Abstract
Papillomaviruses (PVs) demonstrate both tissue and species tropisms. Because PVs replicate only in terminally differentiating epithelium, the recent production of infectious PV particles in 293 cells marks an important breakthrough. In this article, we demonstrate that infectious PV particles produced in 293TT cells can cause papillomatous growths in the natural host animal. Moreover, we show that species-matched PV genomes can be successfully delivered in vivo by a heterologous, species-mismatched PV capsid. Additionally, our results indicate that the addition of the simian virus 40 origin of replication to the papillomavirus genome increases the production of infectious papillomavirus particles by increasing genome amplification in the transfected 293TT cells.
The production of authentic papillomaviruses (PVs) has been problematic for researchers in the field. The species and tissue tropism of this virus has necessitated the isolation of authentic virions from natural lesions in the host animal, from infected xenografts grown in immunocompromised animals, or from infected keratinocytes grown in organotypic raft culture. Recently it was reported that pseudovirus technology (1) can be used to encapsidate human papillomavirus (HPV) genomes within HPV capsids (5). These virus particles were infectious in cell culture systems but were untested in host tissue in vivo.
We applied pseudovirus technology to package cottontail rabbit papillomavirus (CRPV) genomes into homologous and heterologous PV capsids. Because it remains uncertain whether these particles, assembled using 293TT cells, are identical to authentic virions produced in terminally differentiated epithelium, we will refer to these particles as “quasivirions” until their virion-like nature is more fully evaluated. Here we present in vivo evidence that quasivirions containing CRPV genomes encapsidated by either CRPV or HPV-16 capsid proteins are similarly infectious in rabbits. The production of quasivirions in 293TT cells can be achieved using either linear or recircularized CRPV genomes and the addition of the simian virus 40 origin of replication (SV40 ori) to the viral genome increases its amplification in 293TT cells and improves quasivirion production without obviously altering pathology of the CRPV-induced papillomas.
Generation of infectious CRPV particles using 293TT cells.
Using the previously described protocol (1), 293TT producer cells were transfected with circular CRPV genomes together with plasmids expressing codon-modified L1 and L2. Two days posttransfection, the cells were lysed with Brij-58, incubated to allow particle maturation (2), and treated with Benzonase (Sigma) and Plasmid Safe (Epicenter) to destroy unprotected DNA. Cell lysates were centrifuged on an Optiprep density gradient (Accurate Chemical). Fractions (∼300 μl) were collected dropwise from the bottom of the column and analyzed for the presence of capsid proteins by immunoblotting and/or nondenaturing enzyme-linked immunosorbent assay (data not shown). Fractions with appropriate densities and positive for capsid proteins were assayed for their infectivity in RK13 cells by quantitative reverse transcription-PCR (QRT-PCR) measuring viral E1∧E4 transcripts (4). Those fractions capable of generating viral transcripts in inoculated cells were retested for their ability to be neutralized by monoclonal antibodies (MAbs) (CRPV.1A or H16.V5). Fractions (and particles) termed “infectious” both induced the production of E1∧E4 transcripts in the in vitro infection assay and lost this ability in the presence of a specific MAb.
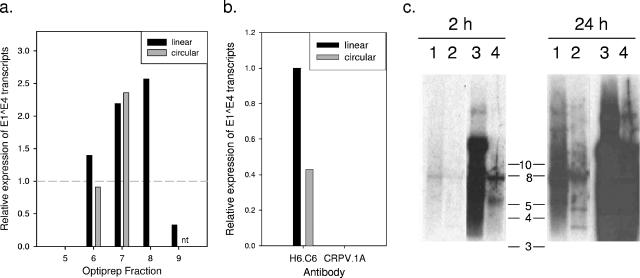
Because each step in the isolation and recircularization of viral genomes from bacterial cloning vectors can result in losses in DNA yield, we examined whether 293TT cells transfected with linear CRPV genomes (cut with SalI in E5) can produce infectious particles. 293TT producer cells were transfected with pCRPVL1, pCRPVL2, and a CRPV genome (either linear or recircularized). Based upon the results of an immunoblot assay for L1 (data not shown), select fractions from the Optiprep gradient were tested for infectivity on RK13 cells (Fig. 1a). Using QRT-PCR to assay infection, we used viral RNA levels as a biological readout for the quantification of infectious particles in these fractions (3). Reproducibly, transfection with the linear versus the recircularized CRPV genomes showed evidence of improved production of quasivirions. Southern blot analysis of CRPV genomes released from infectious particles (using proteinase K and sodium dodecyl sulfate) revealed that encapsidated genomes were circular regardless of the state of input viral DNA (data not shown). Neutralization of particles in these fractions by the MAb CRPV.1A demonstrated capsid-dependent delivery of viral genomes (Fig. 1b). The apparent shift of infectivity toward higher (less dense) fractions seen with the linear genome preparation in this experiment was not reproducible and was likely the result of experimental error.
FIG. 1.
Modification of transfected genomes. (a) Infectious quasivirions can be produced using either linear or circular CRPV genomes and neutralized by a specific MAb. 293TT cells transfected with pCRPVL1, pCRPVL2, and linear (SalI) or recircularized CRPVwt genomes were lysed and treated as described in the text. Following immunoblot analysis for the presence of L1, select fractions were tested using RK13 cells as an assay of infectivity in vitro. “Linear” fractions were collected from the lysates of 293TT cells transfected with linear CRPV genomes, while “circular” fractions were collected from 293TT cells transfected with the recircularized genomes. Vertical bars represent the levels of E1∧E4 transcripts in treated cells (measured by QRT-PCR) relative to those in RK13 cells infected with a laboratory stock of native CRPV (shown as dashed horizontal line). nt, not tested (b) Ascites containing CRPV.1A (specific MAb) or H6.C6 (control MAb) were added to pooled fractions from the experiment above (linear fractions 6 to 8 and circular fractions 6 and 7) at the time of infection of RK13 cells. (c) Addition of SV40 ori to CRPV genomes increases genome concentration in 293TT cells. Cells were transfected with CRPV genomes ± SV40 ori. Aliquots of the purified low-molecular-weight DNAs were cut with EcoRI (nt 1063) to evaluate the state of isolated genomes. Southern blot analysis was performed using a 32P-labeled probe made against the linear CRPVwt genome. Shown are 2-h (left) and 24-h (right) exposures showing uncut CRPVwt (lane 1), EcoRI-digested CRPVwt (lane 2), uncut CRPVori (lane 3), and EcoRI-digested CRPVori (lane 4). The predominant 8-kb band in lane 1 (2 h), together with the substantial 3.5- and 4.5-kb bands in lane 2 (24 h), indicates that many of the CRPVwt genomes are linear in these cells. The position of the predominant band in lane 3 (>10 kb) and the relative absence of 3.5- and 4.5-kb bands in lane 4 reveal that most of the CRPVori genomes are circular.
We hypothesized that the addition of the SV40 ori to the CRPV genome might improve amplification and packaging by 293TT cells. These cells express high levels of large T antigen. To test this hypothesis, the SV40 ori (337 nucleotides [nt]) was cloned into the upstream regulatory region of CRPV at BglII (nt 7663). Reproducibly, transfection of 293TT cells with CRPV genomes containing the SV40 ori (CRPVori) showed a tendency to improve the yield of infectious particles over transfection with wild-type CRPV (CRPVwt) genomes (Table 1).
TABLE 1.
Estimate of the total infectivity in a given preparation of quasivirusa
| Transfected genome | Infectious fractions | Combined vol (μl) | Mean ± SE no. of CRPV doses/fraction (total) |
|---|---|---|---|
| CRPVwt | |||
| Linear | 6-8 | 791 | 12.3 ± 0.167 (36.8) |
| Circular | 6-7 | 850 | 14.4 ± 7.30 (28.8) |
| CRPVori | |||
| Linear | 5-7 | 935 | 43.6 ± 19.6 (130.7) |
| Circular | 5-7 | 1,008 | 17.8 ± 12.8 (48.1) |
Because the dropwise collection of fractions from the density gradient yielded a considerable range of volumes, the total infectivity per preparation was calculated relative to the infectivity present in a laboratory stock of native CRPV virions (used as a positive control in each QRT-PCR assay). To calculate relative infectivity, three sequential calculations were performed. First a CRPV “equivalency” was approximated for each infectious fraction (i.e., x μl of the fraction = 1 μl of CRPV stock) by dividing the volume of each fraction used to infect RK13 cells (50 μl) by the change (fold) in E1^E4 transcript levels relative to the level resulting from infection with 1 dose of CRPV stock. Second, the total volume collected in each fraction was divided by the CRPV “equivalency” to calculate the number of CRPV doses present in each gradient fraction. Finally, the numbers of CRPV doses present in each infectious fraction of the gradient were added together to estimate the total infectivity in the preparation (1 dose = 50 μl of CRPV stock).
To evaluate if CRPVori genomes were amplified to higher levels in this system, 293TT cells were transfected with 15 μg of linearized (SalI cut at nt 4571) CRPV genome ± SV40 ori. Two days posttransfection, the low-molecular-weight DNAs were isolated from monolayers using the QIAPrep Spin Miniprep kit (QIAGEN) according to the protocol previously described (6). Southern blot analysis revealed that the CRPVori genomes were present in much higher quantities in the 293TT cells than were CRPVwt genomes (Fig. 1c). Presumably a higher genome concentration leads to increased genome encapsidation with this system. Although the addition of the SV40 ori to the PV genome may be unnecessary for the production of many PV quasivirus genotypes, this strategy may prove advantageous in the production of some PV types.
In vivo infections using CRPV quasivirions.
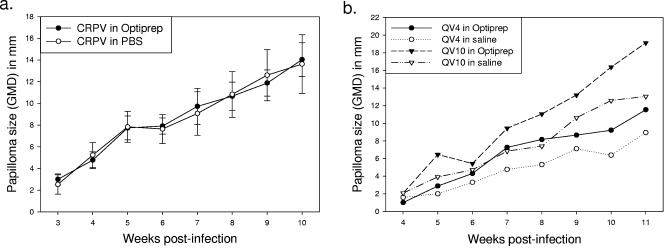
Papilloma appearance and growth rates were similar following infection of rabbit skin with native CRPV virions diluted in Optiprep versus phosphate-buffered saline (Fig. 2a). Additionally, the dialysis of quasivirions into 0.8 M saline (pH 7.0) led to a small decrease in infectivity in vivo, probably due to particle loss (Fig. 2b). We therefore performed all subsequent in vivo experiments using infectious Optiprep fractions.
FIG. 2.
In vivo infections using New Zealand White rabbits. (a) Optiprep has no effect on CRPV infection in vivo. Authentic CRPV virions diluted 100-fold in either phosphate-buffered saline or 33% Optiprep (in 0.8 M saline) were applied to scarified sites on four rabbits (one site each per rabbit). Data shown are mean sizes of papillomas ± standard error (b) To determine whether the infectivity of quasivirions in Optiprep could be improved by dialysis into saline, aliquots from the pooled fractions of two different preparations were dialyzed into 0.8 M saline (pH 7.0) overnight at 4°C using Slide-A-Lyzer dialysis cassettes (Pierce). Quasivirus 4 (QV4) was produced using linear CRPVori genomes and the CRPV capsid proteins. Quasivirus 10 (QV10) was produced using linear CRPVwt genomes and the HPV-16 capsid proteins. Due to volume increases during dialysis, total protein readings (using the Bio-Rad protein assay) were used to approximate the volumes necessary for infection with an equivalent number of particles within each type. Based upon these results, the scarified sites on the skin of an individual rabbit were treated with 10 μl of QV4 or QV10 in Optiprep or alternatively with 63 or 71 μl of QV4 and QV10, respectively, in saline.
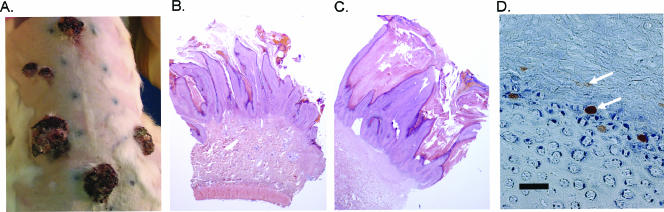
To evaluate the infectivity of quasivirions in vivo, scarified rabbit skin was exposed to aliquots (2 to 75 μl) of various preparations. As shown in Fig. 3, papillomas showing typical morphology and histopathology resulted at sites infected with quasivirions previously found to be infectious in vitro. This includes chimeric particles with CRPV genomes encapsidated by HPV-16 L1 and L2, supporting the hypothesis that the species tropism of PVs is not solely based upon the interactions of the capsid with the target tissue.
FIG. 3.
Papillomas induced by quasivirions at 12 weeks postinfection. (A) Papillomas on the dorsal skin of a New Zealand White rabbit inoculated with various dilutions, constructs, and preparations of quasivirus. This rabbit was never exposed to native CRPV virions. (B) Hematoxylin- and eosin-stained tissue section of a papilloma induced by quasivirions composed of CRPV capsid proteins and CRPVori genomes. (C) Hematoxylin- and eosin-stained tissue section of a papilloma induced by quasivirions composed of HPV-16 capsid proteins and CRPVwt genomes. (D) Expression of CRPV L1 (arrows) in a papilloma induced by chimeric HPV-16/CRPVwt quasivirions is seen following immunohistochemical staining with the MAb CRPV.G4.B. Scale bar, 50 μm.
Because CRPV DNA can directly induce papillomas with this infection model, capsid-dependent infection was evaluated in three ways. First, CRPV genomes were released from quasivirions (using proteinase K and sodium dodecyl sulfate), isolated using a standard phenol-chloroform extraction, and then applied to scarified sites. While sites receiving either 5 μl of quasivirions or the control CRPV DNA produced papillomas, no papillomas were produced at sites receiving the DNA extracted from up to 50 μl of quasivirus stock (equivalent to a 10× dose). Second, the preincubation of CRPV/CRPV and HPV-16/CRPV quasivirions with type-specific neutralizing MAbs (CRPV.4B and H16.V5) prevented papilloma development in a type-specific manner. Third, animals vaccinated with CRPV or HPV-16 L1 virus-like particles (VLPs) prior to inoculation with quasivirions showed type specific immunity (submitted for publication).
Consistent with the in vitro infection experiments, quasivirus preparations made using the CRPVori genomes showed a tendency to be more infectious in vivo both by producing papillomas at a higher percentage of sites and by requiring lower volumes (typically 2 to 5 μl versus 10 to 20 μl). As previously stated, we interpret this to indicate that higher concentrations of CRPV genomes within the 293TT cells facilitate improved yield. PCR analysis of DNA isolated from papillomas generated by CRPVori genomes showed that the SV40 ori was detectable several weeks after infection, indicating that at least a fraction of persistent CRPV genomes retained this heterologous element in their upstream regulatory region (data not shown).
Our studies show that infectious homologous and chimeric PV particles delivering CRPV genomes can be assembled using 293TT cells. These quasivirions can be neutralized in vitro and in vivo by L1-specific antibodies, indicating capsid-mediated delivery of viral genomes. Inoculation of rabbit skin with relatively low volumes of homologous or chimeric particles can induce the growth of papillomatous lesions via capsid-mediated infection of the correct target cells within the wounded epithelium. These results offer further evidence for the virus-like nature of PV particles assembled using pseudovirion technology (1). Our findings demonstrate the potential application of using chimeric quasivirions and animal models both for structural studies (e.g., L1-L2 and capsid-genome interactions) and for the preclinical evaluation of HPV vaccine efficacy. In a second study, we show that rabbits immunized with CRPV or HPV-16 VLPs are protected against homologous or chimeric quasivirions, respectively, while animals vaccinated with HPV-11 VLPs are protected against neither particle (submitted for publication).
Acknowledgments
We thank John Schiller and Chris Buck at NCI for generously providing 293TT cells and expression plasmids containing genes encoding the CRPV and HPV-16 capsid proteins.
These studies were supported by NIH NCI grant CA 47622 and the Jake Gittlen Memorial Golf Tournament. Microscopy studies were also supported by NIH grant CA 40145 (G. Clawson).
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Buck, C. B., D. V. Pastrana, D. R. Lowy, and J. T. Schiller. 2004. Efficient intracellular assembly of papillomaviral vectors. J. Virol. 78:751-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck, C. B., C. D. Thompson, Y.-Y. S. Pang, D. R. Lowy, and J. T. Schiller. 2005. Maturation of papillomavirus capsids. J. Virol. 79:2839-2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Culp, T. D., and N. D. Christensen. 2003. Quantitative RT-PCR assay for HPV infection in cultured cells. J. Virol. Methods 111:135-144. [DOI] [PubMed] [Google Scholar]
- 4.Culp, T. D., and N. D. Christensen. 2004. Kinetics of in vitro adsorption and entry of papillomavirus virions. Virology 319:152-161. [DOI] [PubMed] [Google Scholar]
- 5.Pyeon, D., P. F. Lambert, and P. Ahlquist. 2005. Production of infectious human papillomavirus independently of viral replication and epithelial cell differentiation. Proc. Natl. Acad. Sci. USA 102:9311-9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler, K., T. Bui, R. J. Frisque, A. Grandinetti, and V. R. Nerurkar. 2004. A rapid in vitro polyomavirus DNA replication assay. J. Virol. Methods 122:123-127. [DOI] [PubMed] [Google Scholar]