Abstract
Among several proposed cellular receptors for bovine viral diarrhea virus (BVDV), the low-density lipoprotein (LDL) receptor is of special interest because it is also considered a receptor for the related hepatitis C virus. It has been reported that an anti-LDL receptor monoclonal antibody blocked the infection of bovine cells by BVDV and that the resistance of bovine CRIB cells (cells resistant to infection with BVDV) (E. F. Flores and R. O. Donis, Virology 208:565-575, 1995) to BVDV infection was due to a lack of the LDL receptor (V. Agnello et al., Proc. Natl. Acad. Sci. USA 96:12766-12771, 1999). In connection with our studies on BVDV entry, we reevaluated the putative role of the LDL receptor as a cellular receptor for BVDV. It was first clearly demonstrated that neither of two monoclonal antibodies against the LDL receptor inhibited BVDV infection of two bovine cell lines. Furthermore, the LDL receptor was detected on the surface of CRIB cells. The functionality of the LDL receptor on CRIB cells was demonstrated by the internalization of fluorescently labeled LDL. In conclusion, at present no experimental evidence supports an involvement of the LDL receptor in BVDV invasion.
Bovine viral diarrhea viruses (BVDVs) belong to the genus Pestivirus, which comprises small RNA viruses (40 to 60 nm) and, together with members of the genera Flavivirus and Hepacivirus, constitutes the family Flaviviridae (16). The enveloped virion consists of a message sense single-stranded RNA of about 12,300 nucleotides and four structural proteins, which are the capsid protein and the three glycoproteins Erns, E1, and E2 (23). The host range of pestiviruses is restricted to cloven hoofed animals (Artiodactyla, e.g., ruminants and pigs) in vivo as well as in cell culture. Within the group of cloven hoofed animals, crossing of species barriers by BVDV is frequently observed.
The pathway by which pestiviruses attach to and enter their host cells was recently the object of detailed investigations. BVDV infects host cells via clathrin-dependent endocytosis (14, 15). During cell entry, an additional activation step is required, which likely involves disulfide shuffling in the viral envelope proteins (14). Different cellular receptor molecules have been described for BVDV, namely, CD46, heparan sulfate, and the low-density lipoprotein (LDL) receptor. Recently, we reported that bovine CD46 acts as a cellular receptor for various BVDV strains and isolates (13, 19). Virus binding to this molecule was shown to occur via a minimal essential binding platform that is constituted by two short peptides on antiparallel beta strands within complement control protein 1 (13). Expression of CD46 in porcine cells correlated with a 100-fold increase of susceptibility to BVDV. However, expression of CD46 in nonsusceptible human or murine cells did not confer susceptibility to BVDV infection, although BVDV RNA replication is supported by these cells. These findings suggest that a so-far-unknown coreceptor(s) is required for BVDV infection (19).
Heparan sulfate and other glycosaminoglycans were shown to bind a cluster of basic amino acids within the C-terminal domain of the glycoprotein Erns of BVDV strain Pe515 (11, 12). Erns of classical swine fever virus has also been shown to interact with heparan sulfate after tissue culture adaptation (9). A point mutation resulting in a basic amino acid (Arg476) within the C-terminal domain of Erns was reported to account for an increased affinity to heparan sulfate (10).
An important role of the LDL receptor in BVDV entry was suggested by an inhibitory effect of an anti-LDL receptor antibody on infection of bovine turbinate (BT) cells with BVDV (1). In addition, a bovine cell line which is completely resistant to BVDV infection (6) was shown to lack a functional LDL receptor (1).
The LDL receptor was previously described to act as a cellular receptor for the closely related hepatitis C virus (HCV), based on an association between HCV particles and low-density lipoproteins (2, 4). Further evidence came from the promotion of virus binding to LDL receptor-deficient fibroblasts after expression of the recombinant LDL receptor (20). Finally, HCV binding to Vero cells was significantly inhibited by monoclonal antibodies (MAbs) against the LDL receptor (8).
Role of the LDL receptor in BVDV entry into bovine cells.
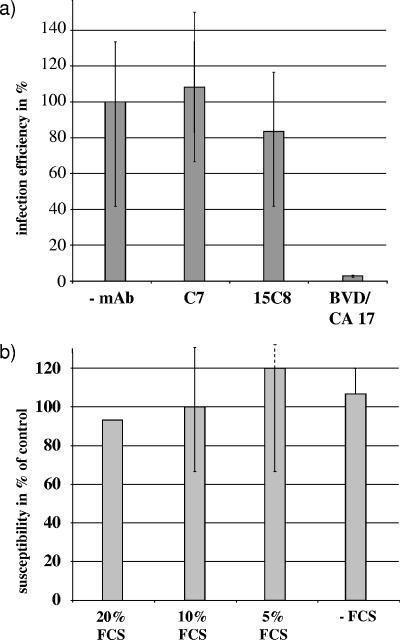
As a first approach to analyze the role of the LDL receptor in BVDV entry, the infection efficiency of BVDV NADL was determined in the absence or presence of two different anti-LDL receptor antibodies; among the latter was clone C7, which was previously reported to inhibit BVDV infection (1). For this purpose, two bovine cell lines were preincubated with 10 μg/ml of different MAbs for 1 h at 4°C. Subsequently, cells were inoculated with a serial dilution of BVDV NADL and washed once with phosphate-buffered saline (PBS) after 1 h at 4°C prior to incubation at 37°C. At 17 h postinfection, the numbers of infected cells were calculated after immunohistochemical detection of viral protein. Preincubation with neither of the anti-LDL receptor MAbs affected infection efficiency on Madin-Darby bovine kidney (MDBK) cells (Fig. 1a) or BT cells (data not shown). This finding contradicts the previously reported observation (1). The discrepancy could be explained by the use of different monoclonal antibodies, different virus strains, or different target cell lines. However, in this study, we used the same monoclonal anti-LDL receptor antibody (clone C7), BVDV strain (NADL), and cell line (BT cells) as those described in the respective study (1).
FIG. 1.
Role of LDL receptor expression in infection of bovine cells by BVDV. (a) Effect of monoclonal antibodies on BVDV plaquing efficiency. MDBK and BT cells were preincubated with 10 μg/ml of different MAbs (anti-LDL receptors C7 and 15C8 and anti-CD46 BVD/CA 17 [21] used as a positive control) for 1 h at 4°C and subsequently inoculated with BVDV NADL. After 1 h at 4°C, cells were washed once with PBS and incubated at 37°C. At 17 h postinfection, cells were fixed and BVDV infection was detected by immunohistochemistry, using an anti-BVDV CP7-5A serum raised in calves (17). The columns represent mean values of triplicate experiments, and bars indicate maximum and minimum values. (b) Effect of LDL receptor upregulation on MDBK cell susceptibility towards BVDV infection. MDBK cells were grown either in serum-free DMEM or in DMEM containing different amounts of FCS for 14 h at 37°C and subsequently inoculated with BVDV NADL. After 1 h at 4°C, cells were washed once with PBS and incubated at 37°C. At 17 h postinfection, cells were fixed and BVDV infection was detected by immunohistochemistry, using MAb 8.12.7 (α-NS3) (5). The number of infected cells preincubated in 10% FCS was set to 100%, and infection efficiency was calculated as a percentage of the control. The columns represent mean values of duplicate experiments, and bars indicate maximum and minimum values.
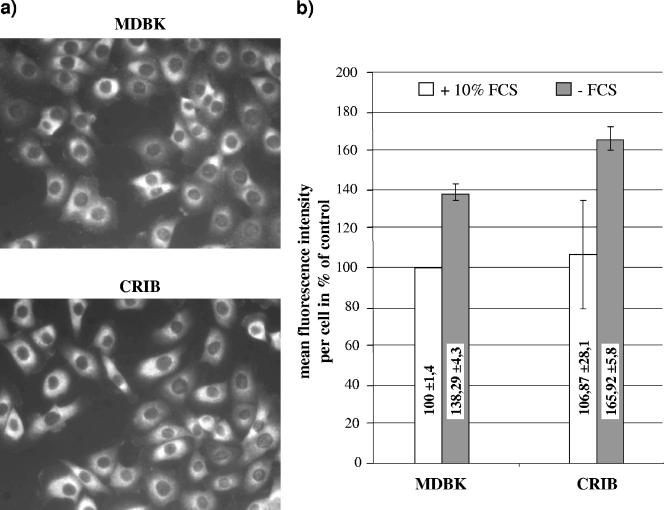
Cell growth in lipoprotein-deficient serum results in upregulation of LDL receptor expression (3). This can also be achieved by depletion of fetal calf serum (FCS) from cell culture medium. To analyze a putative influence of this upregulation on susceptibility towards BVDV infection, MDBK cells were grown in Dulbecco modified Eagle medium (DMEM) containing various amounts of FCS. After 16 h, LDL receptor expression was monitored by flow cytometry, using anti-LDL receptor MAb C7 (Fig. 2b). MDBK cells were detached using PBS containing 5 mM EDTA, pelleted, and fixed with 4% paraformaldehyde for 20 min on ice. Subsequently, the LDL receptor was labeled using anti-LDL receptor MAb clone C7 (30 μg/ml) for 30 min on ice. Afterwards, the cells were washed twice and resuspended with an anti-mouse fluorescein isothiocyanate (FITC) conjugate in PBS for 20 min on ice. The cells were washed twice and analyzed with a FACSCalibur (BD Biosciences, San Jose, CA). Flow cytometric analysis revealed that preincubation with FCS-depleted DMEM elevated the level of LDL receptor expression in MDBK cells by 40% compared to preincubation with DMEM containing 10% FCS.
FIG. 2.
Analysis of LDL receptor expression on CRIB and MDBK cells by (a) indirect immunofluorescence assay and (b) flow cytometry. (a) CRIB and MDBK cells were grown for 16 h at 37°C and fixed with 4% paraformaldehyde in PBS. Antigen was detected by anti-LDL receptor MAb clone C7 (2.5 μg/ml) and an anti-mouse Cy3 conjugate. (b) CRIB and MDBK cells were grown either in serum-free DMEM or in DMEM containing different amounts of FCS for 16 h at 37°C, subsequently detached by being washed in PBS containing 5 mM EDTA, and fixed with 4% paraformaldehyde in PBS. Antigen was detected by anti-LDL receptor MAb clone C7 (30 μg/ml) and an anti-mouse FITC conjugate. A total number of 2,000 events were analyzed with a FACSCalibur. Data analysis was performed using the FCS Express Version 2 software (DeNovo Software). Electronic gates were set according to the negative control included in each test series, defining less than 2% of the cells as positive.
To determine the infection efficiency of BVDV on cells with upregulated LDL receptor expression, MDBK cells preincubated in DMEM containing different amounts of FCS were inoculated with a serial dilution of BVDV NADL. The numbers of infected cells were calculated at 16 h postinfection after immunohistochemical detection of NS3 protein. The differences in infection efficiencies of BVDV NADL on MDBK cells preincubated in DMEM containing different amounts of FCS were negligible, indicating that infection efficiency did not depend on the amount of FCS in the medium and thus upregulation of the LDL receptor (Fig. 1b). Summarizing these data, we conclude that the LDL receptor does not play a pivotal role for BVDV entry into MDBK cells.
Confirmation of CRIB cell phenotype.
Strong evidence for involvement of the LDL receptor in BVDV entry also came from the CRIB cells (cells resistant to infection with BVDV), a cell clone that was derived from MDBK cells (6) and that completely resists infection with any pestivirus (7). It has been reported that the LDL receptor was not detected on CRIB cells by an indirect immunofluorescence assay using anti-LDL receptor monoclonal antibody clone C7; moreover, an LDL uptake assay revealed that fluorescently labeled LDL was not internalized into CRIB cells (1). These results led to the hypothesis that the resistance of CRIB cells to pestivirus infection was due to the lack of the LDL receptor.
The presence and function of the LDL receptor in CRIB cells were reinvestigated. To verify the phenotype of the CRIB cells used in this study (kindly provided by R. O. Donis), MDBK and CRIB cells were infected with serial dilutions of different pestiviruses, including two BVDV-1 strains and the giraffe isolate as well as vesicular stomatitis virus (VSV) and Sindbis virus. Cells were fixed at 17 h postinfection, and the numbers of infected cells were calculated after immunohistochemical detection by using MAb 8.12.7, which recognizes NS3 of all pestiviruses, or polyclonal sera raised against VSV or Sindbis virus (kindly provided by G. Herrler, Hannover, Germany, and G. Wengler, Giessen, Germany). In addition, a plaque assay of both CRIB and MDBK cells was performed with bovine herpesvirus 1 and suid herpesvirus 1. Compared to MDBK cells, CRIB cells were approximately 106-fold less susceptible to the infection with the tested pestiviruses. In contrast, only a minor difference in susceptibility to infection with VSV and Sindbis virus as well as to infection with bovine herpesvirus 1 and suid herpesvirus 1 between CRIB and MDBK cells was observed. The permissiveness of CRIB cells for replication of BVDV RNA was investigated by transfection of a defined amount of in vitro-transcribed BVDV RNA into CRIB and MDBK cells via electroporation (22). Similar numbers of transfected cells were counted after immunohistochemical detection of viral NS3 protein, indicating that both cell lines are equally susceptible to transfection of in vitro-transcribed BVDV RNA and support BVDV RNA replication. The assessment of infectious virus release from transfected CRIB and MDBK cells revealed that similar titers were produced up to 18 h posttransfection, whereas at later times, the yield from CRIB cells was lower than from MDBK cells, apparently due to the lack of a second-cycle infection (data not shown). Taken together, these results confirmed the phenotype of the CRIB cells as described previously (6, 7).
Expression and functionality of LDL receptor in CRIB cells.
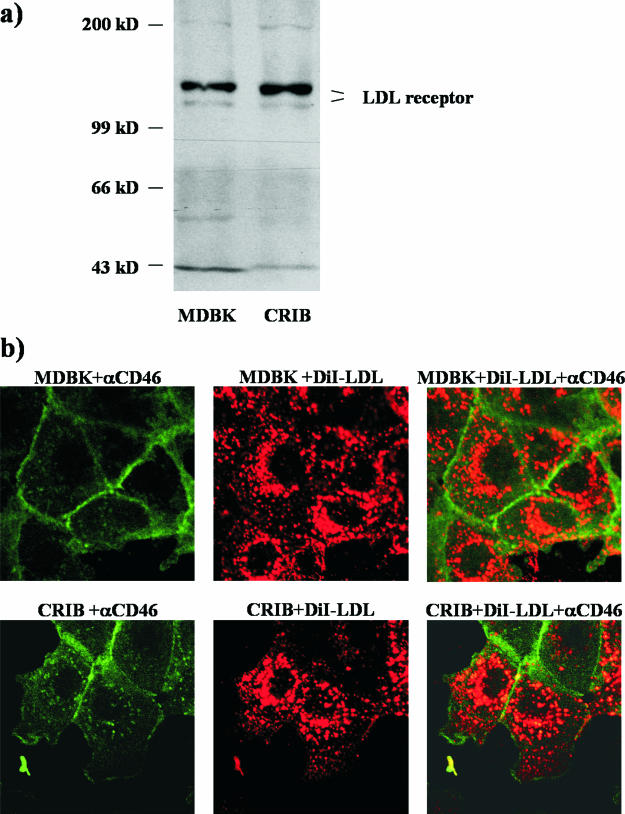
Analysis of LDL receptor expression in CRIB and MDBK cells by indirect immunofluorescence revealed that the LDL receptor was easily detectable on the surface of either cell line by using anti-LDL receptor MAb C7 (Fig. 2a). Neither the distributions nor the signal strengths of the LDL receptor were different between CRIB and MDBK cells, and flow cytometric analysis using anti-LDL receptor MAb C7 revealed similar amounts of LDL receptor expressed at the cell surface of CRIB and MDBK cells (Fig. 2b). To analyze LDL receptor expression at the molecular level, membrane fractions of both cell lines were prepared as described previously (18). Briefly, CRIB and MDBK cells were scraped in homogenization buffer containing 50 mM Tris-HCl, pH 7.4, 50 mM NaCl, 300 mM saccharose, and 1 μl/ml protease inhibitor cocktail, pelleted at 500 × g for 5 min, and resuspended in 10 ml of the same buffer. Cells were homogenized by sonication and then precleared by centrifugation at 800 × g for 10 min. The supernatant was ultracentrifuged at 100,000 × g for 1 h, and the pellet, which contains cellular membranes, was resuspended in 500 μl of the homogenization buffer. Immunoblot analysis revealed that the apparent molecular masses of the LDL receptor molecules from both cell lines were identical and that two bands representing a glycosylated and a nonglycosylated form of the LDL receptor were present in MDBK as well as in CRIB cells (Fig. 3a).
FIG. 3.
The LDL receptor is expressed by CRIB cells and is functional. (a) Membrane fractions of CRIB and MDBK cells were prepared by homogenization and subsequent ultracentrifugation. Membrane fractions of 107 cells were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto nitrocellulose. The blot was probed with anti-LDL receptor MAb 15C8. The two bands correspond to a glycosylated and a nonglycosylated form as described before (18). (b) For CRIB and MDBK cells, medium was replaced by DMEM without serum for 3 h at 37°C. LDL labeled with a fluorescent dye (DiI-LDL) was added to a final concentration of 10 μg/ml for 1 h at 37°C. Cells were washed extensively with PBS and fixed, and as a control, the plasma membrane was stained with a mix of anti-CD46 MAbs, BVD/CA 17, -26, and -27, followed by FITC-conjugated anti-mouse immunoglobulin G. The internalization of DiI-LDL was monitored by confocal microscopy using a Leica DM IRBE microscope.
It has also been described that fluorescently labeled LDL (DiI-LDL; Molecular Probes) was taken up by MDBK but not by CRIB cells (1). This was taken as strong evidence for lack of the LDL receptor on CRIB cells. We reexamined this finding by depleting FCS from the media of MDBK and CRIB cells for 4 h at 37°C to upregulate expression of the LDL receptor. Subsequently DiI-LDL (10 μg/ml) was added for 1 h. Afterwards, cells were fixed with 4% paraformaldehyde in PBS, blocked with PBS containing 0.5% horse serum and 0.5% FCS, and incubated with 1 μg of a mixture of anti-CD46 MAbs followed by FITC-conjugated anti-mouse immunoglobulin G to stain the cell membrane. Cells were analyzed by confocal laser microscopy using a Leica DM IRBE microscope. In both cell lines, fluorescently labeled LDL was taken up and no difference in the intracellular distribution pattern of DiI-LDL in CRIB or MDBK cells was observed (Fig. 3b).
Finally, the influence of LDL receptor upregulation on susceptibility to BVDV infection in CRIB cells was analyzed. CRIB cells were grown in FCS-depleted DMEM as mentioned above for MDBK cells, and upregulation of LDL receptor expression was monitored by flow cytometry as described before. Although deprivation of FCS increased expression of the LDL receptor by 60% (Fig. 2b), CRIB cells did not become susceptible to BVDV infection.
The previously presented evidence that led to the claim of a crucial role of the LDL receptor for BVDV entry (1) included the inhibitory effect of an anti-LDL receptor MAb on the infection of bovine cells with BVDV (1) as well as the observation that resistance of CRIB cells to BVDV infection is due to a lack of the LDL receptor (1). Neither of the two different anti-LDL receptor MAbs inhibited BVDV infection, nor could the resistance of CRIB cells to BVDV infection be attributed to the absence of the LDL receptor. It is evident from these data that the LDL receptor does not play a decisive role in BVDV entry.
We have shown in this study that our CRIB cells phenotypically match those reported previously (6, 7). In contrast, in the study by Agnello et al. (1), no such characterization of CRIB cells has been provided. It is thus conceivable that analysis of a different cell clone may have led to the results presented by Agnello et al. (1).
CRIB cells might turn out to be of outstanding value for the understanding of pestivirus entry, because the defect of this cell clone is clearly localized in the early steps of BVDV invasion (6, 7). Preliminary experiments have shown that CD46 is present at the cell surface of CRIB cells (Fig. 3b). Future studies will aim at the analysis of the detailed role of CD46 and also of heparan sulfate in the resistance of CRIB cells towards BVDV infection. In addition, CRIB cells might facilitate the identification of the putative coreceptor and lead to the elucidation of its role in the pestiviral invasion process.
Acknowledgments
This work was funded by the Deutsche Forschungsgemeinschaft, SFB 535-A18.
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Agnello, V., G. Abel, M. Elfahal, G. B. Knight, and Q. X. Zhang. 1999. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartosch, B., and F. L. Cosset. 2006. Cell entry of hepatitis C virus. Virology 348:1-12. [DOI] [PubMed] [Google Scholar]
- 3.Brown, M. S., and J. L. Goldstein. 1986. A receptor-mediated pathway for cholesterol homeostasis. Science 232:34-47. [DOI] [PubMed] [Google Scholar]
- 4.Cocquerel, L., C. Voisset, and J. Dubuisson. 2006. Hepatitis C virus entry: potential receptors and their biological functions. J. Gen. Virol. 87:1075-1084. [DOI] [PubMed] [Google Scholar]
- 5.Corapi, W. V., R. O. Donis, and E. J. Dubovi. 1990. Characterization of a panel of monoclonal antibodies and their use in the study of the antigenic diversity of bovine viral diarrhea virus. Am. J. Vet. Res. 51:1388-1394. [PubMed] [Google Scholar]
- 6.Flores, E. F., and R. O. Donis. 1995. Isolation of a mutant MDBK cell line resistant to bovine viral diarrhea virus infection due to a block in viral entry. Virology 208:565-575. [DOI] [PubMed] [Google Scholar]
- 7.Flores, E. F., L. C. Kreutz, and R. O. Donis. 1996. Swine and ruminant pestiviruses require the same cellular factor to enter bovine cells. J. Gen. Virol. 77:1295-1303. [DOI] [PubMed] [Google Scholar]
- 8.Germi, R., J. M. Crance, D. Garin, J. Guimet, H. Lortat-Jacob, R. W. Ruigrok, J. P. Zarski, and E. Drouet. 2002. Cellular glycosaminoglycans and low density lipoprotein receptor are involved in hepatitis C virus adsorption. J. Med. Virol. 68:206-215. [DOI] [PubMed] [Google Scholar]
- 9.Hulst, M. M., H. G. P. van Gennip, and R. J. M. Moormann. 2000. Passage of classical swine fever virus in cultured swine kidney cells selects virus variants that bind to heparan sulfate due to a single amino acid change in envelope protein Erns. J. Virol. 74:9553-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hulst, M. M., H. G. P. van Gennip, A. C. Vlot, E. Schooten, A. J. de Smit, and R. J. M. Moormann. 2001. Interaction of classical swine fever virus with membrane-associated heparan sulfate: role for virus replication in vivo and virulence. J. Virol. 75:9585-9595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal, M., H. Flick-Smith, and J. W. McCauley. 2000. Interactions of bovine viral diarrhoea virus glycoprotein E(rns) with cell surface glycosaminoglycans. J. Gen. Virol. 81:451-459. [DOI] [PubMed] [Google Scholar]
- 12.Iqbal, M., and J. W. McCauley. 2002. Identification of the glycosaminoglycan-binding site on the glycoprotein E(rns) of bovine viral diarrhoea virus by site-directed mutagenesis. J. Gen. Virol. 83:2153-2159. [DOI] [PubMed] [Google Scholar]
- 13.Krey, T., A. Himmelreich, M. Heimann, C. Menge, H.-J. Thiel, K. Maurer, and T. Rümenapf. 2006. Function of bovine CD46 as a cellular receptor for bovine viral diarrhea virus is determined by complement control protein 1. J. Virol. 80:3912-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krey, T., H.-J. Thiel, and T. Rümenapf. 2005. Acid-resistant bovine pestivirus requires activation for pH-triggered fusion during entry. J. Virol. 79:4191-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lecot, S., S. Belouzard, J. Dubuisson, and Y. Rouille. 2005. Bovine viral diarrhea virus entry is dependent on clathrin-mediated endocytosis. J. Virol. 79:10826-10829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindenbach, B. D., and C. M. Rice. 2000. Flaviviridae: the viruses and their replication, p. 991-1042. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 4th ed., vol. 1. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 17.Makoschey, B., P. Becher, M. G. Janssen, M. Orlich, H.-J. Thiel, and D. Lutticken. 2004. Bovine viral diarrhea virus with deletions in the 5′-nontranslated region: reduction of replication in calves and induction of protective immunity. Vaccine 22:3285-3294. [DOI] [PubMed] [Google Scholar]
- 18.Martin, G., A. Pilon, C. Albert, M. Valle, D. W. Hum, J. C. Fruchart, J. Najib, V. Clavey, and B. Staels. 1999. Comparison of expression and regulation of the high-density lipoprotein receptor SR-BI and the low-density lipoprotein receptor in human adrenocortical carcinoma NCI-H295 cells. Eur. J. Biochem. 261:481-491. [DOI] [PubMed] [Google Scholar]
- 19.Maurer, K., T. Krey, V. Moennig, H.-J. Thiel, and T. Rümenapf. 2004. CD46 is a cellular receptor for bovine viral diarrhea virus. J. Virol. 78:1792-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monazahian, M., I. Bohme, S. Bonk, A. Koch, C. Scholz, S. Grethe, and R. Thomssen. 1999. Low density lipoprotein receptor as a candidate receptor for hepatitis C virus. J. Med. Virol. 57:223-229. [DOI] [PubMed] [Google Scholar]
- 21.Schelp, C., I. Greiser-Wilke, G. Wolf, M. Beer, V. Moennig, and B. Liess. 1995. Identification of cell membrane proteins linked to susceptibility to bovine viral diarrhoea virus infection. Arch. Virol. 140:1997-2009. [DOI] [PubMed] [Google Scholar]
- 22.Tautz, N., T. Harada, A. Kaiser, G. Rinck, S. Behrens, and H.-J. Thiel. 1999. Establishment and characterization of cytopathogenic and noncytopathogenic pestivirus replicons. J. Virol. 73:9422-9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiel, H.-J., R. Stark, E. Weiland, T. Rümenapf, and G. Meyers. 1991. Hog cholera virus: molecular composition of virions from a pestivirus. J. Virol. 65:4705-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]