Abstract
Human T-cell leukemia virus type 1 (HTLV-1) is the etiologic agent of adult T-cell leukemia, a disease that is triggered after a long latency period. HTLV-1 is known to spread through cell-to-cell contact. In an attempt to study the events in early stages of HTLV-1 infection, we inoculated uninfected human peripheral blood mononuclear cells and the HTLV-1-producing cell line MT-2 into NOD-SCID, common γ-chain knockout mice (human PBMC-NOG mice). HTLV-1 infection was confirmed with the detection of proviral DNA in recovered samples. Both CD4+ and CD8+ T cells were found to harbor the provirus, although the latter population harbored provirus to a lesser extent. Proviral loads increased with time, and inverse PCR analysis revealed the oligoclonal proliferation of infected cells. Although tax gene transcription was suppressed in human PBMC-NOG mice, it increased after in vitro culture. This is similar to the phenotype of HTLV-1-infected cells isolated from HTLV-1 carriers. Furthermore, the reverse transcriptase inhibitors azidothymidine and tenofovir blocked primary infection in human PBMC-NOG mice. However, when tenofovir was administered 1 week after infection, the proviral loads did not differ from those of untreated mice, indicating that after initial infection, clonal proliferation of infected cells was predominant over de novo infection of previously uninfected cells. In this study, we demonstrated that the human PBMC-NOG mouse model should be a useful tool in studying the early stages of primary HTLV-1 infection.
Human T-cell leukemia virus type 1 (HTLV-1) was the first retrovirus shown to be related to human diseases (21, 44), including adult T-cell leukemia (ATL) (50, 51, 58) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (16, 43). The infectivity of free virions is much lower than that of infected cells: transmission is cell mediated (8). Glucose transporter 1 has been identified as an HTLV-1 receptor (35). After infected cells form virological synapses with uninfected cells, the viral genome is transferred into uninfected cells (23). Hence, a salient feature of HTLV-1 infection is that this virus transmits in a cell-to-cell fashion. After infection, HTLV-1 facilitates cell-to-cell transmission by forcing the proliferation of infected cells via the actions of its accessory genes.
In the early stage of HTLV-1 infection, accessory genes including p12, p30, p13, and HBZ, have been reported to be important for in vivo proliferation of infected cells (3, 5, 22, 47). The gene product p12 plays a critical role by releasing calcium from the endoplasmic reticulum to activate nuclear factor of activated T cell-mediated transcription (2). In addition, p12 enhances lymphocyte-associated antigen-1-mediated cell adhesion, which might facilitate cell-to-cell transmission of HTLV-1 (29), and downmodulates the expression of major histocompatibility complex class I antigens (26). p30 has been reported to suppress viral gene transcription by different mechanisms (41). Other functions of p30 have been also demonstrated, such as the enhancement of the transcription of cellular genes associated with cell proliferation (38, 64). In addition, the tax gene is believed to play a central role in proliferation of infected cells by its pleiotropic actions (14, 17, 63). On the other hand, Tax-expressing cells are rapidly eliminated in vivo, since the Tax protein is a major target antigen of cytotoxic T lymphocytes (CTLs) (4, 27). In ATL cells, Tax expression has been shown to be suppressed by several mechanisms (52), strongly suggesting that the loss of Tax expression might be advantageous at the stage of leukemia (36). These studies reveal that the host immune system plays an important role in limiting the proliferation of infected cells. During the long latency period that spans decades, this immune pressure selects those clones with enough alterations to become malignant, eventually leading to the development of ATL.
In vivo studies of HTLV-1 infection have been carried out mainly by inoculating virus-producing or HTLV-1-immortalized cell lines into different animal species (32). Rabbits proved to be an effective model for HTLV-1 infection (1, 65). In addition, monkeys and rats have been used to analyze the in vivo proliferation of HTLV-1-infected cells (7, 55). Furthermore, immunodeficient mouse strains were also able to engraft some HTLV-1-immortalized cell lines (13, 24). These animal models are useful for studying the infection or testing therapeutic agents. However, the early steps of primary HTLV-1 infection remain uninvestigated due to the lack of in vivo experiments using human lymphocytes.
The NOD-SCID (nonobese diabetic-severe combined immunodeficiency), common γ-chain knockout (NOG) mouse was shown to be an excellent recipient for transplantation of human cells due to multiple immune dysfunctions (9, 25, 60). We report here the primary infection of human lymphocytes in this newly developed mouse strain and characterize the infection by measuring proviral load as well as determining the clonality pattern. Furthermore, we tested whether the existing antiretroviral drugs azidothymidine (AZT) and tenofovir blocked primary infection in this mouse model. This small animal model allows us to better understand the mechanism of HTLV-1 infection.
MATERIALS AND METHODS
Cells.
Peripheral blood mononuclear cells (PBMC) were isolated from healthy blood donors by Ficoll-Paque Plus (Pharmacia, Uppsala, Sweden) density gradient centrifugation. MT-2, an HTLV-1-producing cell line (61), was used as the source of virus in all the experiments. MT-2 cells were treated with 50 μg/ml of mitomycin C (MMC) (Kyowa, Tokyo, Japan) for 30 min at 37°C in RPMI 1640 supplemented with 10% fetal bovine serum and antibiotics and washed four times with culture medium prior to inoculation into mice. PBMC of 14 healthy donors were used in the experiments. For in vitro cytotoxicity assays, PBMC were stimulated with phytohemagglutinin (PHA) (Sigma, St. Louis, Mo.) prior to use.
Mice.
The NOG strain of mice, which was generated by backcross matings of C57BL/6J-γcnull mice and NOD/Shi-SCID mice, is homozygous for the SCID mutation and the interleukin 2Rγ allelic mutation. It was previously reported to present multiple immunological dysfunctions that include the absence of T, B, and NK cells and also impaired activity of dendritic cells (25). Mice were purchased from the Central Institute of Experimental Animals (Kanagawa, Japan) and were maintained in microisolator cages under specific-pathogen-free conditions in the animal facility of the Institute for Virus Research, Kyoto University (Kyoto, Japan). Mice were 6 to 7 weeks old at the time of the human PBMC transfer.
Transplantation of human PBMC in NOG mice and infection with HTLV-1.
A total of 107 human PBMC were injected intraperitoneally into each mouse, producing chimeric mice, which we will refer to as hu-PBMC-NOG mice. Three days later, the mice were inoculated intraperitoneally with MMC-treated MT-2 cells (103 or 104 cells/mouse). Spleens and cells obtained from peritoneal lavage were harvested two or four weeks after injection of MT-2 cells. Human mononuclear cells were isolated by Ficoll-Paque Plus (Pharmacia) density gradient centrifugation prior to analysis. The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of Institute for Virus Research, Kyoto University. In each independent experiment, PBMC from a single donor were used.
Quantification of HTLV-1 proviral load.
Genomic DNA was obtained from the samples by standard proteinase K treatment. To quantify the proviral load, we performed a real-time PCR as we described previously (62). The primers for exon 3 of the HTLV-1 tax gene were 5′-GAAGACTGTTTGCCCACCACC-3′ and 5′-TGAGGGTTGAGTGGAACGGA-3′, and the probe was 5′-CACCCGTCACGCTAACAGCCTGGCAA-3′. Genomic DNA (500 ng) was used for real-time PCR in a 50-μl reaction solution prepared with TaqMan Universal PCR master mix (Applied Biosystems, Foster City, CA). The amplification conditions were 50°C for 2 min, 95°C for 10 min, and then 40 cycles of 15 s at 95°C followed by 60 s at 60°C. All experiments were performed and analyzed using the ABI PRISM 7700 sequence detection system (Applied Biosystems). To measure cell equivalents in the input DNA, the recombination activating gene 1 (RAG-1) coding sequence in each sample was also quantified by real-time PCR. The sequences of the primers for RAG-1 exon 2 detection were 5′-CCCACCTTGGGACTCAGTTCT-3′ and 5′-CACCCGGAACAGCTTAAATTTC-3′, and the probe was 5′-CCCCAGATGAAATTCAGCACCCACATA-3′. Amplification conditions were the same as those for tax. The probes were labeled with fluorescent 6-carboxyfluorescein (reporter) at the 5′ end and fluorescent 6-carboxytetramethylrhodamine (quencher) at the 3′ end. All samples were analyzed in duplicate. The DNA of freshly purified ATL cells, which harbor one copy of the HTLV-1 provirus, was used as positive control, and its proviral load was given the value of 100% when used as point of comparison.
IL-PCR.
In order to study the clonality of HTLV-1 infected cells in hu-PBMC-NOG mice, we performed an inverse long PCR (IL-PCR) (10). Briefly, 1 μg of genomic DNA was first digested with EcoRI (TOYOBO, Osaka, Japan) and then self-ligated with T4 DNA ligase (TOYOBO) overnight at 4°C. Circularized DNA was then linearized with MluI (TOYOBO) to prevent amplification of the proviral sequence itself. The resulting DNA was used as template for IL-PCR, performed with LA Taq HS (Takara Bio Inc., Otsu, Japan). Amplification conditions were as follows: 94°C for 2 min; 40 cycles of 94°C for 30 s and 64°C for 10 min; and a final extension at 72°C for 15 min, using a Robocycler thermal cycler (Stratagene, La Jolla, CA). PCR products were electrophoresed in a 1% agarose gel and were then visualized via ethidium bromide staining.
Flow cytometric analysis.
T-cell subsets of splenocytes were analyzed by flow cytometry (EPICS Coulter-Beckman, Fullerton, CA). Briefly, 106 cells were double stained with anti-human CD4-PC5 (Immunotech, Marseille, France) or anti-human CD8-PC5 (Immunotech) and anti-human CD45RO-fluorescein isothiocyanate (FITC) (Immunotech) or anti-human CD25-R-phycoerythrin (Caltag Laboratories, Burlingame, CA). They were also stained with anti-human CD45-FITC (Immunotech) and anti-mouse CD45-phycoerythrin (Immunotech) to assess the predominance of human cells in the recovered splenocytes. Cells were also stained with anti-human CD3-FITC (Sigma) and anti-human CD19-FITC (BD Biosciences, San Jose, CA).
Purification using magnetic beads.
CD4+ and CD8+ T cells were isolated from 107 whole splenocytes by using BD IMag magnetic beads (BD Biosciences) according to the manufacturer's instructions. Positive selection of these T-cell subpopulations was performed using anti-human CD4- and anti-human CD8-conjugated magnetic particles.
Reverse transcriptase PCR (RT-PCR).
RNA was extracted from splenic cells at the time of sacrifice and after 24 h of in vitro culture by using TRIzol reagent (Invitrogen, Carlsbad, CA) according to manufacturer's instructions. One microgram of total RNA was reverse transcribed by using the RNA LA PCR kit (using avian myeloblastosis virus) version 1.1 (Takara) using random primers. One microliter of cDNA was used as the PCR template. The following primers were used: 5′-CCGGCGCTGCTCTCATCCCGG-3′ and 5′-GGCCGAACATAGTCCCCCAGAG-3′ for tax and 5′-GCAGGGGGGAGCCAAAAGGG-3′ and 5′-TGCCAGCCCCAGCGTCAAAG-3′ for the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene. The amplification conditions were as follows: 95°C for 2 min; 40 cycles of 95°C for 30 s, 62°C for 30 s, and 72°C for 30 s; and a final extension at 72°C for 2 min (for tax); 95°C for 3 min; 22 cycles of 95°C for 20 s, 57°C for 30 s, and 72°C for 1 min; and a final extension at 72°C for 7 min (for the GAPDH gene) in a thermal cycler (ASTEC, Fukuoka, Japan). PCR products were electrophoresed in a 2% agarose gel and visualized via ethidium bromide staining. For real-time PCR, an ABI PRISM 7500 sequence detector (Applied Biosystems) was used. Data were analyzed by a comparative cycle threshold method. The level of tax mRNA in the MT-1 cell line was used as a positive control and was assigned a value of 100 arbitrary units.
Sodium bisulfite treatment of genomic DNA.
Sodium bisulfite treatment was performed as previously described (54). Briefly, 1 μg of genomic DNA was denatured in 0.3 N NaOH at 37°C for 15 min, and 1 μg of salmon sperm DNA was added to each sample to act as a carrier. Sodium bisulfite (pH 5.0) and hydroquinone were added to each sample to final concentrations of 3 M and 0.05 mM, respectively, and the reaction mixture was incubated at 55°C for 16 h. Samples were then desalted using the Wizard DNA cleanup system (Promega, Madison, WI). Finally, samples were desulfonated in 0.3 N NaOH at 37°C for 15 min.
COBRA.
For a combined bisulfite restriction analysis (COBRA) (59), different regions of the HTLV-1 provirus were amplified from sodium bisulfite-treated genomic DNA (54). The nested PCRs were performed using FastStart Taq DNA polymerase (Roche, Mannheim, Germany) under the following conditions: 95°C for 5 min; 40 cycles of 30 s at 95°C, 30 s at each annealing temperature, and 30 s at 72°C; and 2 min at 72°C for a final extension. The sequences of the primers used, and their annealing temperatures are as described previously (54). The PCR products were digested for at least 4 h with TaqI restriction enzyme, which resulted in a single recognition site within each product. The digested PCR products were separated in a 3% Nusieve 3:1 agarose (BMA, Rockland, ME) gel. The intensity of each fragment was determined by using a densitograph (version 4.0; ATTO, Tokyo, Japan).
Treatment with reverse transcriptase inhibitors in mice.
hu-PBMC-NOG mice were inoculated with 103 MMC-treated MT-2 cells 3 days after transfer of human PBMC and were then divided into three groups for treatment, with AZT (240 mg/kg of body weight/day) (Nacalai Tesque, Kyoto, Japan), tenofovir (130 mg/kg/day) (kindly provided by Gilead Sciences Inc., CA), or phosphate-buffered saline (PBS). They were treated immediately after MT-2 inoculation for 12 days and then sacrificed to recover spleens and cells from peritoneal lavage for analysis. Tenofovir and AZT were administered intraperitoneally 2 and 3 times a day, respectively. The control group was injected twice a day with PBS. In another experiment, two groups of mice received treatment with AZT for 7 days or tenofovir for 12 days beginning one week after infection with 104 or 103 MT-2 cells/mouse, respectively. Each independent experiment was performed using the PBMC from a single donor.
MTT assay.
The inhibitory effects of tenofovir and AZT on cell growth were assessed by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay, which is based on the reduction of MTT by metabolically active cells to a blue formazan that can be measured spectrophotometrically. PBMC of three different donors (105 cells/well) were cultured in the presence or absence of the RT inhibitors (serial 10-fold dilutions from 5 mM to 0.05 μM) and 20 U/ml of interleukin 2 (kindly provided by Shionogi & Co., Ltd., Osaka, Japan) in a 96-well plate for three days. Twenty microliters of MTT solution (7.5 mg/ml) was added to each well, and the plate was incubated at 37°C for 5 h. One hundred twenty microliters of the medium was removed and 100 μl of acidified isopropanol containing 4% (vol/vol) of Triton X was added to each well to dissolve the formazan crystals. Viability relative to the untreated control was determined. Drug concentrations which inhibited cell growth by 50% (i.e., 50% cytotoxic concentrations) were also calculated from these data. All assays were performed in quadruplicate.
RESULTS
De novo HTLV-1 infection of human PBMC in NOG mice.
In order to establish an in vivo model for primary HTLV-1 infection of human lymphocytes, we chose NOG mice as recipients because they were proven to engraft human cells with high efficiency (25, 60). We first determined the number of MT-2 cells necessary to achieve infection in this new mouse model. We inoculated human PBMC of two different donors intraperitoneally and, three days later, injected different numbers of MMC-treated MT-2 cells, since HTLV-1 transmits efficiently only in a cell-to-cell fashion (23, 45, 61). Two weeks later, cells were recovered from the peritoneal cavity and the spleen of each mouse and proviral load was determined by real-time PCR (Table 1). A total of 103 MT-2 cells was enough to produce a detectable level of proviral load in both groups of NOG mice. Taking these results into account, we decided to use 103 or 104 MT-2 cells in the following experiments. Another group of mice was inoculated with 106 MT-2 cells and sacrificed 3 weeks later, which led to significantly increased proviral loads (Table 1).
TABLE 1.
Proviral load of mice inoculated with different numbers of MT-2 cellsa
| Donor | No. of MT-2 cells in inoculation | Proviral load (%)
|
|
|---|---|---|---|
| Lavage specimen | Spleen | ||
| A | 102 | 0.0 | 0.0 |
| 103 | 0.3 | 0.0 | |
| 104 | 4.2 | 1.2 | |
| B | 102 | 1.1 | 0.2 |
| 103 | 2.5 | 0.4 | |
| 104 | 0.9 | 2.0 | |
| C | 106 | 83.2 | 26.5 |
| 106 | 97.9 | 71.7 | |
| 106 | 90.4 | 53.4 | |
Proviral loads of cells recovered from the peritoneal cavity and spleens 2 (for donors A and B) or 3 (for donor C) weeks after inoculation of the specified numbers of MT-2 cells are shown for mice initially receiving PBMC of three different human donors.
To check the effects of different donor sources on proviral load, we inoculated PBMC from six healthy donors into NOG mice and found that the proportions of subpopulations in T and B lymphocytes did not influence proviral loads at 2 weeks after inoculation of MT-2 cells, and the proviral loads, even in mice inoculated with cells from the same donor, were variable, especially in cells from lavages (Table 2). Regarding provirus loads in spleen cells, variations were not so remarkable. In the following experiments, we used PBMC from a single donor in each experiment.
TABLE 2.
Phenotypes of donor PBMC and proviral loads of cells recovered from infected hu-PBMC-NOG micea
| Donor | Surface markers of donor PBMC (%)b
|
Proviral load (%)c
|
||||
|---|---|---|---|---|---|---|
| CD3 | CD4 | CD8 | CD19 | Lavage specimen | Spleen | |
| D | 69.7 | 61.8 | 18.7 | 12.2 | 3.7 | 0.6 |
| 34.0 | 1.4 | |||||
| 2.8 | 0.5 | |||||
| E | 84.7 | 53.4 | 33.9 | 3.0 | 0.6 | 0.1 |
| 12.6 | 1.0 | |||||
| 11.6 | 0.8 | |||||
| F | 67.0 | 48.0 | 31.9 | 2.8 | 0.2 | 0.0 |
| 2.7 | 0.2 | |||||
| 0.6 | 0.1 | |||||
| G | 74.9 | 43.9 | 37.7 | 1.3 | 7.4 | 0.2 |
| 2.8 | 0.6 | |||||
| H | 80.0 | 62.8 | 18.0 | 1.2 | 2.4 | 0.2 |
| 0.4 | 0.0 | |||||
| I | ND | ND | ND | ND | 20.5 | 2.5 |
| 0.1 | 0.3 | |||||
PBMC from the indicated donors were transferred into NOG mice, and these were sacrificed 2 weeks after inoculation of 104 MMC-treated MT-2 cells.
The percentage of cells positive for the specified markers before transfer into mice is shown for each donor.
The proviral loads of human cells recovered from peritoneal lavage and spleens of the different mice are shown as percentages, calculated as described in Materials and Methods. ND, not determined.
In order to characterize the primary infection with HTLV-1, we inoculated a group of mice with 104 MT-2 cells after the transfer of PBMC and analyzed them in two groups at 2 and 4 weeks postinfection (p.i.). To assess the proportions of human cells in the studied specimens, we stained recovered cells with anti-mouse-CD45 and anti-human-CD45 antibodies and analyzed them by flow cytometry. Human cells accounted for at least 85% of the recovered splenocytes two weeks after the transfer and reached more than 94% in the group analyzed at 4 weeks p.i. (data not shown). The total number of recovered human lymphocytes was much larger than the number initially inoculated. Two weeks after the transfer of 107 human PBMC, we were able to recover (7.7 ± 3.4) × 107 human cells from the spleen of MT-2-inoculated mice and (8.1 ± 2.7) × 107 human cells from the spleen of the control group. These results demonstrate both migration from the peritoneal cavity to the spleen and in vivo cell expansion. There was no significant difference between the numbers of recovered splenocytes from the MT-2-inoculated and the uninoculated control groups, indicating that the cell proliferation was probably due to xenogeneic stimulation. This suggests that, in the early stages, many cells are stimulated to proliferate in the NOG mouse environment regardless of HTLV-1 infection.
In order to confirm HTLV-1 infection, we amplified a fragment of the HTLV-1 pX region by using PCR, and proviral DNA was detected in the cells recovered from the MT-2-inoculated groups of hu-PBMC-NOG mice (data not shown). These PCR products were not derived from contamination of cellular DNA of MT-2 cells, since a PCR specific for one of HTLV-1 integration sites in MT-2 did not detect the provirus (data not shown). Splenocytes tended to have a lower proviral load than cells recovered from the peritoneal cavity. However, the proviral load in the 4-week group was generally greater than that from the 2-week group, suggesting the continuous proliferation of infected cells and propagation of the virus in this mouse model (Table 3).
TABLE 3.
Proviral load of in vivo infected cellsa
| Mouse | Proviral load (%)
|
|
|---|---|---|
| Lavage specimen | Spleen | |
| 2-wk group | ||
| 2W-1 | 3.7 | 0.6 |
| 2W-2 | 34.0 | 1.4 |
| 2W-3 | 2.8 | 0.5 |
| 4-wk group | ||
| 4W-1 | 33.6 | 14.1 |
| 4W-2 | 48.1 | 12.9 |
The percentages of the proviral load, calculated by comparison with a control DNA as described in Materials and Methods are shown for cells recovered from abdominal lavage and cells isolated from spleens of MT-2-inoculated hu-PBMC-NOG mice.
Significant increase in the memory CD4+ T-cell population after HTLV-1 infection.
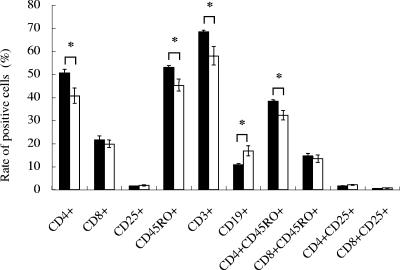
Although HTLV-1 is known to infect many types of cells in vivo (31), the majority of HTLV-1-infected cells are CD4+ memory T cells (46, 62). To determine the effect of HTLV-1 infection on subpopulations of lymphocytes, we studied the expression of surface molecules by flow cytometry. Two weeks after infection, there was a significant increase in the cell population expressing CD4 and CD45RO molecules in the infected group compared to that in the control group (Fig. 1), suggesting that in the infected group of mice, memory CD4+ T cells proliferated. This finding is consistent with observations with HTLV-1 carriers (62). The proviral loads in CD4+ and CD8+ splenic T cells were determined by real-time PCR (Table 4). As previously reported for HTLV-1 carriers, CD8+ T cells were also found to contain the provirus, but to a lesser extent than CD4+ T cells (39, 62). Nevertheless, proviral load tended to increase with time in both subpopulations of T cells (Table 4).
FIG. 1.
Surface marker analysis of splenocytes in hu-PBMC-NOG mice. Splenocytes were isolated from hu-PBMC-NOG mice with or without HTLV-1 infection, and their surface markers were analyzed by flow cytometry. Splenocytes were recovered at 2 weeks p.i. The percentages of cells positive for various surface molecules are shown for MT-2-inoculated hu-PBMC-NOG mice (black bars) and uninfected controls (open bars). Values are means ± standard deviations from groups of three mice. *, P < 0.05 (Student's t test).
TABLE 4.
Proviral load in CD4+ and CD8+ T cellsa
| Mouse | Proviral load (%)
|
|
|---|---|---|
| CD4+ | CD8+ | |
| 2-wk group | ||
| 2W-1 | 0.6 | 0.7 |
| 2W-2 | 4.5 | 1.1 |
| 2W-3 | 1.2 | 0.4 |
| 4-wk group | ||
| 4W-1 | 14.9 | 7.8 |
| 4W-2 | 19.9 | 13.6 |
Human CD4+ and CD8+ T cells were purified from 107 splenocytes of mice sacrificed at 2 or 4 weeks p.i. with the use of magnetic beads. Proviral load was determined by real-time PCR as described in Materials and Methods.
Polyclonal proliferation of HTLV-1-infected cells.
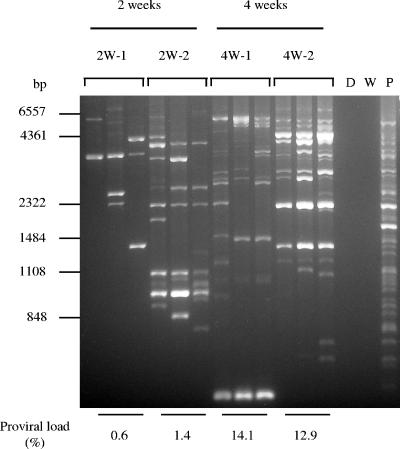
In HTLV-1 carriers, polyclonal proliferation of HTLV-1 infected cells has been detected (10). Therefore, the clonality of HTLV-1-infected cells in hu-PBMC-NOG mice was analyzed by IL-PCR. We analyzed the same DNA samples in triplicate. When the same bands are detected in all three reactions, it means that the number of such clones is high. On the other hand, the stochastic results suggest that these clones are minor in vivo. As shown in Fig. 2, multiple bands were detected by IL-PCR at the 2-week time point, indicating an early polyclonal proliferation. At the 4-week time point, the number of bands increased, as did the intensity of bands corresponding to major clones, suggesting that both the numbers of clones and cell numbers of major clones increased (Fig. 2). We further confirmed the presence of different clones in the same mouse by determining the integration sites of the provirus in the human cells (data not shown).
FIG. 2.
Polyclonal proliferation of HTLV-1-infected cells in the spleens of hu-PBMC-NOG mice (2W-1, 2W-2, 4W-1, and 4W-2). Genomic DNA was isolated from recovered splenocytes and analyzed by IL-PCR as described in Materials and Methods. IL-PCR was performed in triplicate for each DNA sample. Genomic DNA was recovered from splenocytes at 2 or 4 weeks after injection of MT-2 cells. D, DNA of donor PBMC before inoculation; W, water; P, positive control (DNA from PBMC of an HTLV-1 carrier). In addition, proviral load was quantified by real-time PCR as described in Materials and Methods and is shown as a relative percentage.
Profile of proviral DNA methylation in primary HTLV-1 infection.
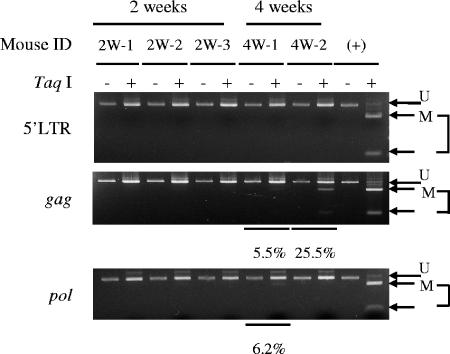
Proviral DNA methylation appears to begin at the internal sequences, such as the gag, pol, and env regions (54), and accumulates in vivo. DNA methylation is thought to disturb viral gene transcription when the 5′ long terminal repeat (LTR) is methylated by inhibiting the binding of transcriptional factors (6). We analyzed the DNA methylation status of the proviral DNA in the cells recovered from the mice (Fig. 3). In the 2-week group, none of the three samples tested presented methylation in the gag, pol, or 5′ LTR regions. However, in the cells recovered from two mice after 4 weeks, the gag regions from both mice were partially methylated, and the pol region from one of the two mice was methylated. These results coincide with our previous findings that CpG motifs within the proviral sequence of HTLV-1 are methylated in a progressive manner, starting from internal regions and then spreading to the 5′ and 3′ ends of the provirus (54).
FIG. 3.
DNA methylation of HTLV-1 provirus. hu-PBMC-NOG mice were sacrificed 2 or 4 weeks after inoculation of MT-2 cells, and DNA methylation in the 5′ LTR, gag, and pol regions was studied by a COBRA assay. (+), positive control; U, intact fragment (unmethylated CpG); M, digested fragments (methylated CpG). Percentages of DNA methylation were calculated by densitography according to the following formula (with the variables as described above): [M/(U + M)] × 100.
Suppression of tax gene transcription in the NOG mouse model.
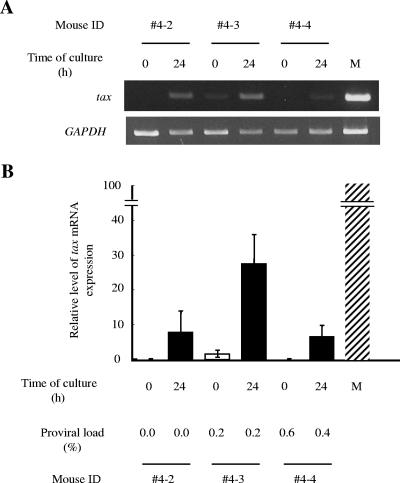
The viral protein Tax is believed to play an important role in the proliferation of infected cells due to its pleiotropic functions (63). However, its expression in vivo has not been detected in most ATL patients (52). When ATL cells are transferred to culture ex vivo, Tax expression can be recovered (21, 30, 57). Viral gene transcription is also suppressed in PBMC of HAM/TSP patients, as well as asymptomatic HTLV-1 carriers (19, 28). We performed an RT-PCR in order to detect tax mRNA in the spleens of infected hu-PBMC-NOG mice sacrificed 2 weeks p.i. (Fig. 4). Transcripts of the tax gene were undetectable in two of the three mice when cells were recovered, while the remaining one showed a low level of expression. In all three cases, there was an increase of tax gene transcription after 24 h of culture in vitro, even without changes in the proviral load (Fig. 4). Since this phenomenon occurs even in hu-PBMC-NOG mice, a factor(s) other than the host immune system must be involved in the suppression of tax gene transcription in vivo.
FIG. 4.
Transcription of the tax gene increases after in vitro culture. Splenocytes of hu-PBMC-NOG mice inoculated with 104 MT-2 cells were recovered 2 weeks after infection. Transcription of the tax gene was quantified by semiquantitative PCR (A) or real-time PCR (B) at recovery and after 24 h of in vitro culture. Proviral loads for the same samples were also measured by real-time PCR. M, MT-1 cells; ID, identification number.
Effect of antiretroviral agents on HTLV-1 infection.
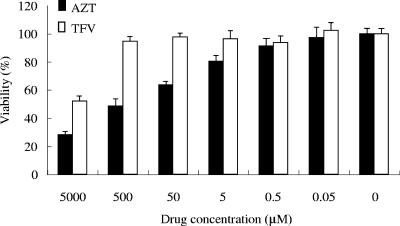
It is well known that HTLV-1 is transmitted through sexual intercourse (49), breast feeding (48), and blood transfusions (42), and for transmission, cell-to-cell contact is thought to be essential. Due to the low capacity of cell-free virus to infect (8, 11), accidental exposures were not thought to confer a high risk of infection, and no prophylactic therapy has been considered. However, the prevalence of HTLV-1 carriers among drug abusers shows that we do need to develop strategies to prevent viral transmission. A previous in vitro study reported that AZT was able to inhibit new HTLV-1 infection of human lymphocytes (37). In addition, it has been reported that tenofovir efficiently inhibited the reverse transcriptase activity of HTLV-1 (20). In order to assess whether a preventive antiretroviral treatment would prove useful in cases of accidental HTLV-1 exposure, we treated hu-PBMC-NOG mice with two reverse transcriptase inhibitors, AZT and tenofovir. The treatment started as soon as MT-2 cells were injected and continued for 12 days. Proviral DNA was undetectable by real-time PCR in the groups of mice treated with AZT or tenofovir (Table 5). Mice seemed to tolerate the treatment without evident signs of toxicity. In the cases where weight loss was seen, it did not exceed 6% of the weight at the time treatment was started (data not shown). However, the number of human cells recovered from spleens of mice receiving AZT treatment was lower than those of the other two groups (Table 5), which indicates that this drug might be also interfering in the proliferation of transferred PBMC. In in vitro assays, we analyzed the cytotoxic effects of AZT and tenofovir on PHA-stimulated human PBMC derived from three different donors. We found that, in a range of concentrations from 5 mM to 0.05 μM, AZT was more toxic than tenofovir when used in incubations for 3 days (Fig. 5). The 50% cytotoxic concentration of AZT was 0.297 ± 0.169 mM, while that of tenofovir was higher than 5 mM. These results indicate that the cytotoxic effect of AZT contributes to suppression of the number of transferred human lymphocytes in our mouse in addition to inhibition of reverse transcriptase.
TABLE 5.
RT inhibitors AZT and tenofovir inhibit de novo infection by HTLV-1a
| Condition or treatment | Mouse | Proviral load (%)
|
Cell count (106) | |
|---|---|---|---|---|
| Lavage specimen | Spleen | |||
| Untreated | C1 | 4.2 | 1.1 | 1.6 |
| C2 | 0.7 | 0.0 | 12.5 | |
| C3 | 0.0 | 0.0 | 19.0 | |
| C4 | 5.9 | 0.6 | 6.0 | |
| C5 | 0.1 | 0.1 | 4.8 | |
| Tenofovir | T1 | 0.0 | 0.0 | 5.2 |
| T2 | 0.0 | 0.0 | 9.2 | |
| T3 | 0.0 | 0.0 | 1.7 | |
| T4 | 0.0 | 0.0 | 8.8 | |
| T5 | 0.0 | 0.0 | 4.0 | |
| AZT | A1 | 0.0 | 0.0 | 2.6 |
| A2 | 0.0 | 0.0 | 3.6 | |
| A3 | 0.0 | 0.0 | 4.5 | |
| A4 | 0.0 | 0.0 | 2.2 | |
| A5 | 0.0 | 0.0 | 2.3 | |
After human PBMC transfer and MT-2 inoculation (103 cells/mouse), mice were immediately subjected to antiretroviral therapy with AZT or tenofovir for 12 days. The control group was injected with PBS instead. Proviral loads were determined in cells recovered from the abdominal cavity and spleens. The total numbers of cells recovered from spleens are also shown.
FIG. 5.
Cytotoxic effects of tenofovir (TFV) and AZT in vitro. Human PBMC were stimulated with PHA for 3 days. Cells were then cultured in medium alone or medium containing the specified concentration of the indicated drug for another three days, at a density of 105 cells/well, in a 96-well plate. Viability was assessed by MTT assay as described in Materials and Methods. The results show the means ± standard deviations of quadruplicate measurements made in one of three representative experiments.
Clonal expansion of infected cells takes place even in the early stages of primary HTLV-1 infection.
It remains undetermined whether clonal proliferation or internal continuous contagion contributes to the increase of HTLV-1-infected cells. To answer this question, hu-PBMC-NOG mice infected with MT-2 cells were treated with tenofovir beginning 1 week after infection. Tenofovir treatment made no significant difference in HTLV-1 proviral load (Table 6), suggesting that clonal proliferation is predominant after HTLV-1 infection. The provirus loads of AZT-treated mice were lower than those of untreated mice, suggesting that the cytotoxic effect of AZT suppressed the provirus loads, as shown in Table 6.
TABLE 6.
Proviral load after treatment with tenofovir or AZT beginning one week after infectiona
| Condition or treatment | Mouse | Proviral load (%)
|
|
|---|---|---|---|
| Lavage specimen | Spleen | ||
| Untreated | U11 | 7.4 | 1.7 |
| U12 | 0.1 | 0.0 | |
| U13 | 15.4 | 1.9 | |
| U14 | 3.8 | 0.7 | |
| Tenofovir | T11 | 0.0 | 0.0 |
| T12 | 0.2 | 0.0 | |
| T13 | 15.9 | 1.8 | |
| T14 | 14.5 | 2.6 | |
| Untreated | U21 | 0.6 | 0.1 |
| U22 | ND | 3.0 | |
| U23 | 12.6 | 1.0 | |
| U24 | 11.6 | 0.8 | |
| AZT | A21 | 0.0 | 0.0 |
| A22 | ND | 0.6 | |
| A23 | 2.5 | 0.0 | |
| A24 | 0.1 | 0.0 | |
After human PBMC transfer and MT-2 inoculation (103 cells/mouse for the tenofovir group, and 104 cells/mouse for the AZT group), mice were left for one week before starting treatment with tenofovir or AZT. The control groups were injected with PBS instead of the drugs. Mice were sacrificed 7 or 12 days after treatment with AZT or tenofovir, respectively. Splenocytes, as well as cells from the abdominal cavity, were recovered for analysis as described in Materials and Methods. ND, not determined.
DISCUSSION
Human immunodeficiency virus type 1 vigorously generates progeny virions through the action of its accessory genes, and the resulting free virions play an important role in its transmission, in addition to cell-to-cell transmission. In contrast, for HTLV-1, the efficiency of transmission by free virions is much lower than that via cell-to-cell contact (8), suggesting that HTLV-1 transmits primarily through the latter mechanism. To facilitate such transmission, instead of producing virions, HTLV-1 increases the number of infected cells by the actions of its accessory genes (17, 63). The finding that mother-to-infant transmission was more frequent in mothers with higher proviral loads indicates that such an increase in the number of infected cells facilitates the transmission of HTLV-1 (33). In vivo studies using animal models show that the early stage of HTLV-1 infection is controlled by accessory genes, including p12, p13, p30, and HBZ genes (3, 5, 22, 47). Thus, although in vivo studies using animal models revealed the importance of accessory genes in replication of HTLV-1 and proliferation of infected cells, the events in the early stages of in vivo transmission in human lymphocytes have not been studied yet due to the lack of an appropriate animal model. Since the metabolisms of nucleosides are quite different among animal species, it is critical to study the effect of reverse transcriptase inhibitors on HTLV-1 in human lymphocytes.
It is widely accepted that the HTLV-1 virion per se is poorly infectious (8, 11) and that cell-to-cell transmission is more efficient both in vivo and in vitro (23, 42, 45, 61). Among drug abusers, HTLV-1 infection has been reported, indicating that HTLV-1 can be transmitted by the sharing of needles (12). Therefore, in cases of accidental exposure to HTLV-1-positive blood, preventive administration of antiretroviral drugs should be considered. In this study, we proved that the administration of a reverse transcriptase inhibitor beginning immediately after exposure can block HTLV-1 transmission. However, a delay in its administration may render it ineffective at preventing HTLV-1 transmission due to the importance of clonal expansion in the biology of this virus.
In particular, whether clonal expansion or internal continuous contagion is important in increasing the number of infected cells still remains unknown. A previous study reported that a reverse transcriptase inhibitor, lamivudine, reduced the proviral load in a patient with HAM/TSP (56), implicating internal contagion in maintaining the number of infected cells in vivo. However, another study reported that lamivudine had no definite effect on proviral load (34). In this study, administering tenofovir to block the spread of infection to new cells did not influence the proviral load in hu-PBMC-NOG mice, even though tenofovir has been reported to be more efficient in inhibiting HTLV-1 replication than lamivudine (20). Taken together, these results suggest that clonal proliferation contributes to the increase of HTLV-1-infected cells more than internal contagion even early in HTLV-1 infection. Recently, one study reported that clonality of HTLV-1-infected cells was variable after seroconversion but it became stable over time, indicating that the host immune system selected certain HTLV-1-infected clones (53). Since there is little or no host immune response to HTLV-1-infected cells in our system, it is possible that clonal proliferation of HTLV-1-infected cells is influenced by their ability to produce HTLV-1-encoded proteins, such as Tax. The factors including integration of the provirus in certain sites of the genome might also contribute to the variable proliferation of infected cells.
Viral gene transcription in HTLV-1-infected cells and ATL cells is suppressed in vivo. However, when they are cultured in vitro, transcription is rapidly recovered (54). Regarding the mechanisms of in vivo suppression, one possibility is that CTLs kill Tax-expressing cells, and the other is that nonimmune factors suppress it. The removal of CD8+ T lymphocytes from PBMC derived from seropositive carriers enhanced Tax expression, suggesting that CTLs were indeed involved in inhibiting Tax expression in vivo (15, 18). On the other hand, a nonimmune factor(s) might be involved in this suppression. In this study, we showed that tax gene transcription was enhanced after in vitro culture. This finding is very similar to the phenomenon in carriers. It is noteworthy that in our system, there is no immune response to HTLV-1, indicating that a nonimmune factor(s) suppresses tax gene expression in vivo. These results suggest that both immune and nonimmune factors may be involved in the silencing of tax gene transcription.
Methylation of proviral DNA is regarded as a kind of host defense mechanism to suppress viral gene expression. However, HTLV-1 utilizes this epigenetic modification to escape the host immune surveillance. In cells immortalized by HTLV-1 in vitro, there was little DNA methylation in the provirus. In humans, on the other hand, DNA methylation accumulated within one year after seroconversion (54). In our system, DNA methylation was detected in the pol and gag regions 4 weeks after inoculation of MT-2 cells, indicating that HTLV-1 provirus is prone to methylation in vivo. Since tax gene transcription is silenced in hu-PBMC-NOG mice as shown in this study, such suppression might promote DNA methylation in vivo. On the other hand, since proliferation of HTLV-1-immortalized T lymphocytes is likely dependent on Tax expression, we speculate that cells with unmethylated provirus have growth advantages. We previously reported that histone H3 was hyperacetylated in the 5′ LTR of ATL cells without tax gene transcription, and such ATL cells transcribed tax gene within one hour after in vitro culture (54). This suggests the presence of a factor(s) inhibitory to tax gene transcription whose inhibition is nullified in in vitro culture. Such a mechanism, with the capacity for quickly switching on and off, would be useful for controlling tax gene transcription in vivo and thus enabling HTLV-1-infected cells to escape the host immune response.
In this study, we established an in vivo system for de novo infection with HTLV-1 and observed that the phenotype of HTLV-1-infected cells resembled that in the carrier state. The limitation of this in vivo system is that the long-term persistence of de novo infection in hu-PBMC-NOG mice cannot be examined, due to the graft-versus-host disease caused by implanted human lymphocytes. On the other hand, its merit is that the severe immune deficiency of this strain allows the vigorous proliferation of human lymphocytes, previously reported to be the result of a hyperactivation of the cells (40), which enables HTLV-1 to rapidly spread by cell-to-cell contact. Therefore, this model system should be a useful tool for analyzing the events in the early stage of HTLV-1 infection in human lymphocytes.
Acknowledgments
We thank Gilead Sciences Inc. for generously providing tenofovir for this study and Linda Kingsbury for excellent proofreading.
This study was supported by a grant-in-aid for scientific research from the Ministry of Education, Science, Sports, and Culture of Japan.
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Akagi, T., I. Takeda, T. Oka, Y. Ohtsuki, S. Yano, and I. Miyoshi. 1985. Experimental infection of rabbits with human T-cell leukemia virus type I. Jpn. J. Cancer Res. 76:86-94. [PubMed] [Google Scholar]
- 2.Albrecht, B., C. D. D'Souza, W. Ding, S. Tridandapani, K. M. Coggeshall, and M. D. Lairmore. 2002. Activation of nuclear factor of activated T cells by human T-lymphotropic virus type 1 accessory protein p12I. J. Virol. 76:3493-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnold, J., B. Yamamoto, M. Li, A. J. Phipps, I. Younis, M. D. Lairmore, and P. L. Green. 2006. Enhancement of infectivity and persistence in vivo by HBZ, a natural antisense coded protein of HTLV-1. Blood 107:3976-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bangham, C. R., and M. Osame. 2005. Cellular immune response to HTLV-1. Oncogene 24:6035-6046. [DOI] [PubMed] [Google Scholar]
- 5.Collins, N. D., G. C. Newbound, B. Albrecht, J. L. Beard, L. Ratner, and M. D. Lairmore. 1998. Selective ablation of human T-cell lymphotropic virus type 1 p12I reduces viral infectivity in vivo. Blood 91:4701-4707. [PubMed] [Google Scholar]
- 6.Datta, S., N. H. Kothari, and H. Fan. 2000. In vivo genomic footprinting of the human T-cell leukemia virus type 1 (HTLV-1) long terminal repeat enhancer sequences in HTLV-1-infected human T-cell lines with different levels of Tax I activity. J. Virol. 74:8277-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Debacq, C., J. M. Heraud, B. Asquith, C. Bangham, F. Merien, V. Moules, F. Mortreux, E. Wattel, A. Burny, R. Kettmann, M. Kazanji, and L. Willems. 2005. Reduced cell turnover in lymphocytic monkeys infected by human T-lymphotropic virus type 1. Oncogene 24:7514-7523. [DOI] [PubMed] [Google Scholar]
- 8.Derse, D., S. A. Hill, P. A. Lloyd, H. Chung, and B. A. Morse. 2001. Examining human T-lymphotropic virus type 1 infection and replication by cell-free infection with recombinant virus vectors. J. Virol. 75:8461-8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dewan, M. Z., K. Terashima, M. Taruishi, H. Hasegawa, M. Ito, Y. Tanaka, N. Mori, T. Sata, Y. Koyanagi, M. Maeda, Y. Kubuki, A. Okayama, M. Fujii, and N. Yamamoto. 2003. Rapid tumor formation of human T-cell leukemia virus type 1-infected cell lines in novel NOD-SCID/γcnull mice: suppression by an inhibitor against NF-κB. J. Virol. 77:5286-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etoh, K., S. Tamiya, K. Yamaguchi, A. Okayama, H. Tsubouchi, T. Ideta, N. Mueller, K. Takatsuki, and M. Matsuoka. 1997. Persistent clonal proliferation of human T-lymphotropic virus type I-infected cells in vivo. Cancer Res. 57:4862-4867. [PubMed] [Google Scholar]
- 11.Fan, N., J. Gavalchin, B. Paul, K. H. Wells, M. J. Lane, and B. J. Poiesz. 1992. Infection of peripheral blood mononuclear cells and cell lines by cell-free human T-cell lymphoma/leukemia virus type I. J. Clin. Microbiol. 30:905-910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feigal, E., E. Murphy, K. Vranizan, P. Bacchetti, R. Chaisson, J. E. Drummond, W. Blattner, M. McGrath, J. Greenspan, and A. Moss. 1991. Human T cell lymphotropic virus types I and II in intravenous drug users in San Francisco: risk factors associated with seropositivity. J. Infect. Dis. 164:36-42. [DOI] [PubMed] [Google Scholar]
- 13.Feuer, G., J. A. Zack, W. J. Harrington, Jr., R. Valderama, J. D. Rosenblatt, W. Wachsman, S. M. Baird, and I. S. Chen. 1993. Establishment of human T-cell leukemia virus type I T-cell lymphomas in severe combined immunodeficient mice. Blood 82:722-731. [PubMed] [Google Scholar]
- 14.Franchini, G., R. Fukumoto, and J. R. Fullen. 2003. T-cell control by human T-cell leukemia/lymphoma virus type 1. Int. J. Hematol. 78:280-296. [DOI] [PubMed] [Google Scholar]
- 15.Furuta, R. A., K. Sugiura, S. Kawakita, T. Inada, S. Ikehara, T. Matsuda, and J. Fujisawa. 2002. Mouse model for the equilibration interaction between the host immune system and human T-cell leukemia virus type 1 gene expression. J. Virol. 76:2703-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gessain, A., F. Barin, J. C. Vernant, O. Gout, L. Maurs, A. Calender, and G. de The. 1985. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet ii:407-410. [DOI] [PubMed] [Google Scholar]
- 17.Grassmann, R., M. Aboud, and K. T. Jeang. 2005. Molecular mechanisms of cellular transformation by HTLV-1 Tax. Oncogene 24:5976-5985. [DOI] [PubMed] [Google Scholar]
- 18.Hanon, E., S. Hall, G. P. Taylor, M. Saito, R. Davis, Y. Tanaka, K. Usuku, M. Osame, J. N. Weber, and C. R. Bangham. 2000. Abundant tax protein expression in CD4+ T cells infected with human T-cell lymphotropic virus type I (HTLV-I) is prevented by cytotoxic T lymphocytes. Blood 95:1386-1392. [PubMed] [Google Scholar]
- 19.Hanon, E., J. C. Stinchcombe, M. Saito, B. E. Asquith, G. P. Taylor, Y. Tanaka, J. N. Weber, G. M. Griffiths, and C. R. Bangham. 2000. Fratricide among CD8(+) T lymphocytes naturally infected with human T cell lymphotropic virus type I. Immunity 13:657-664. [DOI] [PubMed] [Google Scholar]
- 20.Hill, S. A., P. A. Lloyd, S. McDonald, J. Wykoff, and D. Derse. 2003. Susceptibility of human T cell leukemia virus type I to nucleoside reverse transcriptase inhibitors. J. Infect. Dis. 188:424-427. [DOI] [PubMed] [Google Scholar]
- 21.Hinuma, Y., Y. Gotoh, K. Sugamura, K. Nagata, T. Goto, M. Nakai, N. Kamada, T. Matsumoto, and K. Kinoshita. 1982. A retrovirus associated with human adult T-cell leukemia: in vitro activation. Gann 73:341-344. [PubMed] [Google Scholar]
- 22.Hiraragi, H., S. J. Kim, A. J. Phipps, M. Silic-Benussi, V. Ciminale, L. Ratner, P. L. Green, and M. D. Lairmore. 2006. Human T-lymphotropic virus type 1 mitochondrion-localizing protein p13II is required for viral infectivity in vivo. J. Virol. 80:3469-3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Igakura, T., J. C. Stinchcombe, P. K. Goon, G. P. Taylor, J. N. Weber, G. M. Griffiths, Y. Tanaka, M. Osame, and C. R. Bangham. 2003. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science 299:1713-1716. [DOI] [PubMed] [Google Scholar]
- 24.Imada, K., A. Takaori-Kondo, T. Akagi, K. Shimotohno, K. Sugamura, T. Hattori, H. Yamabe, M. Okuma, and T. Uchiyama. 1995. Tumorigenicity of human T-cell leukemia virus type I-infected cell lines in severe combined immunodeficient mice and characterization of the cells proliferating in vivo. Blood 86:2350-2357. [PubMed] [Google Scholar]
- 25.Ito, M., H. Hiramatsu, K. Kobayashi, K. Suzue, M. Kawahata, K. Hioki, Y. Ueyama, Y. Koyanagi, K. Sugamura, K. Tsuji, T. Heike, and T. Nakahata. 2002. NOD/SCID/gamma(c) (null) mouse: an excellent recipient mouse model for engraftment of human cells. Blood 100:3175-3182. [DOI] [PubMed] [Google Scholar]
- 26.Johnson, J. M., C. Nicot, J. Fullen, V. Ciminale, L. Casareto, J. C. Mulloy, S. Jacobson, and G. Franchini. 2001. Free major histocompatibility complex class I heavy chain is preferentially targeted for degradation by human T-cell leukemia/lymphotropic virus type 1 p12I protein. J. Virol. 75:6086-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kannagi, M., S. Harada, I. Maruyama, H. Inoko, H. Igarashi, G. Kuwashima, S. Sato, M. Morita, M. Kidokoro, M. Sugimoto, et al. 1991. Predominant recognition of human T cell leukemia virus type I (HTLV-I) pX gene products by human CD8+ cytotoxic T cells directed against HTLV-I-infected cells. Int. Immunol. 3:761-767. [DOI] [PubMed] [Google Scholar]
- 28.Kannagi, M., S. Matsushita, H. Shida, and S. Harada. 1994. Cytotoxic T cell response and expression of the target antigen in HTLV-I infection. Leukemia 8(Suppl. 1):S54-S59. [PubMed] [Google Scholar]
- 29.Kim, S. J., A. M. Nair, S. Fernandez, L. Mathes, and M. D. Lairmore. 2006. Enhancement of LFA-1-mediated T cell adhesion by human T lymphotropic virus type 1 p12I1. J. Immunol. 176:5463-5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinoshita, T., M. Shimoyama, K. Tobinai, M. Ito, S. Ito, S. Ikeda, K. Tajima, K. Shimotohno, and T. Sugimura. 1989. Detection of mRNA for the tax1/rex1 gene of human T-cell leukemia virus type I in fresh peripheral blood mononuclear cells of adult T-cell leukemia patients and viral carriers by using the polymerase chain reaction. Proc. Natl. Acad. Sci. USA 86:5620-5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koyanagi, Y., Y. Itoyama, N. Nakamura, K. Takamatsu, J. Kira, T. Iwamasa, I. Goto, and N. Yamamoto. 1993. In vivo infection of human T-cell leukemia virus type I in non-T cells. Virology 196:25-33. [DOI] [PubMed] [Google Scholar]
- 32.Lairmore, M. D., L. Silverman, and L. Ratner. 2005. Animal models for human T-lymphotropic virus type 1 (HTLV-1) infection and transformation. Oncogene 24:6005-6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li, H. C., R. J. Biggar, W. J. Miley, E. M. Maloney, B. Cranston, B. Hanchard, and M. Hisada. 2004. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J. Infect. Dis. 190:1275-1278. [DOI] [PubMed] [Google Scholar]
- 34.Machuca, A., B. Rodes, and V. Soriano. 2001. The effect of antiretroviral therapy on HTLV infection. Virus Res. 78:93-100. [DOI] [PubMed] [Google Scholar]
- 35.Manel, N., F. J. Kim, S. Kinet, N. Taylor, M. Sitbon, and J. L. Battini. 2003. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell 115:449-459. [DOI] [PubMed] [Google Scholar]
- 36.Matsuoka, M., and K. T. Jeang. 2005. Human T-cell leukemia virus type I at age 25: a progress report. Cancer Res. 65:4467-4470. [DOI] [PubMed] [Google Scholar]
- 37.Matsushita, S., H. Mitsuya, M. S. Reitz, and S. Broder. 1987. Pharmacological inhibition of in vitro infectivity of human T lymphotropic virus type I. J. Clin. Investig. 80:394-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michael, B., A. M. Nair, H. Hiraragi, L. Shen, G. Feuer, K. Boris-Lawrie, and M. D. Lairmore. 2004. Human T lymphotropic virus type-1 p30II alters cellular gene expression to selectively enhance signaling pathways that activate T lymphocytes. Retrovirology 1:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagai, M., M. B. Brennan, J. A. Sakai, C. A. Mora, and S. Jacobson. 2001. CD8(+) T cells are an in vivo reservoir for human T-cell lymphotropic virus type I. Blood 98:1858-1861. [DOI] [PubMed] [Google Scholar]
- 40.Nakata, H., K. Maeda, T. Miyakawa, S. Shibayama, M. Matsuo, Y. Takaoka, M. Ito, Y. Koyanagi, and H. Mitsuya. 2005. Potent anti-R5 human immunodeficiency virus type 1 effects of a CCR5 antagonist, AK602/ONO4128/GW873140, in a novel human peripheral blood mononuclear cell nonobese diabetic-SCID, interleukin-2 receptor gamma-chain-knocked-out AIDS mouse model. J. Virol. 79:2087-2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicot, C., M. Dundr, J. M. Johnson, J. R. Fullen, N. Alonzo, R. Fukumoto, G. L. Princler, D. Derse, T. Misteli, and G. Franchini. 2004. HTLV-1-encoded p30II is a post-transcriptional negative regulator of viral replication. Nat. Med. 10:197-201. [DOI] [PubMed] [Google Scholar]
- 42.Okochi, K., and H. Sato. 1984. Transmission of ATLV (HTLV-I) through blood transfusion. Princess Takamatsu Symp. 15:129-135. [PubMed] [Google Scholar]
- 43.Osame, M., K. Usuku, S. Izumo, N. Ijichi, H. Amitani, A. Igata, M. Matsumoto, and M. Tara. 1986. HTLV-I associated myelopathy, a new clinical entity. Lancet i:1031-1032. [DOI] [PubMed] [Google Scholar]
- 44.Poiesz, B. J., F. W. Ruscetti, A. F. Gazdar, P. A. Bunn, J. D. Minna, and R. C. Gallo. 1980. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 77:7415-7419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Popovic, M., P. S. Sarin, M. Robert-Gurroff, V. S. Kalyanaraman, D. Mann, J. Minowada, and R. C. Gallo. 1983. Isolation and transmission of human retrovirus (human T-cell leukemia virus). Science 219:856-859. [DOI] [PubMed] [Google Scholar]
- 46.Richardson, J. H., A. J. Edwards, J. K. Cruickshank, P. Rudge, and A. G. Dalgleish. 1990. In vivo cellular tropism of human T-cell leukemia virus type 1. J. Virol. 64:5682-5687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silverman, L. R., A. J. Phipps, A. Montgomery, L. Ratner, and M. D. Lairmore. 2004. Human T-cell lymphotropic virus type 1 open reading frame II-encoded p30II is required for in vivo replication: evidence of in vivo reversion. J. Virol. 78:3837-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugiyama, H., H. Doi, K. Yamaguchi, Y. Tsuji, T. Miyamoto, and S. Hino. 1986. Significance of postnatal mother-to-child transmission of human T-lymphotropic virus type-I on the development of adult T-cell leukemia/lymphoma. J. Med. Virol. 20:253-260. [DOI] [PubMed] [Google Scholar]
- 49.Tajima, K., S. Tominaga, and T. Suchi. 1986. Malignant lymphomas in Japan: epidemiological analysis on adult T-cell leukemia/lymphoma. Hematol. Oncol. 4:31-44. [DOI] [PubMed] [Google Scholar]
- 50.Takatsuki, K. 2005. Discovery of adult T-cell leukemia. Retrovirology 2:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takatsuki, K., T. Uchiyama, K. Sagawa, and J. Yodoi. 1977. Adult T cell leukemia in Japan, p. 73-77. In S. Seno, F. Takaku, and S. Irino (ed.), Topic in hematology. The 16th International Congress of Hematology. Excerpta Medica, Amsterdam, The Netherlands.
- 52.Takeda, S., M. Maeda, S. Morikawa, Y. Taniguchi, J. Yasunaga, K. Nosaka, Y. Tanaka, and M. Matsuoka. 2004. Genetic and epigenetic inactivation of tax gene in adult T-cell leukemia cells. Int. J. Cancer 109:559-567. [DOI] [PubMed] [Google Scholar]
- 53.Tanaka, G., A. Okayama, T. Watanabe, S. Aizawa, S. Stuver, N. Mueller, C. C. Hsieh, and H. Tsubouchi. 2005. The clonal expansion of human T lymphotropic virus type 1-infected T cells: a comparison between seroconverters and long-term carriers. J. Infect. Dis. 191:1140-1147. [DOI] [PubMed] [Google Scholar]
- 54.Taniguchi, Y., K. Nosaka, J. Yasunaga, M. Maeda, N. Mueller, A. Okayama, and M. Matsuoka. 2005. Silencing of human T-cell leukemia virus type I gene transcription by epigenetic mechanisms. Retrovirology 2:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tateno, M., N. Kondo, T. Itoh, T. Chubachi, T. Togashi, and T. Yoshiki. 1984. Rat lymphoid cell lines with human T cell leukemia virus production. I. Biological and serological characterization. J. Exp. Med. 159:1105-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor, G. P., S. E. Hall, S. Navarrete, C. A. Michie, R. Davis, A. D. Witkover, M. Rossor, M. A. Nowak, P. Rudge, E. Matutes, C. R. Bangham, and J. N. Weber. 1999. Effect of lamivudine on human T-cell leukemia virus type 1 (HTLV-1) DNA copy number, T-cell phenotype, and anti-Tax cytotoxic T-cell frequency in patients with HTLV-1-associated myelopathy. J. Virol. 73:10289-10295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uchiyama, T. 1997. Human T cell leukemia virus type I (HTLV-I) and human diseases. Annu. Rev. Immunol. 15:15-37. [DOI] [PubMed] [Google Scholar]
- 58.Uchiyama, T., J. Yodoi, K. Sagawa, K. Takatsuki, and H. Uchino. 1977. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood 50:481-492. [PubMed] [Google Scholar]
- 59.Xiong, Z., and P. W. Laird. 1997. COBRA: a sensitive and quantitative DNA methylation assay. Nucleic Acids Res. 25:2532-2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yahata, T., K. Ando, Y. Nakamura, Y. Ueyama, K. Shimamura, N. Tamaoki, S. Kato, and T. Hotta. 2002. Functional human T lymphocyte development from cord blood CD34+ cells in nonobese diabetic/Shi-scid, IL-2 receptor gamma null mice. J. Immunol. 169:204-209. [DOI] [PubMed] [Google Scholar]
- 61.Yamamoto, N., M. Okada, Y. Koyanagi, M. Kannagi, and Y. Hinuma. 1982. Transformation of human leukocytes by cocultivation with an adult T cell leukemia virus producer cell line. Science 217:737-739. [DOI] [PubMed] [Google Scholar]
- 62.Yasunaga, J., T. Sakai, K. Nosaka, K. Etoh, S. Tamiya, S. Koga, S. Mita, M. Uchino, H. Mitsuya, and M. Matsuoka. 2001. Impaired production of naive T lymphocytes in human T-cell leukemia virus type I-infected individuals: its implications in the immunodeficient state. Blood 97:3177-3183. [DOI] [PubMed] [Google Scholar]
- 63.Yoshida, M. 2001. Multiple viral strategies of HTLV-1 for dysregulation of cell growth control. Annu. Rev. Immunol. 19:475-496. [DOI] [PubMed] [Google Scholar]
- 64.Zhang, W., J. W. Nisbet, B. Albrecht, W. Ding, F. Kashanchi, J. T. Bartoe, and M. D. Lairmore. 2001. Human T-lymphotropic virus type 1 p30II regulates gene transcription by binding CREB binding protein/p300. J. Virol. 75:9885-9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao, T. M., B. Hague, D. L. Caudell, R. M. Simpson, and T. J. Kindt. 2005. Quantification of HTLV-I proviral load in experimentally infected rabbits. Retrovirology 2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]