Abstract
Immunoglobulin A (IgA) monoclonal antibodies (MAbs) directed at the conserved inner core protein VP6 of rotavirus, such as the IgA7D9 MAb, provide protective immunity in adult and suckling mice when delivered systemically. While these antibodies do not have traditional in vitro neutralizing activity, they could mediate their antiviral activity either by interfering with the viral replication cycle along the IgA secretory pathway or by acting at mucosal surfaces as secretory IgA and excluding virus from target enterocytes. We sought to determine the critical step at which antirotaviral activity was initiated by the IgA7D9 MAb. The IgA7D9 MAb appeared to directly interact with purified triple-layer viral particles, as shown by immunoprecipitation and immunoblotting. However, protection was not conferred by passively feeding mice with the secretory IgA7D9 MAb. This indicates that the secretory IgA7D9 MAb does not confer protection by supplying immune exclusion activity in vivo. We next evaluated the capacity of polymeric IgA7D9 MAb to neutralize rotavirus intracellularly during transcytosis. We found that when polymeric IgA7D9 MAb was applied to the basolateral pole of polarized Caco-2 intestinal cells, it significantly reduced viral replication and prevented the loss of barrier function induced by apical exposure of the cell monolayer to rotavirus, supporting the conclusion that the antibody carries out its antiviral activity intracellularly. These findings identify a mechanism whereby the well-conserved immunodominant VP6 protein can function as a target for heterotypic antibodies and protective immunity.
Rotavirus (RV) is the main cause of severe diarrhea in young infants worldwide and is responsible for 611,000 deaths per year (36). Two vaccines based on attenuated live viruses have recently completed successful phase III clinical trials and showed efficacy in preventing severe RV diarrhea caused by multiple serotypes (45, 50). However, the relevant immune effectors required for achieving protection against diverse circulating strains are still a matter of considerable debate. While CD8+ T cells facilitate the timely clearance of rotavirus infection, B cells with the appropriate pattern of mucosal homing receptors and antibody production have been demonstrated as crucial for protection from reinfection (17, 24).
Neutralizing antibodies against RV that block viral replication in cell culture are felt to play a major role in defense. A variety of passive antibody transfer studies in animals (28), as well as immunization studies using selected rotavirus reassortants in humans (7), have demonstrated a clear role for VP4 and VP7 in protective immunity. Neutralizing antibodies are directed against the outer shell VP4 and VP7 proteins, whose variable antigenic specificities define multiple serotypes, assigned as P and G serotypes, respectively (41). Worldwide, the G1-to-G4 serotypes associated with P4 and P8 and the more recently described G9/P6 or P8 serotypes are globally important rotavirus G/P combinations in humans (47). This diversity suggests that VP4- and VP7-encoding segments from many strains might have to be included in vaccines to provide broad immunity to reinfection with multiple serotypes. However, a body of evidence from animal studies indicates that either humoral or cell-mediated immunity directed at the intermediate capsid VP6 protein can also mediate protection, at least in some animal models (3, 5, 15, 32, 48). Of note, one of the two recently introduced human rotavirus vaccine only contains a single VP4 and VP7 serotype specificity but is clearly able to induce heterotypic protective immunity (45). Interestingly VP6, which represents 51% of the virion by mass, is antigenically conserved among most circulating group A RV strains and could, therefore, provide heterotypic protection (40).
In mucosae, polymeric immunoglobulin A (pIgA), comprising four heavy and light chains and the associated J chain, binds to the polymeric Ig receptor (pIgR) at the basolateral pole of epithelial cells. Following vectorial transport to the apical surface, it is released in the lumen attached to a proteolytic product of the pIgR called secretory component (SC), giving rise to secretory IgA (sIgA) (8). It is generally accepted that sIgA prevents microbial infections by forming immune complexes responsible for “immune exclusion” (i.e., the prevention of pathogen interaction with the target mucosal cell). The possible mechanisms involved in immune exclusion include competition for adhesion sites on target cells, blockade of motility of bacterial pathogens, and trapping in the mucus layer for which sIgA-immune complexes display strong affinity (43). In a previous study, we showed that the polymeric Ig secretory pathway plays a key role in the protection induced by intranasal immunization with virus-like particles (VLP) made exclusively of VP2 and VP6 proteins (VLP2/6) (48). Two plausible mechanisms could explain these findings: (i) the anti-VP6 pIgA could interfere with the viral life cycle during its intracellular transcytosis along the pIgR vesicular pathway, or (ii) the anti-VP6 sIgA could react with triple-shelled particles in the gut lumen through holes in the capsid or via areas of partial decapsidation (49) and thus exclude the antibody-particle complexes in the intestinal mucus layer and prevent infection.
Several anti-VP6 IgA monoclonal antibodies (MAbs), including the IgA7D9 MAb, when used in hybridomas in backpack experiments, were found protective in mice and could clear chronic infection in SCID mice (3). However, the same IgA MAb lacking SC was not protective when fed orally and was not neutralizing in classical in vitro assays using nonpolarized cells (3, 15). At the molecular level, anti-VP6 IgA7D9 MAb blocked viral transcription in vitro by introducing a conformational change in the VP6 trimer (15). Furthermore, IgA7D9 MAb competed with VP7 in binding to VP6, thus potentially perturbing VP7 assembly onto VP6 (20). Although suggesting intracellular neutralization, these in vivo and in vitro experiments provided only indirect information as to the possible mechanism of IgA7D9 MAb-mediated protection in the physiological context.
In order to directly determine at which biological step anti-VP6 IgA MAb interferes with rotavirus production, we tested the antiviral function of purified pIgA7D9 MAb and SIgA7D9 MAb in two complementary experimental settings: (i) SIgA7D9 MAb, assembled by association of purified SC and pIgA7D9 MAb, was delivered orally to mice, and its capacity to inhibit virus replication in the intestine was examined; (ii) the intracellular neutralizing potential of pIgA7D9 MAb was evaluated during transport from the basolateral compartment of a polarized epithelial cell monolayer mimicking the intestinal barrier.
MATERIALS AND METHODS
Preparation of pIgA7D9 and SIgA7D9.
Ascites fluid (30 ml) was obtained from BALB/c mice inoculated intraperitoneally (i.p.) with 2 × 106 IgA7D9 hybridoma cells (3). Hybridoma IgA2A10 (anti-VP4 rhesus monkey rotavirus [RRV] MAb) (19) and hybridoma IgAC5 (anti-Shigella flexneri lipopolysaccharide [LPS] MAb) (39) were grown in Celline-350 cartridges (Vitaris, Baar, Switzerland) in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM glutamine, 2 mM sodium pyruvate, 10 mM HEPES (pH 7.0), 0.1 mM folic acid, 100 U/ml penicillin, and 100 μg/ml streptomycin. Preparations were loaded onto two successive Sephacryl S-300 columns (1 m by 2.6 cm; Amersham Biosciences), equilibrated and run in phosphate-buffered saline (PBS), coupled to an AktaPrime automated fraction collector (13). Samples of column fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under nonreducing conditions, and those containing pIgA, as confirmed by immunoblotting using anti-alpha chain and anti-J chain antisera (42), were pooled. Purified SC was obtained from stably transfected CHO cells (38).
Reconstitution of sIgA was performed by combining, in 5 ml of PBS, 4 mg of purified pIgA with 1.2 mg of purified SC (13). The excess SC was separated by fast protein liquid chromatography using a Superdex 200 column (1 m by 1.6 cm; Amersham Biosciences). The association between SC and pIgA was confirmed by subjecting the protein to SDS-PAGE under nonreducing conditions that allowed the detection of covalent and noncovalent sIgA complexes following immunoblotting with rabbit anti-SC antiserum (26).
Viruses.
For the in vivo study, a crude stock of wild-type murine RV which causes epizootic diarrhea of infant mice (Cambridge ECw, P[16], G3) was prepared as intestinal homogenates from 4-day-old BALB/c neonates. The titer of ECw in 6-week-old adult mice was determined as previously described (14) and was found to correspond to 1 × 108 shedding doses 50 (SD50) per ml. For the in vitro study, RRV stock was generated in MA104 cells after a 24-h preincubation of the cells in a serum-free culture medium. Viruses were treated with 0.5 μg of trypsin per ml for 30 min, and MA104 cell monolayers were infected at a multiplicity of infection (MOI) of 1. After 1 h of adsorption at ambient temperature with gentle shaking, the inoculum was removed, and infected cells were incubated in fresh medium containing 0.5 μg of trypsin per ml. After complete cytopathic effect was obtained (usually within 5 days), the cultures were submitted to three freeze-thaw cycles, and cell debris was cleared by centrifugation at 2,200 × g. Virus titer in the supernatant was determined by plaque assay on MA104 cells as previously described (6).
Immunoprecipitation of RV by different molecular forms of IgA7D9 MAb.
Purified rotavirus triple-layer particles (TLP) (50 μg) (25) were incubated for 2 h at ambient temperature with 50 and 500 μg of pIgA7D9 MAb or 62.5 and 625 μg of SIgA7D9 MAb, respectively, in a final volume of 800 μl of PBS. Goat anti-mouse IgA (Sigma, St. Louis, MO) diluted 1:500 was added, and the mixture was left with gentle shaking for 1 h prior to addition of 50 μl of protein G-Sepharose (settled beads) equilibrated in PBS. After 1 h at ambient temperature, the beads were washed three times with TENT (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 150 mM NaCl, 0.05% Triton X-100). Bound proteins were eluted by boiling in SDS-PAGE loading buffer (50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 10% glycerol, 2% SDS, 0.1% bromophenol blue) and loaded onto a 12% polyacrylamide gel in 0.1% SDS. Following transfer onto a polyvinylidene fluoride membrane, nonspecific binding sites were blocked by incubation in 5% nonfat dry milk in PBS-0.05% Tween 20 (Bio-Rad, Hercules, CA), and the membrane was probed for 2 h using the 5.73 MAb to VP4/VP8* (33) diluted 1:500 in 0.5% nonfat dry milk in PBS-0.05% Tween 20. Bound MAb was detected using horseradish peroxidase-conjugated goat anti-mouse IgG (1:3,000; Sigma) followed by treatment with chemiluminescence reagents (Amersham Biosciences) and exposure to autoradiography films.
In vivo immune exclusion assay.
BALB/c mice (6 weeks old; Charles River Laboratories) were shown to be free of RV-specific antibodies prior to vaccination by an enzyme-linked immunosorbent assay (ELISA) on serum samples. They were housed in an A3 animal facility (Unité d'Expérimentation Animale Rongeurs, Jouy-en-Josas, France) under the 78-20 license delivered by the “Direction des Services Vétérinaires de Versailles” (France). Each mouse was individually marked. The assay was performed early in the morning in order to take advantage of the relatively higher stomach pH at that time of the day.
In order to examine the in vivo protective efficacy of purified pIgA7D9 MAb via the secretory pathway, a group of three mice was inoculated i.p. twice (1 mg, then 400 μg) with pIgA7D9 MAb in PBS 16 and 2.5 h before viral challenge, respectively. Groups of six mice were used for testing the luminal neutralization of RV. Two hours before challenge and right after oral administration of 1.3% buffering bicarbonate, preparations of SIgA7D9 MAb (500 μg), pIgA7D9 MAb (400 μg), SC alone (200 μg), or PBS were given orally by esophageal gavage in a final volume of 400 μl. Five minutes before challenge, the gavages were repeated with doses representing one third of the initial dose. The mice were infected with 1 × 102 SD50 of ECw murine virus after administration of 100 μl 1.3% buffering bicarbonate (48). Feces were then collected for each mouse in order to monitor SIgA7D9 MAb transit. From challenge to day 7 (time point at which no virus could be detected in the control PBS group), stools were harvested on absorbent paper from individual mice placed in independent cages on a daily basis. The feces were kept at −20°C until processed for analysis of the quantity of RV. The experiment was done three times.
Detection of SIgA7D9 MAb in feces.
Two fecal specimens were suspended in 500 μl of a mixture of 10 mM Tris (pH 7.4), 140 mM NaCl, 10 mM CaCl2, 25 mM EDTA, 0.05% Tween 20, and 1% protease inhibitor cocktail (Complete Mini; Roche, Basel, Switzerland). After a 45-min incubation on ice, the fecal suspensions were frozen at −80°C, thawed, and spun down at 13,000 × g, and the crude supernatants were tested for the presence of anti-VP6 activity by ELISA (48). Serial dilutions of reconstituted complexes of SIgA7D9 MAb were used to establish a standard curve. The sensitivity of the assay was 1 ng/ml. Feces were considered positive when the optical density (OD) value reached 0.2 or above. The results were expressed as nanograms of SIgA7D9 MAb (inferred from the standard curve) per ml of stool specimen.
Detection of RV antigens in feces.
Detection of group A RV antigens in mouse feces was performed using a commercial ELISA (IDEIA RV; Dako, Ely, Cambridgeshire, United Kingdom) according to the manufacturer's recommendations. Stools from noninfected mice were included as control samples and gave a mean OD450 value of 0.02. The total fecal virus shedding for each mouse was calculated by integrating the area under the shedding curve defined by the OD450 of each feces sample (y axis) over the 7-day period of feces collection (x axis), using Microcal Origin 5.0 software (37). Protection was determined by reduction of viral shedding relative to that of the PBS-treated group.
Cells and culture conditions.
Human colonic adenocarcinoma Caco-2 cells were grown in Dulbecco's modified Eagle's medium (4.5% glucose; Sigma) supplemented with 10% FBS (Sigma), 10 mM HEPES (pH 7.0), 1% nonessential amino acids, 1% sodium pyruvate, 2 mM glutamine, 0.1% transferrin, 100 U/ml penicillin, and 100 μg/ml streptomycin. For establishment of polarized monolayers, Caco-2 cells cultivated to 80% confluency were seeded on Snapwell filters (diameter, 12 mm; pore size, 0.4 μm; Corning Costar Corporation, Cambridge, MA) at a density of 2 × 105 cells/cm2. The formation of a polarized Caco-2 cell monolayer at week 3 was established by morphology and monitoring of the transepithelial electrical resistance (TER) using a Millicell-ERS apparatus (Millipore, Bedford, MA) (10). The TER values ranged between 350 and 400 Ω × cm2. Embryonic rhesus monkey kidney MA104 cells were grown in Earle's minimal essential medium (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 20 mM HEPES (pH 7.0), 2 mM glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin. All cell lines were maintained at 37°C in a 5% humidified CO2 incubator.
Virus infection of, and intracellular neutralization in, polarized Caco-2 cells.
An RRV virus inoculum was activated for 30 min by treatment with 0.5 μg of trypsin per ml. Caco-2 cells grown on Transwell filters were cultured without FBS in the apical compartment for 24 h and were then infected apically with the trypsin-treated inoculum at an MOI of 2 or 10 for 1 h at ambient temperature. The inoculum was then removed, and FBS-free fresh medium containing 0.5 μg of trypsin per ml was added. Infected cells were incubated at 37°C for the indicated experimental time and processed postinfection to measure stability of TER (20 h). Equimolar amounts of pIgA (5 μg/ml) or monomeric IgA (mIgA; 2.5 μg/ml) antibody were added 4 h prior to infection in the basolateral compartment. Virus release in the apical cell culture medium from infected filter-grown Caco-2 cells in the presence of basolateral antibodies was determined at 20 h postinfection by plaque assay on MA104 cells as previously described (6). The experiment was done twice under each condition in triplicate.
Cell viability measurements.
Polarized monolayers of Caco-2 cells were washed once with PBS to remove desquamated cells (usually less than 10 to 15% at 24 h postinfection), Snapwell filters were detached from the insert, and cells attached to the membrane were exposed to a solution of 0.25% trypsin-1 mM EDTA (Sigma) for 5 min. Cells were recovered by centrifugation, resuspended in FBS-free medium containing 0.1% trypan blue, and counted using a hemacytometer. Percent viability was assessed using the formula [(total cells − stained cells)/total cells] × 100.
RESULTS
Purified pIgA7D9 and SIgA7D9 interact with TLP.
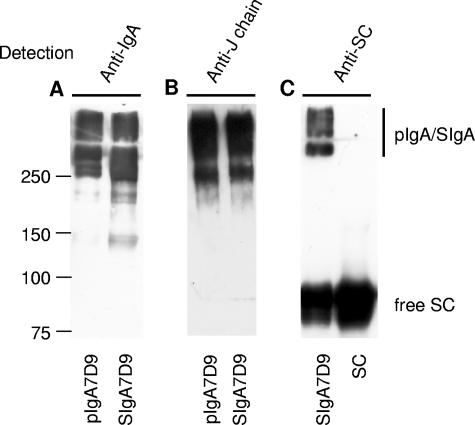
We first prepared biochemically defined pIgA7D9 and SIgA7D9 MAbs for subsequent in vivo oral administration. Ascitic fluid preparations of pIgA7D9 MAb were separated by size exclusion chromatography, the pIgA fraction was isolated, a portion was associated in vitro with SC, and the resulting SIgA7D9 MAb complex was purified by another size exclusion chromatography as described previously (13). The quality of the preparations was assessed by electrophoresis under nonreducing conditions followed by immunodetection analysis. Most SIgA7D9 and pIgA7D9 MAb was found in the polymeric form (Fig. 1A) containing covalently bound J chain (Fig. 1B). SIgA7D9 MAb was made of covalently assembled complexes, with some SC not covalently bound (Fig. 1C), as previously reported (26).
FIG. 1.
Western blot analysis of the pIgA7D9 MAb, SIgA7D9 MAb, and SC preparations. Polymeric IgA7D9 MAb, reconstituted SIgA7D9 MAb, and SC were purified by size-exclusion chromatography and analyzed by Western blotting under nonreducing conditions. (A) pIgA7D9 and SIgA7D9 MAbs were revealed with horseradish peroxidase-coupled anti-murine IgA serum. (B) Both polymeric forms contained the J chain, reflecting proper assembly. (C) Expected covalent and noncovalent association of SC and pIgA7D9 MAb was confirmed upon immunodetection with anti-SC antiserum. Molecular mass markers (kDa) are indicated alongside the sample lanes.
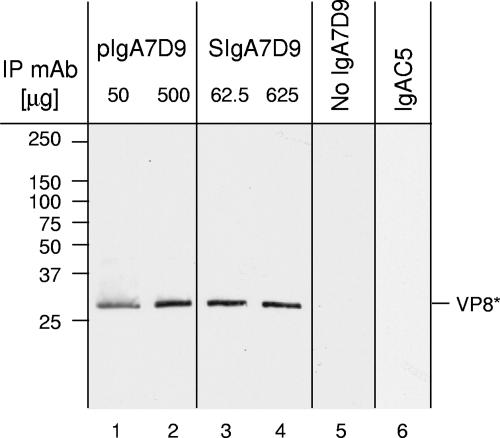
Preliminary experiments demonstrated that cesium chloride density gradient-purified TLP were recognized by the pIgA7D9 MAb in ELISA (data not shown). However, a small proportion of double-layered particles are generally present in a purified-TLP preparation, and thus a positive signal in ELISA does not necessarily indicate that the anti-VP6 7D9 MAb actually reacts with TLP. Therefore, immunoprecipitation studies of RV with pIgA7D9 MAb or SIgA7D9 MAb were conducted. Detection of a 28-kDa band corresponding to VP4-derived VP8* was used to establish that TLP were bound by both pIgA7D9 MAb and SIgA7D9 MAb (Fig. 2). Together, these data suggest that immune complexes can form between IgA7D9 and RV TLP, with TLP being mostly complete yet containing some partially uncoated viruses, as observed by electron microscopy (data not shown). Hence, it was reasonable to determine if such anti-VP6 antibodies were capable of offering passive protection in mice after oral feeding.
FIG. 2.
Interaction between RV TLP and pIgA7D9 or SIgA7D9 MAbs. Immunoprecipitation (IP) of RV TLP was performed with two quantities (50 μg and 500 μg) of pIgA7D9 MAb and equimolar amounts (62.5 μg and 625 μg) of SIgA7D9 MAb. Following protein separation by SDS-PAGE, detection of VP8* by specific 5.73 MAb indicates that TLP are recognized by the two molecular forms of the anti-VP6 MAb, namely pIgA7D9 MAb (lanes 1 and 2) and SIgA7D9 MAb (lanes 3 and 4). No signal was observed in the absence of IgA7D9 MAb (lane 5) or with 500 μg of irrelevant pIgAC5 MAb (lane 6).
Inhibition of viral replication by pIgA7D9 does not involve immune exclusion in vivo.
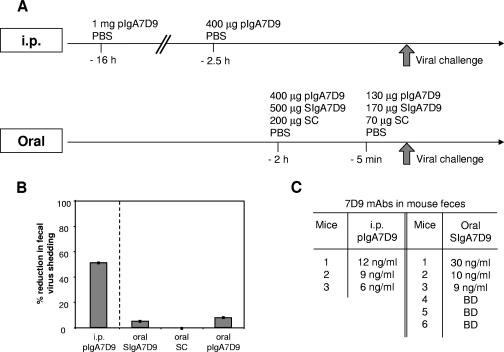
Two different protocols of passive administration were used to assess in vivo whether protection mediated by purified pIgA7D9 can be achieved. We first injected i.p. purified pIgA7D9 MAb into three mice 16 h (1 mg) and 2.5 h (400 μg) before viral challenge (Fig. 3A, i. p.). This treatment led to a 51.0% ± 0.7% reduction of viral shedding (Fig. 3B) as compared to the level in mice given PBS (P < 0.005) in accordance with previously published data (15). Importantly, anti-VP6 activity was detected in the stools of the inoculated mice at the time of challenge and corresponded to 12, 9, and 6 ng/ml SIgA7D9 MAb (Fig. 3C). This raises the possibility that the protection observed is mediated by SIgA7D9 through immune exclusion in the intestinal lumen. However, in a prior work, passive oral administration of pIgA did not block viral replication in mice (3). We postulated that in the previous oral feeding studies, the normal IgA molecular form present in secretions, i.e., sIgA, was not used, and hence stability of the orally administered antibody molecule might not have been ideal. For optimal assessment of oral delivery, mice (six per group) were fed (2 h and 5 min before challenge) with SIgA7D9 MAb (500 μg and 170 μg), pIgA7D9 MAb (400 μg and 130 μg), SC (200 μg and 70 μg), or PBS (Fig. 3A, oral). No significant reduction in viral shedding was observed in any of the SIgA7D9 MAb-fed mice nor in the pIgA7D9 MAb- and SC-fed groups (Fig. 3B). Protection was not seen despite the fact that three out of six mice had, at the time of challenge, amounts of SIgA7D9 MAb in the stools comparable to those found in the i.p.-injected group (30, 10, and 9 ng/ml feces; Fig. 3C). Overall, these data show that protection mediated by the anti-VP6 IgA7D9 MAb does not rely on immune exclusion in the intestinal lumen, thus prompting us to investigate intracellular neutralization mechanisms.
FIG. 3.
Oral feeding with SIgA7D9 does not reduce viral shedding. (A) Time frame summarizing the kinetics of i.p. and oral passive administration of the various molecular forms of pIgA7D9 and SIgA7D9. (B) Mean percentages of reduction of virus shedding (± standard error) compared to the PBS group are reported from BALB/c mice inoculated i.p. with pIgA7D9 MAb or dosed orally with SIgA7D9, pIgA7D9 MAb, and SC. A statistically significant difference was only achieved for viral shedding in the group receiving pIgA7D9 MAb i.p. (Student's t test, P < 0.005). Similar results were obtained in two other independent experiments. (C) Level of IgA7D9 MAb in mouse feces measured 2 h after passive administration of pIgA7D9 MAb i.p. or SIgA7D9 MAb orally. BD, below the level of detection.
Intracellular neutralization of RV by IgA7D9 MAb in polarized Caco-2 intestinal epithelial monolayers.
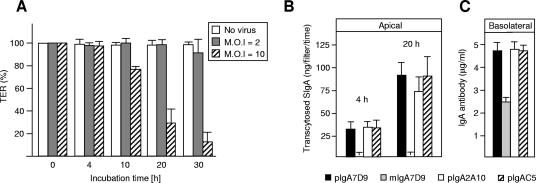
As direct luminal virus neutralization was not detected in vivo using either pIgA7D9 or SIgA7D9 MAbs, we sought to examine the possibility of intracellular neutralization of the virus during transcytosis of the antibody. To address this issue, we took advantage of the observation made by Jourdan et al. (23) that following apical infection of Caco-2 cell polarized monolayers, RRV is released almost exclusively from the apical surface (99.9%) without any cell lysis. We thus postulated that addition of test pIgA MAb to the basolateral compartment might contribute to virus neutralization during transcytosis. We then determined the optimal MOI that ensures infection of polarized Caco-2 cells and subsequent apical release within a time frame appropriate for assessing possible protection by transcytosed MAb. Figure 4A indicates that an MOI of 10 led to a significant drop in TER at 20 h postinfection, confirming that viral replication taking place within the epithelial cells affects the integrity of the monolayer. Apical incubation of polarized Caco-2 cells with medium lacking FBS did not artifactually perturb the TER of the monolayer, which remained stable during the time course of analysis (data not shown). We then defined the kinetics of transcytosis for pIgA MAb (7D9, C5, and 2A10). We demonstrated that transport of IgA began within 4 h and was sustained at 20 h (Fig. 4B). This indicates that pIgA antibodies on their way to the apical surface are likely to be present within Caco-2 cells after 4 h. We found that transport of pIgA MAb was highly specific, resulting in the release of up to 100 ng per filter of sIgA MAb in the apical compartment (Fig. 4B); as expected, no mIgA7D9 MAb unable to be bound by pIgR was recovered in the apical chamber (Fig. 4B) and the amount of remaining IgA in the basal chamber at 20 h indicates that the antibodies were not in limiting quantities (Fig. 4C).
FIG. 4.
Intracellular neutralization of RRV by pIgA7D9 MAb. (A) Stability of the Caco-2 monolayer TER exposed to different virus MOI as a function of time. (B) Transcytosis of the various IgAs used in this study and molecular forms thereof across Caco-2 cell monolayers was measured by ELISA as the appearance of SIgA in the apical chamber 4 and 20 h after addition of the purified IgA MAbs in the basolateral compartment (B). (C) The amount of IgA present in the basolateral chamber (5 μg/ml) is not limiting in the assay, as measured at 20 h.
We then examined the effect of MAb on the maintenance of Caco-2 cell polarization in the presence of apically administered RRV. In comparison with cells incubated with the virus alone, cells transporting pIgA7D9 MAb exhibited preserved TER similarly to noninfected Caco-2 cells (Table 1). Monomeric IgA7D9 MAb not capable of transcytosis and nonspecific pIgAC5 did not protect Caco-2 cells from infection, leading to a drop in TER (Table 1). The anti-VP4 pIgA2A10 MAb, which is capable of neutralizing apical rotavirus (44), showed only little if any effect on preservation of TER stability (Table 1). Appropriate cell viability was preserved at the end of the assay (Table 1).
TABLE 1.
Release of RRV from polarized Caco-2 epithelial cellsa
| Exptl setup for apical compartment | Antibody addition (basolateral compartment)b | % TERc | % Cell viability on filtersd | Virus titer (PFU/ml) |
|---|---|---|---|---|
| RRV (MOI = 10) | No antibody | 26 ± 3 | 88 | 1.5 × 107-2.75 × 107 |
| pIgA7D9 (5 μg/ml) | 98 ± 5 | 93 | 5.75 × 103-12.5 × 103 | |
| mIgA7D9 (2.5 μg/ml)e | 26 ± 2 | 95 | 1.1 × 107-3.5 × 107 | |
| pIgAC5 (5 μg/ml) | 27 ± 4 | 90 | 1.75 × 107-4.5 × 107 | |
| pIgA2A10 (5μg/ml) | 38 ± 4 | 88 | 2.5 × 105-6 × 105 | |
| No virus | No antibody | 100 | 94 |
Monolayers of 24-day-old Caco-2 cells grown on Transwell filters were infected with RRV at an MOI of 10 PFU/cell. At 20 h postinfection, media were collected from apical chambers and analyzed for virus content by plaque assay.
MAbs were added 4 h prior to infection.
TER values are expressed as the percentage of TER measured in the absence of viral infection fixed at 100%.
Cell viability is expressed as the percentage of total live cells on membrane filters.
IgA7D9 used in the form of a monomer was added at 2.5 μg/ml to yield an equimolar amount to polymeric IgA antibodies in the basolateral chamber.
The impact of MAb transport during infection was further assessed by measuring the level of the viral progeny secreted into the apical chamber. In the absence of MAb, a rotavirus titer above 107 PFU/ml was found in the apical well, which decreased to 103 to 104 PFU/ml in the presence of pIgA7D9 MAb (Table 1). Values in the same range as for virus alone were observed using either mIgA7D9 or IgAC5 (Table 1). Incubation with anti-VP4 IgA2A10 yielded a 50- to 100-fold decrease in apical viral titer (approximately 105 PFU/ml), which is likely explained by rapid transcytosis and subsequent extracellular neutralization of a portion of the virus in the apical chamber, as previously reported (44). This intracellular neutralization phenomenon could be reproduced using polarized intestinal HT29 cells (data not shown). The sum of these data indicates that pIgA7D9 MAb acts intracellularly during transcytosis across epithelial cells.
DISCUSSION
Our study demonstrates that the IgA7D9 MAb directed to the VP6 inner core protein can neutralize rotavirus intracellularly, most likely by an encounter with viral particles within the host cell. The luminal secretory form of the same IgA did not show any inhibition of viral replication in vivo after oral feeding, arguing for the primary effect of the antibody taking place inside cells. From a more general point of view, our findings further support the notion that transcytosing pIgA antibodies, together with the documented role of CD4 T cells (4, 31, 32), contribute to the VP6-dependent heterotypic protection observed in vivo.
In the course of this work, we confirmed prior observations that pIgA7D9 MAb given i.p. interferes with viral replication (15). In the present study, purified pIgA and not crude ascites fluid was tested, further strengthening the conclusion that inhibition of viral replication was due to bona fide effects of IgA7D9 MAb and not to other nonspecific activities potentially present in ascites fluid or secreted from 7D9 hybridoma cells. In addition, the observed reduction of viral shedding supports the notion that the molecular form of the pIgA7D9 MAb used to prepare SIgA7D9 MAb is biologically active. Administration of only two intraperitoneal doses (1.4 mg in total) of antibody is the most likely explanation for the finding that viral replication was not more completely abrogated. In fact, Feng et al. (15) documented that full protection was observed after daily injections of 3 mg IgA7D9 MAb over a 9-day period and that only limited protection was seen with a lower dose.
One of our working hypotheses to explain the protective efficacy of the IgA7D9 MAb was that it might interact with infectious particles via spaces in the viral outer shell or other forms of exposed VP6 and thereby could anchor the viral immune complex in the mucus and prevent infection. However, our experiments demonstrated that we could not achieve protection by passive oral administration of SIgA7D9 MAb alone, as would be expected if our working hypothesis was correct. It remains possible that polyclonal sIgA with a broader spectrum of action or directed against other viral antigens, including VP4 and VP7, might work by this mechanism. Actually, oral administration of milk from RV-immunized dams protected suckling mice against challenge (34); protective activity was detected in both the anti-rotavirus IgA and IgG fractions, but IgA was more potent in vivo than IgG (34). The use of a milk concentrate prepared from rotavirus-hyperimmunized cows resulted in a significant reduction in the duration of viral excretion in infants hospitalized for acute rotavirus gastroenteritis (1, 12, 21). However, milk from dams immunized with VLP2/6 did not prevent diarrhea in suckling offspring (9), while milk from VLP2/6/7-vaccinated mothers was protective, indicating that sIgA directed at VP7, but not at VP6, could be implicated in mediating immune exclusion.
One can argue that the amount of SIgA7D9 MAb (670 μg) given orally might not have been sufficient to confer protection by passive administration. However, the concentration of fecal SIgA7D9 recovered after oral delivery of SIgA7D9 in our experiments was in the same order of magnitude as the amount of specific fecal sIgA observed following nasal vaccination with VP6 (31). It represents 35 times more antibody than the amount used to passively protect against a Shigella infection via the nasal route in mice (38). Furthermore, 100 times less anti-VP4 or -VP7 IgG, an isotype not optimal for protection at mucosal surfaces, was sufficient to protect newborn mice from RV challenge after oral administration (35). While passive administration of sIgA is not strictly comparable to the physiological transport of pIgA across the mucosae, it results in the location of sIgA in mucus lining epithelial cells (38) and does lead to comparable levels of VP6-reacting sIgA in feces. Taken together, our data support the notion that IgA7D9 MAb acts against rotavirus predominantly inside cells in vivo.
In most cases where intracellular neutralization by IgA has been described, for human immunodeficiency virus (HIV) (2, 22), Sendai virus (18), and influenza virus (29), the active IgA molecule was directed at a viral protein known to be the target of extracellularly acting neutralizing antibodies. However, in the case of RV, such neutralizing antibodies directed to VP4 (44) did not exhibit intracellular neutralization activity. Conversely, we show here that the anti-VP6 IgA7D9, which is unable to inhibit viral replication in a classic neutralization assay, can indeed mediate intracellular neutralization. The mechanism involved is most probably associated with the pIgR-mediated transcystosis pathway. Polymeric Ig receptor-mediated transcytosis of polymeric IgA occurs via a vesicular pathway divided into three steps: step 1, internalization from the basolateral plasma membrane into basolateral early endosomes; step 2, translocation from the basolateral early endosomes to the apical recycling endosomes (ARE); and step 3, delivery from the ARE to the apical plasma membrane (27). Step 3 is rate-limiting, resulting in accumulation of pIgA-pIgR in the ARE. We propose that VP6, although first synthesized in the cytosol, encounters IgA either before or after new viral protein synthesis, leading to impediment of replication. This hypothesis will be addressed in future studies. Rotavirus particles enter the cell through a still-debated mechanism, but experiments showing that infection is a dynamin-dependent phenomenon (46) strongly support the hypothesis that RV is initially internalized via endocytosis. Thus, IgA7D9 MAb associated with pIgR could complex VP6 during transepithelial transport in the ARE, the compartment where IgA-mediated intracellular neutralization of HIV has been shown to occur (2) and where LPS signaling in epithelial cells can also be blocked (16). As previously hypothesized, IgA7D9 MAb could impede viral transcription by causing subtle architectural rearrangements within the VP6 capsid layer, reducing the enzymatic activity of the viral RNA polymerase in the core (15). The fact that this antitranscriptional property was limited to IgA7D9 MAb (15) may explain why all anti-VP6 antibodies are not capable of intracellular neutralization (44). Actually, the structure of the VP6 epitope recognized by the anti-VP6 IgA is probably crucial for intracellular neutralization to occur (44). This might also explain why J chain-dependent protection was observed when mice were immunized with VLP2/6 (48) and not with recombinant chimeric VP6 (31). Alternatively, recent studies have clearly identified a secretory transport pathway of RV from the endoplasmic reticulum, through the endoplasmic reticulum-Golgi intermediate compartment that eventually leads to rotavirus secretion from the apical surface of the cell; transcytosis of IgA7D9 might interact with VP6 during this phase of assembly and release (11).
Heterotypic protection observed after rotavirus infection can involve several mechanisms. Using a specific anti-VP6 IgA (IgA7D9), we show that intracellular neutralization can be one of these mechanisms. Since humoral immunity has been implicated in protection induced after live virus immunization (17, 30), it is likely that heterotypic protection with anti-VP6 IgA MAb occurs primarily after natural infection or live virus vaccination. However, when protection was generated by recombinant VP6 (5, 32) or by VLP2/6 (M. Conner, unpublished results), CD4 T cells were found to play a pivotal role. Finally, heterotypic neutralizing antibodies to VP7 and VP4 (28) may also contribute to broad protection. Thus, heterotypic immunity probably involves several distinct immunological pathways, and this work illustrates that one anti-VP6 IgA MAb acts via an intracellular neutralization mechanism.
Acknowledgments
We thank Ningguo Feng for providing ascites fluid, Franco Ruggeri for the kind gift of 2A10 hybridoma cells, Matthieu Pillot and the animal facility staff for invaluable help with mouse work and Maria Christina Jaimes for helpful advice regarding the in vitro system. Critical readings of the manuscript by Daniel Speiser and Monica McNeal are gratefully acknowledged.
I.S.-C. and B.C. thank the PAI-Germaine de Staël-SATW exchange program (SAMW-03-001). B.C. is supported by grant no. 3200-109545 from the Swiss Science Research Foundation, and I.S.-C. is supported by INRA institutional funds. Work on this study was also supported by a grant from the Veterans Administration (H.G.).
Footnotes
This work is dedicated to the memory of Jean Cohen.
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Bogstedt, A. K., K. Johansen, H. Hatta, M. Kim, T. Casswall, L. Svensson, and L. Hammarstrom. 1996. Passive immunity against diarrhoea. Acta Paediatr. 85:125-128. [DOI] [PubMed] [Google Scholar]
- 2.Bomsel, M., M. Heyman, H. Hocini, S. Lagaye, L. Belec, C. Dupont, and C. Desgranges. 1998. Intracellular neutralization of HIV transcytosis across tight epithelial barriers by anti-HIV envelope protein dIgA or IgM. Immunity 9:277-287. [DOI] [PubMed] [Google Scholar]
- 3.Burns, J. W., M. Siadat-Pajouh, A. A. Krishnaney, and H. B. Greenberg. 1996. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 272:104-107. [DOI] [PubMed] [Google Scholar]
- 4.Choi, A. H.-C., M. Basu, M. M. McNeal, J. D. Clements, and R. L. Ward. 1999. Antibody-independent protection against rotavirus infection of mice stimulated by intranasal immunization with chimeric VP4 or VP6 protein. J. Virol. 73:7574-7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, A. H. C., M. Basu, M. M. McNeal, J. Flint, J. L. VanCott, J. D. Clements, and R. L. Ward. 2000. Functional mapping of protective domains and epitopes in the rotavirus VP6 protein. J. Virol. 74:11574-11580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciarlet, M., and M. K. Estes. 1999. Human and most animal rotavirus strains do not require the presence of sialic acid on the cell surface for efficient infectivity. J. Gen. Virol. 80:943-948. [DOI] [PubMed] [Google Scholar]
- 7.Clark, H. F., P. A. Offit, R. W. Ellis, D. Krah, A. R. Shaw, J. J. Eiden, M. Pichichero, and J. J. Treanor. 1996. WC3 reassortant vaccines in children. Arch. Virol. Suppl. 12:187-198. [DOI] [PubMed] [Google Scholar]
- 8.Corthésy, B., and F. Spertini. 1999. Secretory immunoglobulin A: from mucosal protection to vaccine development. Biol. Chem. 380:1251-1262. [DOI] [PubMed] [Google Scholar]
- 9.Coste, A., J.-C. Sirard, K. Johansen, J. Cohen, and J. P. Kraehenbuhl. 2000. Nasal immunization of mice with virus-like particles protects offspring against rotavirus diarrhea. J. Virol. 74:8966-8971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cottet, S., I. Corthésy-Theulaz, F. Spertini, and B. Corthésy. 2002. Microaerophilic conditions permit to mimic in vitro events occurring during in vivo Helicobacter pylori infection and to identify Rho/Ras-associated proteins in cellular signaling. J. Biol. Chem. 277:33978-33986. [DOI] [PubMed] [Google Scholar]
- 11.Cuadras, M. A., B. B. Bordier, J. L. Zambrano, J. E. Ludert, and H. B. Greenberg. 2006. Dissecting rotavirus particle-raft interaction with small interfering RNAs: insights into rotavirus transit through the secretory pathway. J. Virol. 80:3935-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson, G. P., P. B. Whyte, E. Daniels, K. Franklin, H. Nunan, P. I. McCloud, A. G. Moore, and D. J. Moore. 1989. Passive immunisation of children with bovine colostrum containing antibodies to human rotavirus. Lancet ii:709-712. [DOI] [PubMed] [Google Scholar]
- 13.Favre, L. I., F. Spertini, and B. Corthésy. 2003. Simplified procedure to recover recombinant antigenized secretory IgA to be used as a vaccine vector. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 786:143-151. [DOI] [PubMed] [Google Scholar]
- 14.Feng, N., J. W. Burns, L. Bracy, and H. B. Greenberg. 1994. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J. Virol. 68:7766-7773. (Erratum, 69:3246, 1995.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng, N., J. A. Lawton, J. Gilbert, N. Kuklin, P. Vo, B. V. Prasad, and H. B. Greenberg. 2002. Inhibition of rotavirus replication by a non-neutralizing, rotavirus VP6-specific IgA mAb. J. Clin. Investig. 109:1203-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez, M. I., T. Pedron, R. Tournebize, J. C. Olivo-Marin, P. J. Sansonetti, and A. Phalipon. 2003. Anti-inflammatory role for intracellular dimeric immunoglobulin a by neutralization of lipopolysaccharide in epithelial cells. Immunity 18:739-749. [DOI] [PubMed] [Google Scholar]
- 17.Franco, M. A., and H. B. Greenberg. 1995. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J. Virol. 69:7800-7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujioka, H., S. N. Emancipator, M. Aikawa, D. S. Huang, F. Blatnik, T. Karban, K. DeFife, and M. B. Mazanec. 1998. Immunocytochemical colocalization of specific immunoglobulin A with Sendai virus protein in infected polarized epithelium. J. Exp. Med. 188:1223-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giammarioli, A. M., E. R. Mackow, L. Fiore, H. B. Greenberg, and F. M. Ruggeri. 1996. Production and characterization of murine IgA monoclonal antibodies to the surface antigens of rhesus rotavirus. Virology 225:97-110. [DOI] [PubMed] [Google Scholar]
- 20.Gilber, J. M., N. Feng, J. T. Patton, and H. B. Greenberg. 2001. Rotavirus assembly—interaction of surface protein VP7 with middle layer protein VP6. Arch. Virol. 146:1155-1171. [DOI] [PubMed] [Google Scholar]
- 21.Hilpert, H., H. Brussow, C. Mietens, J. Sidoti, L. Lerner, and H. Werchau. 1987. Use of bovine milk concentrate containing antibody to rotavirus to treat rotavirus gastroenteritis in infants. J. Infect. Dis. 156:158-166. [DOI] [PubMed] [Google Scholar]
- 22.Huang, Y. T., A. Wright, X. Gao, L. Kulick, H. Yan, and M. E. Lamm. 2005. Intraepithelial cell neutralization of HIV-1 replication by IgA. J. Immunol. 174:4828-4835. [DOI] [PubMed] [Google Scholar]
- 23.Jourdan, N., M. Maurice, D. Delautier, A. M. Quero, A. L. Servin, and G. Trugnan. 1997. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J. Virol. 71:8268-8278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuklin, N. A., L. Rott, N. Feng, M. E. Conner, N. Wagner, W. Muller, and H. B. Greenberg. 2001. Protective intestinal anti-rotavirus B cell immunity is dependent on alpha 4 beta 7 integrin expression but does not require IgA antibody production. J. Immunol. 166:1894-1902. [DOI] [PubMed] [Google Scholar]
- 25.Ludert, J. E., M. C. Ruiz, C. Hidalgo, and F. Liprandi. 2002. Antibodies to rotavirus outer capsid glycoprotein VP7 neutralize infectivity by inhibiting virion decapsidation. J. Virol. 76:6643-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lullau, E., S. Heyse, H. Vogel, I. Marison, U. von Stockar, J. P. Kraehenbuhl, and B. Corthésy. 1996. Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. J. Biol. Chem. 271:16300-16309. [DOI] [PubMed] [Google Scholar]
- 27.Luton, F., M. H. Cardone, M. Zhang, and K. E. Mostov. 1998. Role of tyrosine phosphorylation in ligand-induced regulation of transcytosis of the polymeric Ig receptor. Mol. Biol. Cell 9:1787-1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsui, S. M., P. A. Offit, P. T. Vo, E. R. Mackow, D. A. Benfield, R. D. Shaw, L. Padilla-Noriega, and H. B. Greenberg. 1989. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to the heterotypic neutralization domain of VP7 and the VP8 fragment of VP4. J. Clin. Microbiol. 27:780-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazanec, M. B., C. L. Coudret, and D. R. Fletcher. 1995. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 69:1339-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeal, M. M., K. S. Barone, M. N. Rae, and R. L. Ward. 1995. Effector functions of antibody and CD8+ cells in resolution of rotavirus infection and protection against reinfection in mice. Virology 214:387-397. [DOI] [PubMed] [Google Scholar]
- 31.McNeal, M. M., S. C. Stone, M. Basu, J. A. Bean, J. D. Clements, B. A. Hendrickson, A. H. Choi, and R. L. Ward. 2006. Protection against rotavirus shedding after intranasal immunization of mice with a chimeric VP6 protein does not require intestinal IgA. Virology 346:338-347. [DOI] [PubMed] [Google Scholar]
- 32.McNeal, M. M., J. L. VanCott, A. H. C. Choi, M. Basu, J. A. Flint, S. C. Stone, J. D. Clements, and R. L. Ward. 2002. CD4 T cells are the only lymphocytes needed to protect mice against rotavirus shedding after intranasal immunization with a chimeric VP6 protein and the adjuvant LT(R192G). J. Virol. 76:560-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nejmeddine, M., G. Trugnan, C. Sapin, E. Kohli, L. Svensson, S. Lopez, and J. Cohen. 2000. Rotavirus spike protein VP4 is present at the plasma membrane and is associated with microtubules in infected cells. J. Virol. 74:3313-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Offit, P. A., and H. F. Clark. 1985. Protection against rotavirus-induced gastroenteritis in a murine model by passively acquired gastrointestinal but not circulating antibodies. J. Virol. 54:58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Offit, P. A., R. D. Shaw, and H. B. Greenberg. 1986. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J. Virol. 58:700-703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parashar, U. D., C. J. Gibson, J. S. Bresse, and R. I. Glass. 2006. Rotavirus and severe childhood diarrhea. Emerg. Infect. Dis. 12:304-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parez, N., C. Fourgeux, A. Mohamed, C. Dubuquoy, M. Pillot, A. Dehee, A. Charpilienne, D. Poncet, I. Schwartz-Cornil, and A. Garbarg-Chenon. 2006. Rectal immunization with rotavirus virus-like particles induces systemic and mucosal humoral immune responses and protects mice against rotavirus infection. J. Virol. 80:1752-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Phalipon, A., A. Cardona, J. P. Kraehenbuhl, L. Edelman, P. J. Sansonetti, and B. Corthésy. 2002. Secretory component: a new role in secretory IgA-mediated immune exclusion in vivo. Immunity 17:107-115. [DOI] [PubMed] [Google Scholar]
- 39.Phalipon, A., M. Kaufmann, P. Michetti, J. M. Cavaillon, M. Huerre, P. Sansonetti, and J. P. Kraehenbuhl. 1995. Monoclonal immunoglobulin A antibody directed against serotype-specific epitope of Shigella flexneri lipopolysaccharide protects against murine experimental shigellosis. J. Exp. Med. 182:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad, B. V., and W. Chiu. 1994. Structure of rotavirus. Curr. Top. Microbiol. Immunol. 185:9-29. [DOI] [PubMed] [Google Scholar]
- 41.Ramig, R. F. 1997. Genetics of the rotaviruses. Annu. Rev. Microbiol. 51:225-255. [DOI] [PubMed] [Google Scholar]
- 42.Rindisbacher, L., S. Cottet, R. Wittek, J. P. Kraehenbuhl, and B. Corthésy. 1995. Production of human secretory component with dimeric IgA binding capacity using viral expression systems. J. Biol. Chem. 270:14220-14228. [DOI] [PubMed] [Google Scholar]
- 43.Rojas, R., and G. Apodaca. 2002. Immunoglobulin transport across polarized epithelial cells. Nat. Rev. Mol. Cell Biol. 3:944-955. [DOI] [PubMed] [Google Scholar]
- 44.Ruggeri, F. M., K. Johansen, G. Basile, J. P. Kraehenbuhl, and L. Svensson. 1998. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J. Virol. 72:2708-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ruiz-Palacios, G. M., I. Perez-Schael, F. R. Velazquez, H. Abate, T. Breuer, S. C. Clemens, B. Cheuvart, F. Espinoza, P. Gillard, B. L. Innis, Y. Cervantes, A. C. Linhares, P. Lopez, M. Macias-Parra, E. Ortega-Barria, V. Richardson, D. M. Rivera-Medina, L. Rivera, B. Salinas, N. Pavia-Ruz, J. Salmeron, R. Ruttimann, J. C. Tinoco, P. Rubio, E. Nunez, M. L. Guerrero, J. P. Yarzabal, S. Damaso, N. Tornieporth, X. Saez-Llorens, R. F. Vergara, T. Vesikari, A. Bouckenooghe, R. Clemens, B. De Vos, and M. O'Ryan. 2006. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N. Engl. J. Med. 354:11-22. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez-San Martin, C., T. López, C. F. Arias, and S. López. 2004. Characterization of rotavirus cell entry. J. Virol. 78:2310-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santos, N., and Y. Hoshino. 2005. Global distribution of rotavirus serotypes/genotypes and its implication for the development and implementation of an effective rotavirus vaccine. Rev. Med. Virol. 15:29-56. [DOI] [PubMed] [Google Scholar]
- 48.Schwartz-Cornil, I., Y. Benureau, H. Greenberg, B. A. Hendrickson, and J. Cohen. 2002. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J. Virol. 76:8110-8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tosser, G., T. Delaunay, E. Kohli, J. Grosclaude, P. Pothier, and J. Cohen. 1994. Topology of bovine rotavirus (RF strain) VP6 epitopes by real-time biospecific interaction analysis. Virology 204:8-16. [DOI] [PubMed] [Google Scholar]
- 50.Vesikari, T., D. O. Matson, P. Dennehy, P. Van Damme, M. Santosham, Z. Rodriguez, M. J. Dallas, J. F. Heyse, M. G. Goveia, S. B. Black, H. R. Shinefield, C. D. Christie, S. Ylitalo, R. F. Itzler, M. L. Coia, M. T. Onorato, B. A. Adeyi, G. S. Marshall, L. Gothefors, D. Campens, A. Karvonen, J. P. Watt, K. L. O'Brien, M. J. DiNubile, H. F. Clark, J. W. Boslego, P. A. Offit, and P. M. Heaton. 2006. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N. Engl. J. Med. 354:23-33. [DOI] [PubMed] [Google Scholar]