic endothelial cells by activating c-Src. Biochem. Biophys. Res. Commun. 325:314-319. [DOI] [PubMed] [Google Scholar]
- 40.Naranatt, P. P., H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 64:72-84. [DOI] [PubMed] [Google Scholar]
- 41.Olofsson, B., E. Korpelainen, M. S. Pepper, S. J. Mandriota, K. Aase, V. Kumar, Y. Gunji, M. M. Jeltsch, M. Shibuya, K. Alitalo, and U. Eriksson. 1998. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl. Acad. Sci. USA 95:11709-11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park, J. E., H. H. Chen, J. Winer, K. A. Houck, and N. Ferrara. 1994. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 269:25646-25654. [PubMed] [Google Scholar]
- 43.Podgrabinska, S., P. Braun, P. Velasco, B. Kloos, M. S. Pepper, and M. Skobe. 2002. Molecular characterization of lymphatic endothelial cells. Proc. Natl. Acad. Sci. USA 99:16069-16074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poole, L. J., Y. Yu, P. S. Kim, Q. Z. Zheng, J. Pevsner, and G. S. Hayward. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3395-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raghunand, N., R. A. Gatenby, and R. J. Gillies. 2003. Microenvironmental and cellular consequences of altered blood flow in tumours. Br. J. Radiol. 76:S11-S22. [DOI] [PubMed] [Google Scholar]
- 46.Salceda, S., and J. Caro. 1997. Hypoxia-inducible factor 1α (HIF-1α) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J. Biol. Chem. 272:22642-22647. [DOI] [PubMed] [Google Scholar]
- 47.Samaniego, F., P. D. Markham, R. Gendelman, Y. Watanabe, V. Kao, K. Kowalski, J. A. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi's sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 152:1433-1443. [PMC free article] [PubMed] [Google Scholar]
- 48.Sato, M., T. Tanaka, T. Maeno, Y. Sando, T. Suga, Y. Maeno, H. Sato, R. Nagai, and M. Kurabayashi. 2002. Inducible expression of endothelial PAS domain protein-1 by hypoxia in human lung adenocarcinoma A549 cells. Role of Src family kinases-dependent pathway. Am. J. Respir. Cell Mol. Biol. 26:127-134. [DOI] [PubMed] [Google Scholar]
- 49.Semenza, G. L. 1999. Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1. Annu. Rev. Cell Dev. Biol. 15:551-578. [DOI] [PubMed] [Google Scholar]
- 50.Skobe, M., L. F. Brown, K. Tognazzi, R. K. Ganju, B. J. Dezube, K. Alitalo, and M. Detmar. 1999. Vascular endothelial growth factor-C (VEGF-C) and its receptors KDR and flt-4 are expressed in AIDS-associated Kaposi's sarcoma. J. Investig. Dermatol. 113:1047-1053. [DOI] [PubMed] [Google Scholar]
- 51.Sodhi, A., S. Montaner, V. Patel, M. Zohar, C. Bais, E. A. Mesri, and J. S. Gutkind. 2000. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1α. Cancer Res. 60:4873-4880. [PubMed] [Google Scholar]
- 52.Soulier, J., L. Grollet, E. Oksenhendler, P. Cacoub, D. Cazals-Hatem, P. Babinet, M. F. d'Agay, J. P. Clauvel, M. Raphael, L. Degos, and et al. 1995. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood 86:1276-1280. [PubMed] [Google Scholar]
- 53.Staskus, K. A., W. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Haase. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sturzl, M., H. Brandstetter, C. Zietz, B. Eisenburg, G. Raivich, D. P. Gearing, N. H. Brockmeyer, and P. H. Hofschneider. 1995. Identification of interleukin-1 and platelet-derived growth factor-B as major mitogens for the spindle cells of Kaposi's sarcoma: a combined in vitro and in vivo analysis. Oncogene 10:2007-2016. [PubMed] [Google Scholar]
- 55.Takeda, N., K. Maemura, Y. Imai, T. Harada, D. Kawanami, T. Nojiri, I. Manabe, and R. Nagai. 2004. Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ. Res. 95:146-153. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka, T., H. Akiyama, H. Kanai, M. Sato, S. Takeda, K. Sekiguchi, T. Yokoyama, and M. Kurabayashi. 2002. Endothelial PAS domain protein 1 (EPAS1) induces adrenomedullin gene expression in cardiac myocytes: role of EPAS1 in an inflammatory response in cardiac myocytes. J. Mol. Cell. Cardiol. 34:739-748. [DOI] [PubMed] [Google Scholar]
- 57.Tian, H., S. L. McKnight, and D. W. Russell. 1997. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 11:72-82. [DOI] [PubMed] [Google Scholar]
- 58.Venetsanakos, E., A. Mirza, C. Fanton, S. R. Romanov, T. Tlsty, and M. McMahon. 2002. Induction of tubulogenesis in telomerase-immortalized human microvascular endothelial cells by glioblastoma cells. Exp. Cell Res. 273:21-33. [DOI] [PubMed] [Google Scholar]
- 59.Wang, G. L., B. H. Jiang, E. A. Rue, and G. L. Semenza. 1995. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc. Natl. Acad. Sci. USA 92:5510-5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang, G. L., and G. L. Semenza. 1995. Purification and characterization of hypoxia-inducible factor 1. J. Biol. Chem. 270:1230-1237. [DOI] [PubMed] [Google Scholar]
- 61.Wang, H. W., M. W. Trotter, D. Lagos, D. Bourboulia, S. Henderson, T. Makinen, S. Elliman, A. M. Flanagan, K. Alitalo, and C. Boshoff. 2004. Kaposi sarcoma herpesvirus-induced cellular reprogramming contributes to the lymphatic endothelial gene expression in Kaposi sarcoma. Nat. Genet. 36:687-693. [DOI] [PubMed] [Google Scholar]
- 62.Webster-Cyriaque, J. 2002. Development of Kaposi's sarcoma in a surgical wound. N. Engl. J. Med. 346:1207-1210. [DOI] [PubMed] [Google Scholar]
- 63.Yang, L., J. R. Kowalski, X. Zhan, S. M. Thomas, and F. W. Luscinskas. 2006. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ. Res. 98:394-402. [DOI] [PubMed] [Google Scholar]