Abstract
The UL11 gene of herpes simplex virus type 1 encodes a 96-amino-acid tegument protein that is myristylated, palmitylated, and phosphorylated and is found on the cytoplasmic faces of nuclear, Golgi apparatus-derived, and plasma membranes of infected cells. Although this protein is thought to play a role in virus budding, its specific function is unknown. Purified virions were found to contain ∼700 copies of the UL11 protein per particle, making it an abundant component of the tegument. Moreover, comparisons of cell-associated and virion-associated UL11 showed that packaging is selective for underphosphorylated forms, as has been reported for several other tegument proteins. Although the mechanism by which UL11 is packaged is unknown, previous studies have identified several sequence motifs in the protein that are important for membrane binding, intracellular trafficking, and interaction with UL16, another tegument protein. To ascertain whether any of these motifs are needed for packaging, a transfection/infection-based assay was used in which mutant forms of the protein must compete with the wild type. In this assay, the entire C-terminal half of UL11 was found to be dispensable. In the N-terminal half, the sites of myristylation and palmitylation, which enable membrane-binding and Golgi apparatus-specific targeting, were found to be essential for efficient packaging. The acidic cluster motif, which is not needed for Golgi apparatus-specific targeting but is involved in recycling the protein from the plasma membrane and for the interaction with UL16, was found to be essential, too. Thus, something other than mere localization of UL11 to Golgi apparatus-derived membranes is needed for packaging. The critical factor is unlikely to be the interaction with UL16 because other mutants that fail to bind this protein (due to removal of the dileucine-like motif or substitutions with foreign acidic clusters) were efficiently packaged. Collectively, these results suggest that UL11 packaging is not driven by a passive mechanism but instead requires trafficking through a specific pathway.
Herpes simplex virus type 1 (HSV-1) contains more than 40 different proteins, and these are partitioned into three compartments: the nucleocapsid, the surrounding but poorly defined tegument layer, and the glycoprotein-containing lipid envelope. Although the mechanisms by which the virion components come together to produce an infectious particle are not understood, the overall morphogenesis pathway is well defined (32). In brief, DNA-containing capsids are assembled in the nucleus and then transported into the cytoplasm by budding and fusion events at the inner and outer nuclear membranes, respectively. The cytoplasmic nucleocapsids are subsequently transported to Golgi apparatus-derived membranes, where the viral glycoproteins accumulate and maturation budding takes place. Tegument proteins are thought to be added to the nucleocapsid in an orderly fashion at various steps along the transport pathway and during budding. Vesicles containing the mature virions subsequently fuse with the plasma membrane, enabling release into the extracellular medium.
One of the smallest HSV-1 components is the lipid modified, 96-amino-acid tegument protein, UL11 (Fig. 1). All herpesviruses encode a homolog of this protein (32), but in all cases, the function is unknown. Viruses that fail to express their UL11 homolog invariably exhibit defects in maturation budding and accumulate nucleocapsids in the cytoplasm (2, 10, 22, 23, 26, 27, 48, 51, 52); hence, it is possible that this protein is a component of the budding machinery. However, it is also possible that it serves indirectly (e.g., perhaps by recruiting components of the actual machinery to the site of budding).
FIG. 1.
Sequence of the UL11 protein. The motifs that are important for membrane binding (myristylation and palmitylation) and membrane trafficking (dileucine and acidic cluster) are indicated along with the amino acid substitutions of mutants used in this study.
UL11 is synthesized on free ribosomes and is cotranslationally modified with myristate, a 14-carbon fatty acid that enables binding of proteins to the cytoplasmic faces of cellular membranes (11, 26, 29, 42, 45). In infected cells, it accumulates on nuclear and Golgi apparatus-derived membranes (1), but when expressed in the absence of all other viral proteins, it is found primarily on the Golgi apparatus (7). Recent studies have shown that UL11 has at least four sequence motifs that influence its subcellular localization, all of which map to the first half of the protein and are found in the homologs from other herpesviruses (Fig. 1). In addition to the N-terminal myristylation site, which enables the protein to bind weakly and nonspecifically to membranes (11, 29, 42), there are three nearby cysteines, at least one of which is posttranslationally modified with palmitate, a 16-carbon fatty acid that enables tight membrane binding and Golgi apparatus accumulation (24, 45). A dileucine-like (LI) motif and an acidic cluster (DIESEEE) enable UL11 to be recycled from post-Golgi apparatus locations, and in the absence either of these, higher levels of the protein accumulate on the plasma membrane (7, 24, 25). The LI and DIESEEE are also needed for binding to UL16 (25, 56), another poorly-understood tegument protein (3, 37, 41). Moreover, mutants in which the DIESEEE sequence is replaced with foreign acidic clusters (those from Nef or furin) fail to bind UL16 even though they are capable of recycling (25).
The goal of the experiments described here was to ascertain which features of UL11 are needed for virion packaging. Because the critical 5′ half of the UL11 gene overlaps with UL12, the product of which is essential for transport of capsids from the nucleus (50, 60), it was not feasible to construct recombinant viruses carrying the mutations of interest. For the same reason, previously described “null” mutants (2, 27) were not of use because they actually express large N-terminal fragments of UL11, which would complicate the interpretation of packaging studies. Therefore, the experiments below made use of a transfection/infection-based assay in which mutant forms of UL11 must compete with the wild type (wt) for packaging. The results suggest that packaging is not driven by a passive mechanism but instead requires transport of UL11 through a specific pathway.
MATERIALS AND METHODS
Cells and viruses.
Vero and A7 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal bovine serum, penicillin (65 μg/ml), and streptomycin (131 μg/ml). Cells infected with the KOS strain of HSV-1 (53) were grown in DMEM supplemented with 2% fetal bovine serum, 25 mM HEPES buffer, glutamine (0.3 μg/ml), penicillin, and streptomycin.
Antibodies.
UL11-specific antiserum was derived from rabbits and has been described previously (25). Purified VP16- and GFP-specific antibodies (derived from rabbits) were obtained from Clontech (product no. 3844-1 and 8367-1, respectively).
UL11 constructs.
All of the green fluorescent protein (GFP)-tagged UL11 derivatives used in this study have been described previously (24, 25). To produce constructs that have a different C-terminal tag, a small sequence encoding an epitope from hemagglutinin (HA [YPYDVPDYA]) followed by a stop codon was inserted in place of the gfp cassette using the flanking Asp718I and NotI sites within the various UL11-GFP constructs. To create this coding sequence, two oligonucleotides were annealed (5′-GTACCG1CTACCCGTACGACGTCCCAGACTACGCTTAGATAACTGATGC-3′ and 5′-GGCCGCATCAGTTATCTAAGCGTAGTCTGGGACGTCGTACGGGTAGCG-3′) that are fully complementary except for 4 nucleotides at each 5′ end (producing Asp718I and NotI sticky ends).
Confocal microscopy.
Vero cells were transfected with plasmids encoding GFP-tagged UL11 chimeras using the Lipofectamine 2000 reagent (Invitrogen). At 18 h posttransfection, cells were infected with HSV-1 at a multiplicity of infection (MOI) of 30, and after an additional 15 to 20 h, they were visualized using a Zeiss laser-scanning microscope with a helium-argon laser (488 peak excitation).
Quantitation of UL11 copy number.
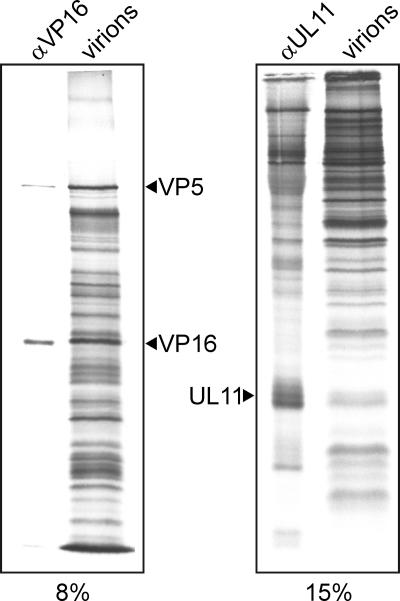
Confluent monolayers of Vero cells (eight 100-mm plates) were infected at an MOI of 0.01. At 12 to 16 h postinfection, the cells were metabolically labeled with methionine-free l-[35S]cysteine (70 μCi/ml; >1,000 Ci/mmol) in DMEM that otherwise lacked cysteine. When cytopathic effects were visible (24 to 36 h), the medium was collected and centrifuged at 2,000 × g for 10 min to remove cells and debris. Virions were pelleted from the supernatant by centrifugation at 25,000 rpm for 1.5 h in an SW28 rotor and purified in a 5 to 15% Ficoll gradient, as described previously (54). Equal amounts of purified virions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) in 8% (to detect VP5 and VP16) or 15% (to detect UL11) gels, which were subsequently dried and subjected to autoradiography with Kodak X-Omat AR5 film for 5 to 7 days to detect radiolabeled proteins. PhosphorImager analysis was also performed to quantitate the relative amounts of UL11, VP5, and VP16. To ensure that the correct bands were identified, samples of the three proteins were immunoprecipitated from [35S]cysteine-labeled, infected-cell lysates (1/5 of a 100-mm plate) as described previously (61) and loaded on the gels to serve as markers. To calculate UL11 copy number, raw PhosphorImager readings for UL11, VP5, and VP16 were first normalized by dividing by the number of cysteines in each protein (4, 25, and 6, respectively). Normalized VP5 and VP16 readings were divided by their known copy numbers (960 and 1,647, respectively) to give values that represent normalized PhosphorImager units/copy of protein, and the normalized UL11 reading was divided by those values to reveal the number of UL11 proteins per virion.
Analysis of UL11 phosphorylation.
Confluent monolayers of Vero cells (one 35-mm plate) were infected at an MOI of 30. At 5 h postinfection, cells were starved for 15 min in cysteine-free, methionine-free DMEM and then metabolically labeled for 3 h with 85 μCi/ml (>1,000 Ci/mmol) of EXPRE35S35S protein labeling mix (Perkin-Elmer). UL11 proteins were immunoprecipitated from cell lysates prepared with radioimmunoprecipitation assay buffer containing protease inhibitors (Sigma; product no. P8340). Collected proteins (still on the protein A-agarose beads) were either left untreated or were treated with 100 U of lambda protein phosphatase (New England Biolabs) for 1.5 h in the absence or presence of the phosphatase inhibitors sodium vanadate (200 μM) and NaF (50 mM). The labeled UL11 proteins were then separated by SDS-PAGE in 15% gels and quantitated using a Phosphorimager.
Analysis of UL11 packaging.
To compare the relative levels of packaging for wt and mutant derivatives of UL11, a transfection/infection-based assay was developed. Semiconfluent monolayers of Vero cells were transfected with UL11-GFP expression vectors (2 to 20 μg of DNA per 100-mm plate for each construct) using Lipofectamine 2000 (Invitrogen). Human melanoma (A7) cells, which are much more readily transfected than Vero cells, were found to be unsuitable for the packaging assay since they release free GFP into the medium, presumably due to membrane blebbing (data not shown). To ensure equal expression levels, each vector was titrated prior to the start of the experiment. At 18 h posttransfection, the cells were infected at an MOI of 30, and after an additional 22 h, the medium was collected and cell debris was removed, as described above. Extracellular virions (in 8 ml) were purified by centrifugation (1 h at 26,000 rpm in an SW41 rotor) through a 30% (wt/vol) sucrose cushion (1.7 ml). To examine the expression levels, infected-cell lysates were prepared by scraping the monolayers into PBS, pelleting at 2,000 × g for 10 min, and adding sample buffer. The cell lysates and virions were separated by SDS-PAGE in 12% gels and analyzed by Western blotting using antibodies specific for GFP (to detect UL11-GFP chimeras) and VP16 (to ensure equal loading of samples). Using densitometry, the packaging efficiency was quantitated by dividing the amount of UL11-GFP protein in the medium by the amount in the cell lysate (both normalized for VP16). To ensure that the Western blots were in the linear range of the assay, different amounts of each sample (e.g., 1/10, 1/5, and 1/2 of the total) were loaded onto the gels. The method of Western blotting was chosen over radiolabeling-immunoprecipitation assays because it was less expensive and produced fewer interfering background bands.
RESULTS
Characterization of UL11 within extracellular virions.
Two attributes of UL11 that are relevant to its packaging are the number of copies and the number of differentially modified species present in the virion. The number of UL11 molecules in radiolabeled, purified virions was determined by comparing it to proteins of known copy number (Fig. 2 and Table 1). Using VP5 (960 copies) and VP16 (1,647 copies) as standards (38, 39, 49, 62), it was estimated that approximately 700 copies of UL11 are packaged per particle, making it an abundant component of the tegument.
FIG. 2.
Determination of UL11 copy number. Equal amounts of radiolabeled, gradient-purified HSV-1 virions were separated by SDS-PAGE on 8% and 15% polyacrylamide gels, and proteins were visualized by autoradiography. VP5 was identified in these preparations based on its unique size, whereas VP16 and UL11 were identified by immunoprecipitating the respective proteins from radiolabeled, infected-cell lysate using specific antibodies: anti-VP16 (αVP16) and anti-UL11 (αUL11). All three proteins were quantitated using PhosphorImager analysis and normalized for the number of cysteines in each protein.
TABLE 1.
Average number of UL11 molecules per virion
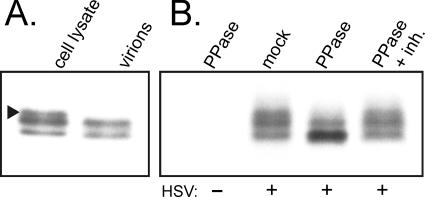
HSV-1-infected cells contain at least three species of UL11 as analyzed by SDS-PAGE (1, 24-27). To determine which forms are packaged, purified virions were examined by Western blotting. Only the two faster-migrating species of UL11 were found (Fig. 3A), suggesting that either these two species are selectively packaged or the slowest-migrating species is actively excluded. Alternatively, all three forms might be packaged with a subsequent alteration taking place to eliminate the upper species.
FIG. 3.
Phosphorylation state of UL11 in purified virions. (A) Determination of which UL11 species are packaged. Proteins from gradient-purified virions were separated by SDS-PAGE on 15% polyacrylamide gels alongside infected-cell lysate, electrotransferred onto nitrocellulose membranes, and then analyzed by Western blotting using UL11-specific antiserum. The arrowhead denotes the slowest-migrating UL11 species which is present in infected-cell lysate, but not in purified virions. (B) Analysis of UL11 phosphorylation. Infected Vero cells were metabolically labeled with [35S]methionine/cysteine for 3 h and lysed. UL11 protein was immunoprecipitated and treated with phosphatase for 1.5 h in the absence or presence (+ inh.) of inhibitors. Proteins were then separated by SDS-PAGE on 15% polyacrylamide gels and analyzed by autoradiography and PhosphorImager analysis.
A previous report has demonstrated that UL11 is phosphorylated when expressed in transfected cells as a fusion protein with GFP (24). To determine whether virally encoded UL11 is also modified and whether the three species represent different phosphorylated states of the protein, immunoprecipitated proteins from infected-cell lysates were treated with protein phosphatase in the presence or absence of inhibitors. Treatment with the enzyme resulted in 65% and 30% decreases in the upper and middle species of UL11, respectively, and a 100% increase in the fastest-migrating species (Fig. 3B and Table 2). The addition of inhibitors abrogated the observed changes in band intensities, suggesting that phosphatase itself, and not the treatment conditions, was responsible for the effects. These data suggest that the two slower-migrating species are phosphorylated forms of UL11, whereas the fastest-migrating species is unmodified. Attempts to confirm these findings by labeling with 32Pi were unsuccessful due to difficulties in resolving the three 32P-labeled UL11 species (data not shown). Taken together, these data suggest that the most highly phosphorylated form of UL11 is not found within virions. This finding is similar to what has been recently reported with the cytomegalovirus homolog of UL11, pp28 (20).
TABLE 2.
Phosphatase treatment of UL11 species
| Treatment | % Radioactivity in UL11 banda:
|
Total countsb | ||
|---|---|---|---|---|
| Upper | Middle | Lower | ||
| Mock | 33 ± 2 | 35 ± 2 | 32 ± 1 | 100,666 |
| PPase | 12 ± 1 | 25 ± 1 | 63 ± 2 | 117,528 |
| PPase + inhibitor | 25 ± 3 | 36 ± 4 | 39 ± 7 | 101,538 |
Relative position of the gel bands within the UL11 triplet. The percentage of radioactivity found in each band was calculated, and the average of results four independent experiments ± standard deviation is shown.
Combined counts for the three UL11 species averaged from the four experiments.
Development of the UL11 packaging assay.
While it would be of interest to study the packaging of UL11 mutants in the complete absence of the wild-type protein, overlap of the first 30% of UL11 gene with the essential UL12 gene impedes the construction of recombinant viruses that carry the mutant alleles of interest. For the same reason, all previously reported UL11-“null” mutants still express substantial portions of the protein (2, 27), and it is not known what effects these would have on packaging of trans-expressed UL11. Therefore, to ascertain whether any of the sequence motifs in UL11 are required for packaging, a transfection/infection-based assay was developed in which mutant constructs must compete with the wild-type, virally encoded protein (see Materials and Methods). In brief, Vero cells were transfected with various UL11-fusion constructs and subsequently infected with HSV-1. Relative levels of protein present in the cell lysates and extracellular virions were then quantitated by Western blotting with antisera specific for the tag (to detect the trans-expressed UL11 constructs) or VP16 (to correct for errors in cell harvesting and gel loading).
Preliminary experiments revealed that trans-expressed UL11 is indeed competitive for virion packaging, and the efficiency was the same whether the C-terminal tag was GFP or an epitope from HA (data not shown). Packaging was found to increase proportionally with increased levels of expression (Fig. 4A); however, densitometry measurements revealed that at low levels, the Western blot assay ceased to be linear. Therefore, all of the UL11-GFP derivatives used in this study were titrated to express at high levels and in equal amounts (data not shown). Moreover, antibodies specific for UL11 were not used since alterations in the short (96 amino acids) UL11 sequence seemed likely to eliminate epitopes and adversely affect the assay.
FIG. 4.
UL11 packaging assay. (A) Vero cells transfected with wild-type UL11.GFP were infected with HSV-1 at 18 h posttransfection. After another 22 h, extracellular virions were purified from the medium by centrifugation through a 30% (wt/vol) sucrose cushion. Virion and infected-cell lysates were separated by SDS-PAGE in 12% gels and analyzed on Western blots using either anti-GFP serum (to detect UL11-GFP chimeras) or anti-VP16 serum (as a harvesting and loading control). (B) Packaging of a UL11 truncation mutant. Vero cells were transfected with the indicated UL11-GFP constructs or GFP alone and subsequently infected with HSV-1. The cell lysate and medium were then harvested and analyzed as described for panel A. (C) Packaging efficiency. Using densitometry, packaging efficiency was quantitated by dividing the amount of UL11-GFP protein in the medium (normalized for VP16) by the amount in the cell lysate (normalized for VP16). In each experiment, the wt UL11-GFP construct was set at 100% packaging efficiency. Shown is the average of four independent experiments.
Subcellular localization of UL11-GFP constructs in transfected/infected cells.
The eight constructs used in this study were wt UL11, UL11.Δ51-96, Myr(−).UL11, UL11.CCC(−), UL11.AC(−), UL11.nefAC, UL11.furAC, and UL11.L18A/I19A (Fig. 1). Because these were previously characterized in cells that expressed no other viral proteins (24), it was important to ascertain whether their subcellular locations are altered in the context of an infection. To address this, transfected Vero cells were infected with HSV-1 and subsequently visualized by confocal microscopy. For the most part, all of the constructs exhibited the expected patterns (Fig. 5) (data not shown). For instance, wt UL11, UL11.Δ51-96, UL11.AC(−), UL11.nefAC, UL11.furAC, and UL11.L18A/I19A localized strongly to a perinuclear location previously identified as Golgi apparatus related (24), whereas Myr(−).UL11 and UL11.CCC(−) failed to accumulate in this region of the cell. In addition, the UL11.AC(−) and UL11.L18A/I19A constructs accumulated on the plasma membrane (Fig. 5), presumably due to their inability to be efficiently retrieved from the cell surface during plasma membrane-to-Golgi apparatus trafficking (24). The only difference found by microscopy was the appearance of wild-type UL11-GFP on the nuclear membranes of infected but not transfected cells (Fig. 5) (data not shown). This nuclear targeting, which has been observed previously by means of immunofluorescence in fixed cells (1), was abolished by removing either the acidic cluster or dileucine motif, suggesting that these sequences provide UL11 with nuclear targeting specificity in Vero cells, presumably through interactions with other viral proteins.
FIG. 5.
Localization of UL11-GFP derivatives in HSV-1-infected cells. Vero cells transfected with the indicated constructs were infected with HSV-1 at 18 h posttransfection, and after an additional 15 to 20 h, the cells were visualized by confocal microscopy.
Packaging of UL11 mutants.
The C-terminal half of UL11 has been found to be dispensable for all known properties of the protein, including membrane binding, intracellular trafficking, and interaction with UL16 (7, 24, 25). To determine whether it is also dispensable for virion incorporation, the N-terminal half of UL11 was fused to GFP (UL11.Δ51-96) and examined in the packaging assay. Unlike GFP, which was not detected within extracellular virions, UL11.Δ51-96 was packaged to almost the same level as wt UL11 (Fig. 4B and C). This finding suggests that all information required for packaging is contained within the first half of the protein. The small reduction observed with UL11.Δ51-96 is likely the result of having GFP positioned much closer to the packaging signals, but this hypothesis was not tested further.
The N-terminal half of UL11 contains two sites that are modified with fatty acids, and these work together to enable binding to Golgi apparatus-derived membranes. Cotranslational addition of myristate enables weak but nonspecific binding to membranes, while the subsequent addition of palmitate (following the initial membrane-binding event) enables accumulation in a perinuclear compartment (24). While it seemed likely that proper targeting would be needed for packaging, it was possible that interactions with another viral protein (e.g., UL16) would enable UL11 to be packaged in a membrane-independent manner. To address this, mutants defective for one or both modifications [UL11.CCC(−) and Myr(−).UL11, respectively] were tested, and these failed to be packaged in an efficient manner (Fig. 6A and B). Although this suggests that UL11 must be on Golgi apparatus-derived membranes to be packaged efficiently, a small but reproducible amount of Myr(−).UL11 was incorporated at a level above that seen with GFP.
FIG. 6.
Analysis of membrane-binding mutants. (A) Packaging was measured as described in the legend to Fig. 4B. (B) Packaging efficiency was calculated as described in the legend to Fig. 4C. Shown is the average of three independent experiments.
The N-terminal half of UL11 also contains acidic cluster and dileucine-like (LI) motifs (Fig. 1), both of which are required for plasma membrane-to-Golgi apparatus trafficking and the interaction with UL16 (7, 24, 25). Nevertheless, mutants lacking either of these signals [UL11.AC(−) and UL11.L18A/I19A, respectively] still accumulate at the perinuclear compartment in transfected (24, 25) and transfected/infected cells (Fig. 5). Thus, if localization to this compartment is sufficient to enable passive packaging of UL11, then constructs lacking either motif should still be packaged like wt UL11-GFP. This was not found to be the case. The LI mutant actually exhibited enhanced packaging in most experiments, while the acidic cluster mutant was highly defective (Fig. 7A and B). Packaging was restored by inserting the well-characterized acidic clusters from furin (57) or human immunodeficiency virus type 1 (HIV-1) Nef (43) in place of the native motif (mutants UL11.furAC and UL11.nefAC, respectively), thus providing gain-of-function evidence for a role of these motifs in packaging. The ability of UL11.furAC, UL11.nefAC, and UL11.L18A/I19A to be packaged efficiently despite their inability to bind to UL16 (25) suggests that interactions between these two tegument proteins are not required for packaging.
FIG. 7.
Analysis of membrane-trafficking mutants. (A) Packaging was measured as described in the legend to Fig. 4B. (B) Packaging efficiency was calculated as described in the legend to Fig. 4C. Shown is the average of three independent experiments.
DISCUSSION
The experiments described here address the packaging mechanism for the UL11 protein, a component of the tegument that is thought to play a role in virus budding. Because of overlap with the upstream UL12 gene, which encodes the essential alkaline nuclease, the construction and characterization of mutant viruses were not feasible, and instead, a transfection/infection assay was used in which variants of UL11 must compete with the virus-encoded, wild-type protein.
Limitations of the transfection/infection assay.
The mixed population of UL11 species inherent in the assay raises at least three unanswered questions. First, does wild-type UL11 assist in the packaging of any of the trans-expressed mutants or is it truly just a competitor? So far, no evidence for interactions among UL11 molecules has been observed in GST-UL11 pull-down experiments (25) or in yeast two-hybrid assays (56), but a multimerization domain could await discovery. Second, does trans-expressed UL11-GFP reduce the packaging of virus-encoded UL11 (i.e., is there a maximum of ∼700 molecules of UL11 permitted per virion)? This is a difficult question to answer because most virions produced in the assay come from untransfected Vero cells, and these create a large background that obscures the analysis of the small virion population that contains UL11-GFP. One way to address this would be to isolate and infect cell lines that express different levels of UL11-GFP. Third, do any of the mutants have a dominant-negative effect on virus assembly and release? Again, the large background of virions produced by untransfected cells makes this question difficult to address, but it needs to be kept in mind for those mutants that exhibit reduced levels of packaging. In spite of these uncertainties, the assay has provided much information regarding the mechanism of UL11 packaging.
Requirements for UL11 packaging.
All of the information required for packaging is present within the first half of UL11. This region contains determinants for membrane binding, intracellular trafficking, and interaction with UL16 (7, 24, 25). Whether the second half of the protein is entirely dispensable for infectivity remains to be seen. Previously constructed “null” mutants still express the first ∼30% of the protein and exhibit a 1,000-fold reduction in the release of extracellular virions (2, 27). Thus, it will be of interest to learn whether infectivity is normal when only the second half of the gene is missing and is further reduced when a true null mutant is created.
Multiple sequence motifs within the first half of UL11 were found to have functions in packaging. N-terminal myristylation is required for membrane binding (7), and not surprisingly, this modification was also found to be critically important for packaging. Nevertheless, a small, yet reproducible, fraction of Myr(−).UL11 was found in extracellular virions. This is likely the result of an interaction with other viral components (e.g., capsid-associated UL16), rather than a nonspecific incorporation resulting from overexpression, because GFP-only molecules were not packaged when produced at the same level.
Palmitylation was also found to be important for UL11 packaging. This modification adds a 16-carbon, saturated fatty acid to cysteine residues, usually near the N or C termini of target proteins (45). Similar to various cellular and viral proteins, including UL51 of HSV-1 (40), palmitylation of UL11 is required for proper targeting to Golgi apparatus-derived membranes (24). Thus, the reduced packaging of UL11.CCC(−) is most likely the result of not accumulating at the site of virus budding. Interestingly, palmitylation has also been found to be important for the targeting of many cellular (e.g., CD4, G proteins, and Src family kinases) and viral (e.g., HIV-1 Gag) proteins to detergent-resistant, lipid-raft domains within various membrane compartments (4, 17, 30, 34, 59). In addition to serving as sites for signal transduction events, these locations are thought to function as platforms for virus assembly and budding (12). Although HSV-1 has not been reported to assemble on detergent-resistant, lipid-raft domains, it is possible that palmitylation enables UL11 to target specifically to raft domains at, or en route to, the site of budding. Studies are under way to determine whether or not UL11 associates with rafts when expressed by itself or with other virion components.
The most striking findings from this study were seen when the membrane-trafficking motifs in the first half of UL11 were altered. In particular, packaging failed with the acidic cluster deletion mutant but was enhanced with the LI mutant. These results are particularly interesting because both mutants accumulate at a perinuclear location that resembles that for the wild type. Although it is possible that the acidic cluster mutant has a structural defect that merely precludes packaging, the ability of foreign acidic clusters of different sequences (furAC) and/or lengths (nefAC) to restore virion incorporation argues against that hypothesis. Moreover, loss of the interaction with UL16 does not explain the phenotype because constructs that have foreign acidic clusters or lack the LI motif are readily packaged even though they also are unable to participate in this interaction (24, 25).
Because acidic clusters and LI motifs are known to be involved in membrane trafficking (6, 21, 47, 58), it is possible is that UL11 must traverse a particular pathway, perhaps by way of the plasma membrane, as a prerequisite to packaging. This pathway may enable the formation of essential protein-protein interactions or posttranslational modifications. In this model, UL11 has two available pathways it can enter, depending upon which trafficking signal is recognized by the sorting machinery. Recognition of the acidic cluster would lead to virion packaging, while recognition of the LI motif would serve another purpose. Hence, loss of the acidic cluster would block packaging and loss of the LI would enhance it by eliminating the competing pathway.
Precedence for dual-pathway trafficking is provided by the HIV-1 Nef protein, which has several similarities to UL11. Nef is small (205 residues), is peripherally bound to membranes via myristate (but not palmitate), has dileucine and acidic cluster motifs for trafficking between the trans-Golgi network and the plasma membrane, and has an affinity for lipid rafts (15). Unlike UL11, it is quite clear why Nef travels to the plasma membrane: to downregulate cellular surface proteins, most notably CD4 (the receptor for HIV-1) and major histocompatibility complex class I (MHC-I [needed for immune surveillance]). The mechanisms by which these two tasks are accomplished are different (15). After binding CD4, the dileucine motif of Nef links the complex to clathrin-mediated endocytic machinery (18, 19, 46), thereby sending the proteins down a pathway for degradation in lysosomes. In contrast, after binding MHC-I, the acidic cluster is recognized by PACS-1, a protein that in turn links the complex to clathrin adaptors and results in downregulation to the trans-Golgi network (5, 13, 43, 58). With this in mind, it is easy to imagine that UL11 might have a role in downregulating viral or host proteins from the cell surface, with one pathway leading to the site of maturation budding.
Although the experiments described here represent the first packaging analyses for a UL11 homologue, the importance of endocytosis for the packaging of other herpesvirus proteins has been considered in a variety of studies. Some of these have shown that endocytosed proteins can be packaged, but this route is not essential. A few examples of this are provided by HSV-1 gB (14) and pseudorabies virus gE and Us9 (8, 9). However, there are other examples of viral proteins for which endocytosis appears to be required for packaging. Examples of this are gE from varicella-zoster virus (28, 33) and gB from human cytomegalovirus, for which an acidic cluster motif is required (44, 55). Further studies of the trafficking pathways used by herpesvirus proteins are needed to provide mechanistic explanations for these differences.
Phosphorylation states of UL11.
Although the proteins of the tegument generally exist in multiple phosphorylated forms, evidence provided here and elsewhere shows that only the underphosphorylated species are found in purified virions (16, 20, 31). Combined with data showing that phosphorylation promotes dissociation of tegument proteins following entry, a model has been proposed in which HSV-1 utilizes phosphorylation as a reversible mechanism to assemble and dissociate the tegument at different stages of viral replication (35, 36).
The acidic cluster of UL11 (Fig. 1) contains the major site of phosphorylation (24), and it is possible that use of this serine would be inhibitory for packaging. Indeed, the ability of the nefAC chimera to be packaged even though it lacks this residue is consistent with the proposed model. However, at least one other site of phosphorylation must exist in UL11 (24), and this might be the controlling residue. Alternatively, it is possible that fully phosphorylated forms are not actually excluded from packaging but instead are recognized by a phosphatase within the virion. Once the other site or sites of phosphorylation are identified in UL11, it will be interesting to see whether substitutions that mimic constitutive phosphorylation (e.g., serine-to-glutamic acid changes) will interfere with packaging or not.
Acknowledgments
Special thanks are extended to our colleagues Eric Callahan, Michael Brignati, and Nicholas Baird for helpful discussions.
This work was supported by NIH grants to J.W.W. (CA47482) and R.J.C. (CA42460). J.S.L. was supported by NIH training grant CA60395.
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Baines, J. D., R. J. Jacob, L. Simmerman, and B. Roizman. 1995. The herpes simplex virus 1 UL11 proteins are associated with cytoplasmic and nuclear membranes and with nuclear bodies of infected cells. J. Virol. 69:825-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baines, J. D., and B. Roizman. 1992. The UL11 gene of herpes simplex virus 1 encodes a function that facilitates nucleocapsid envelopment and egress from cells. J. Virol. 66:5168-5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baines, J. D., P. L. Ward, G. Campadelli-Fiume, and B. Roizman. 1991. The UL20 gene of herpes simplex virus 1 encodes a function necessary for viral egress. J. Virol. 65:6414-6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya, J., A. Repik, and P. R. Clapham. 2006. Gag regulates association of human immunodeficiency virus type 1 envelope with detergent-resistant membranes. J. Virol. 80:5292-5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C. H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853-866. [DOI] [PubMed] [Google Scholar]
- 6.Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. [DOI] [PubMed] [Google Scholar]
- 7.Bowzard, J. B., R. J. Visalli, C. B. Wilson, J. S. Loomis, E. M. Callahan, R. J. Courtney, and J. W. Wills. 2000. Membrane targeting properties of a herpesvirus tegument protein-retrovirus Gag chimera. J. Virol. 74:8692-8699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brideau, A. D., T. Del Rio, E. J. Wolffe, and L. W. Enquist. 1999. Intracellular trafficking and localization of the pseudorabies virus Us9 type II envelope protein to host and viral membranes. J. Virol. 73:4372-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brideau, A. D., L. W. Enquist, and R. S. Tirabassi. 2000. The role of virion membrane protein endocytosis in the herpesvirus life cycle. J. Clin. Virol. 17:69-82. [DOI] [PubMed] [Google Scholar]
- 10.Britt, W. J., M. Jarvis, J.-Y. Seo, D. Drummond, and J. Nelson. 2004. Rapid genetic engineering of human cytomegalovirus by using a lambda phage linear recombination system: demonstration that pp28 (UL99) is essential for production of infectious virus. J. Virol. 78:539-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buser, C. A., C. T. Sigal, M. D. Resh, and S. McLaughlin. 1994. Membrane binding of myristylated peptides corresponding to the NH2 terminus of Src. Biochemistry 33:13093-13101. [DOI] [PubMed] [Google Scholar]
- 12.Chazal, N., and D. Gerlier. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67:226-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crump, C. M., Y. Xiang, L. Thomas, F. Gu, C. Austin, S. A. Tooze, and G. Thomas. 2001. PACS-1 binding to adaptors is required for acidic cluster motif-mediated protein traffic. EMBO J. 20:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Zarate, I. B. O., K. Kaelin, and F. Rozenberg. 2004. Effects of mutations in the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B on intracellular transport and infectivity. J. Virol. 78:1540-1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 16.Elliott, G., D. O'Reilly, and P. O'Hare. 1996. Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology 226:140-145. [DOI] [PubMed] [Google Scholar]
- 17.Fragoso, R., D. Ren, X. Zhang, M. W. Su, S. J. Burakoff, and Y. J. Jin. 2003. Lipid raft distribution of CD4 depends on its palmitoylation and association with Lck, and evidence for CD4-induced lipid raft aggregation as an additional mechanism to enhance CD3 signaling. J. Immunol. 170:913-921. [DOI] [PubMed] [Google Scholar]
- 18.Janvier, K., Y. Kato, M. Boehm, J. R. Rose, J. A. Martina, B. Y. Kim, S. Venkatesan, and J. S. Bonifacino. 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 gamma-sigma1 and AP-3 delta-sigma3 hemicomplexes. J. Cell Biol. 163:1281-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jin, Y. J., C. Y. Cai, X. Zhang, H. T. Zhang, J. A. Hirst, and S. J. Burakoff. 2005. HIV Nef-mediated CD4 down-regulation is adaptor protein complex 2 dependent. J. Immunol. 175:3157-3164. [DOI] [PubMed] [Google Scholar]
- 20.Jones, T. R., and S.-W. Lee. 2004. An acidic cluster of human cytomegalovirus UL99 tegument protein is required for trafficking and function. J. Virol. 78:1488-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchhausen, T. 2002. Single-handed recognition of a sorting traffic motif by the GGA proteins. Nat. Struct. Biol. 9:241-244. [DOI] [PubMed] [Google Scholar]
- 22.Kopp, M., H. Granzow, W. Fuchs, B. Klupp, and T. C. Mettenleiter. 2004. Simultaneous deletion of pseudorabies virus tegument protein UL11 and glycoprotein M severely impairs secondary envelopment. J. Virol. 78:3024-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopp, M., H. Granzow, W. Fuchs, B. G. Klupp, E. Mundt, A. Karger, and T. C. Mettenleiter. 2003. The pseudorabies virus UL11 protein is a virion component involved in secondary envelopment in the cytoplasm. J. Virol. 77:5339-5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loomis, J. S., J. B. Bowzard, R. J. Courtney, and J. W. Wills. 2001. Intracellular trafficking of the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 75:12209-12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomis, J. S., R. J. Courtney, and J. W. Wills. 2003. Binding partners for the UL11 tegument protein of herpes simplex virus type 1. J. Virol. 77:11417-11424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacLean, C. A., B. Clark, and D. J. McGeoch. 1989. Gene UL11 of herpes simplex virus type 1 encodes a virion protein which is myristylated. J. Gen. Virol. 70:3147-3157. [DOI] [PubMed] [Google Scholar]
- 27.MacLean, C. A., A. Dolan, F. E. Jamieson, and D. J. McGeoch. 1992. The myristylated virion proteins of herpes simplex virus type 1: investigation of their role in the virus life cycle. J. Gen. Virol. 73:539-547. [DOI] [PubMed] [Google Scholar]
- 28.Maresova, L., T. J. Pasieka, E. Homan, E. Gerday, and C. Grose. 2005. Incorporation of three endocytosed varicella-zoster virus glycoproteins, gE, gH, and gB, into the virion envelope. J. Virol. 79:997-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLaughlin, S., and A. Aderem. 1995. The myristoyl-electrostatic switch: a modulator of reversible protein-membrane interactions. Trends Biochem. Sci. 20:272-276. [DOI] [PubMed] [Google Scholar]
- 30.Melkonian, K. A., A. G. Ostermeyer, J. Z. Chen, M. G. Roth, and D. A. Brown. 1999. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J. Biol. Chem. 274:3910-3917. [DOI] [PubMed] [Google Scholar]
- 31.Meredith, D. M., J. A. Lindsay, I. W. Halliburton, and G. R. Whittaker. 1991. Post-translational modification of the tegument proteins (VP13 and VP14) of herpes simplex virus type 1 by glycosylation and phosphorylation. J. Gen. Virol. 72:2771-2775. [DOI] [PubMed] [Google Scholar]
- 32.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 33.Moffat, J., C. Mo, J. J. Cheng, M. Sommer, L. Zerboni, S. Stamatis, and A. M. Arvin. 2004. Functions of the C-terminal domain of varicella-zoster virus glycoprotein E in viral replication in vitro and skin and T-cell tropism in vivo. J. Virol. 78:12406-12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moffett, S., D. A. Brown, and M. E. Linder. 2000. Lipid-dependent targeting of G proteins into rafts. J. Biol. Chem. 275:2191-2198. [DOI] [PubMed] [Google Scholar]
- 35.Morrison, E. E., A. J. Stevenson, Y. F. Wang, and D. M. Meredith. 1998. Differences in the intracellular localization and fate of herpes simplex virus tegument proteins early in the infection of Vero cells. J. Gen. Virol. 79:2517-2528. [DOI] [PubMed] [Google Scholar]
- 36.Morrison, E. E., Y.-F. Wang, and D. M. Meredith. 1998. Phosphorylation of structural components promotes dissociation of the herpes simplex virus type 1 tegument. J. Virol. 72:7108-7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nalwanga, D., S. Rempel, B. Roizman, and J. D. Baines. 1996. The UL16 gene product of herpes simplex virus 1 is a virion protein that colocalizes with intranuclear capsid proteins. Virology 226:236-242. [DOI] [PubMed] [Google Scholar]
- 38.Newcomb, W. W., J. C. Brown, F. P. Booy, and A. C. Steven. 1989. Nucleocapsid mass and capsomer protein stoichiometry in equine herpesvirus 1: scanning transmission electron microscopic study. J. Virol. 63:3777-3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Newcomb, W. W., B. L. Trus, F. P. Booy, A. C. Steven, J. S. Wall, and J. C. Brown. 1993. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J. Mol. Biol. 232:499-511. [DOI] [PubMed] [Google Scholar]
- 40.Nozawa, N., T. Daikoku, T. Koshizuka, Y. Yamauchi, T. Yoshikawa, and Y. Nishiyama. 2003. Subcellular localization of herpes simplex virus type 1 UL51 protein and role of palmitoylation in Golgi apparatus targeting. J. Virol. 77:3204-3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oshima, S., T. Daikoku, S. Shibata, H. Yamada, F. Goshima, and Y. Nishiyama. 1998. Characterization of the UL16 gene product of herpes simplex virus type 2. Arch. Virol. 143:863-880. [DOI] [PubMed] [Google Scholar]
- 42.Peitzsch, R. M., and S. McLaughlin. 1993. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry 32:10436-10443. [DOI] [PubMed] [Google Scholar]
- 43.Piguet, V., L. Wan, C. Borel, A. Mangasarian, N. Demaurex, G. Thomas, and D. Trono. 2000. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hubinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 45.Resh, M. D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451:1-16. [DOI] [PubMed] [Google Scholar]
- 46.Rose, J. J., K. Janvier, S. Chandrasekhar, R. P. Sekaly, J. S. Bonifacino, and S. Venkatesan. 2005. CD4 down-regulation by HIV-1 and simian immunodeficiency virus (SIV) Nef proteins involves both internalization and intracellular retention mechanisms. J. Biol. Chem. 280:7413-7426. [DOI] [PubMed] [Google Scholar]
- 47.Schapiro, F. B., T. T. Soe, W. G. Mallet, and F. R. Maxfield. 2004. Role of cytoplasmic domain serines in intracellular trafficking of furin. Mol. Biol. Cell 15:2884-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schimmer, C., and A. Neubauer. 2003. The equine herpesvirus 1 UL11 gene product localizes to the trans-Golgi network and is involved in cell-to-cell spread. Virology 308:23-36. [DOI] [PubMed] [Google Scholar]
- 49.Schrag, J. D., B. V. Prasad, F. J. Rixon, and W. Chiu. 1989. Three-dimensional structure of the HSV1 nucleocapsid. Cell 56:651-660. [DOI] [PubMed] [Google Scholar]
- 50.Shao, L., L. M. Rapp, and S. K. Weller. 1993. Herpes simplex virus 1 alkaline nuclease is required for efficient egress of capsids from the nucleus. Virology 196:146-162. [DOI] [PubMed] [Google Scholar]
- 51.Silva, M. C., J. Schroer, and T. Shenk. 2005. Human cytomegalovirus cell-to-cell spread in the absence of an essential assembly protein. Proc. Natl. Acad. Sci. USA 102:2081-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Silva, M. C., Q.-C. Yu, L. Enquist, and T. Shenk. 2003. Human cytomegalovirus UL99-encoded pp28 is required for the cytoplasmic envelopment of tegument-associated capsids. J. Virol. 77:10594-10605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith, K. O. 1964. Relationship between the envelope and the infectivity of herpes simplex virus. Proc. Soc. Exp. Biol. Med. 115:814-816. [DOI] [PubMed] [Google Scholar]
- 54.Szilagyi, J. F., and C. Cunningham. 1991. Identification and characterization of a novel non-infectious herpes simplex virus-related particle. J. Gen. Virol. 72:661-668. [DOI] [PubMed] [Google Scholar]
- 55.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vittone, V., E. Diefenbach, D. Triffett, M. W. Douglas, A. L. Cunningham, and R. J. Diefenbach. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 79:9566-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voorhees, P., E. Deignan, E. van Donselaar, J. Humphrey, M. S. Marks, P. J. Peters, and J. S. Bonifacino. 1995. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J. 14:4961-4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wan, L., S. S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. L. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205-216. [DOI] [PubMed] [Google Scholar]
- 59.Webb, Y., L. Hermida-Matsumoto, and M. D. Resh. 2000. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 275:261-270. [DOI] [PubMed] [Google Scholar]
- 60.Weller, S. K., M. R. Seghatoleslami, L. Shao, D. Rowse, and E. P. Carmichael. 1990. The herpes simplex virus type 1 alkaline nuclease is not essential for viral DNA synthesis: isolation and characterization of a lacZ insertion mutant. J. Gen. Virol. 71:2941-2952. [DOI] [PubMed] [Google Scholar]
- 61.Wills, J. W., R. C. Craven, and J. A. Achacoso. 1989. Creation and expression of myristylated forms of Rous sarcoma virus Gag protein in mammalian cells. J. Virol. 63:4331-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, Y., and J. L. C. McKnight. 1993. Herpes simplex virus type 1 UL46 and UL47 deletion mutants lack VP11 and VP12 or VP13 and VP14, respectively, and exhibit altered viral thymidine kinase expression. J. Virol. 67:1482-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]