Abstract
Lentiviral Nef proteins are key factors for pathogenesis and are known to downregulate functionally important molecules, including CD4 and major histocompatibility complex class I (MHC-I), from the surfaces of infected cells. Recently, we demonstrated that Nef reduces cell surface levels of the human immunodeficiency virus type 1 (HIV-1) entry coreceptor CCR5 (N. Michel, I. Allespach, S. Venzke, O. T. Fackler, and O. T. Keppler, Curr. Biol. 15:714-723, 2005). Here, we report that Nef downregulates the second major HIV-1 coreceptor, CXCR4, from the surfaces of HIV-infected primary CD4 T lymphocytes with efficiencies comparable to those of the natural CXCR4 ligand, stromal cell-derived factor-1 alpha. Analysis of a panel of mutants of HIV-1SF2 Nef revealed that the viral protein utilized the same signature motifs for downmodulation of CXCR4 and MHC-I, including the proline-rich motif P73P76P79P82 and the acidic cluster motif E66E67E68E69. Expression of wild-type Nef, but not of specific Nef mutants, resulted in a perinuclear accumulation of the coreceptor. Remarkably, the carboxy terminus of CXCR4, which harbors the classical motifs critical for basal and ligand-induced receptor endocytosis, was dispensable for the Nef-mediated reduction of surface exposure. Functionally, the ability of Nef to simultaneously downmodulate CXCR4 and CD4 correlated with maximum-level protection of Nef-expressing target cells from fusion with cells exposing X4 HIV-1 envelopes. Furthermore, the Nef-mediated downregulation of CXCR4 alone on target T lymphocytes was sufficient to diminish cells' susceptibility to X4 HIV-1 virions at the entry step. The downregulation of chemokine coreceptors is a conserved activity of Nef to modulate infected cells, an important functional consequence of which is an enhanced resistance to HIV superinfection.
In order to fuse with the plasma membrane and enter a target cell, most isolates of the primate lentiviruses human immunodeficiency virus type 1 (HIV-1), HIV-2, and simian immunodeficiency virus (SIV) require the sequential interaction of the viral envelope (Env) glycoprotein with the main cell surface receptor CD4 and, subsequently, with a human chemokine coreceptor. In particular, an isolate's ability to utilize the seven-transmembrane G-protein-coupled chemokine receptors CCR5 and/or CXCR4 as an entry coreceptor critically influences HIV transmission, viral persistence, T-cell depletion, and other aspects of virus pathogenesis (54, 55). In vivo, CCR5-using (R5) HIV-1 strains constitute the almost exclusively transmitted viral species, and R5 viruses predominate in the early phase of infection. CXCR4-using (X4) HIV-1 strains emerge in approximately 50% of infected individuals at later stages, and these X4 viruses are typically associated with a rapid CD4 T-cell decline and disease progression (55). The cell surface density of CD4 as well as that of CXCR4 or CCR5 on target cells can be limiting for infection in vitro and in vivo (52, 70).
Nef proteins of HIV-1, HIV-2, and SIV augment virus replication in vivo and contribute to the pathogenic potentials of these primate lentiviruses (20, 37, 39). Numerous activities have been attributed to this accessory viral protein (4, 24). Nef lowers the threshold of T-cell activation, resulting in an enhanced viral replication (22, 62, 72), and Nef enhances the infectivity of HIV virions (2). Furthermore, Nef modulates surface levels of multiple immune receptors, including CD4, CD8, major histocompatibility complex class I (MHC-I), the invariant chain of immature MHC-II (CD74), DC-SIGN, CD80, CD86, and mature MHC-II (14, 23, 63, 65-67). Concerning the specificity of Nef's effect on cell surface receptors, a recent comprehensive study found that levels of the immune receptors ICAM-3, LFA-1 (CD11a/CD18), and CD2 are not affected by HIV-1 Nef expression in infected T cells (68). Since Nef has no enzymatic activity, all of these different activities depend on the ability to serve as an adapter for a number of cellular proteins. Nef recruits distinct cellular machineries via specialized protein interaction motifs and thereby manipulates the intracellular trafficking of individual cell surface receptors (69). CD4 downregulation by Nef occurs primarily via clathrin-mediated endocytosis and requires the interaction of an LL or ED motif in the carboxy-terminal flexible loop of Nef with components of the cell sorting machinery (1, 19, 25, 45), but effects of Nef on CD4 molecules prior to their insertion in the plasma membrane have also been observed (59). Both mechanisms, accelerated endocytosis and perturbation of anterograde transport, are also employed by Nef to reduce cell surface levels of MHC-I molecules (33, 63). Alanine mutation of the Src homology 3 (SH3) binding motif of Nef (amino acids P76P79 [NefAxxA]) or alanine mutation of the acidic cluster motif (amino acids E66E67E68E69 [NefE4A]), required for the interaction with phosphofurin acidic cluster sorting protein 1 and the AP-1/MHC-I complex (26, 46, 56, 74), almost completely abrogates Nef's ability to reduce MHC-I cell surface levels. Functionally, the reductions of cell surface expression of MHC-I and mature MHC-II have been proposed as immune evasion mechanisms that prevent recognition of HIV infected cells by cytotoxic T lymphocytes (17, 21) and limit the presentation of HIV antigenic peptides (67), respectively. In contrast, the profound reduction of surface-exposed CD4 in infected cells, which is mediated by the HIV proteins Env, Vpu, and Nef, is thought to facilitate virus replication in infected cells by interfering with several undesirable consequences for the virus: induction of proapoptotic signals delivered through CD4, triggering of inhibitory signals for viral transcription, the reduction of viral infectivity by incorporation of cell surface receptors into the viral envelope, and HIV superinfection (for reviews, see references 42 and 44). Regarding the latter, viral superinfection is thought to be deleterious for the infected cell due to toxicity of accumulating unintegrated viral genomes (53, 57).
Recently, we identified the downregulation of the CCR5 coreceptor as a novel and highly conserved activity of Nef (49). Functionally, downregulation of CCR5 and CD4 by Nef was genetically separable and synergized to efficiently protect cells from R5 HIV-1 superinfection at the level of fusion of the viral envelope with the plasma membrane. In the current study we investigated whether Nef affects cell surface levels of the second major HIV-1 chemokine coreceptor, CXCR4, in infected CD4 T cells and whether Nef-mediated downregulation of CXCR4 contributes to superinfection interference.
MATERIALS AND METHODS
Plasmids.
Plasmids encoding the HIV-1SF2 wild-type (wt) Nef or the mutant NefEDAA, NefLLAA, NefAxxA, or NefE4A as green fluorescent protein (GFP) fusion protein or bicistronically are based on the pEGFP-N1 or pIRES2-EGFP vector (both from Clontech), respectively (32, 40, 49). Nef alleles of HIV-1SF2, HIV-1NL4-3, HIV-1NA7, HIV-2NEP, and SIVMac239 were expressed using the bicistronic GFP expression vector pCG (49). The plasmid pcDNA3.1YFP was obtained from Invitrogen, and construction of the plasmid pmRFP-N1 has been described elsewhere (7). The generation of expression plasmids or original sources for CD4, CXCR4, vesicular stomatitis virus G protein (VSV-G), Marburg virus glycoprotein (GP), HIV-1NL4-3 Env, HIV-1HXB-2 Env, HIV-1R9Δenv, and HIV-1NL4-3 have been reported previously (36, 49). pBRHIV-1NL4-3 internal ribosome entry site (IRES)-GFP plasmids (Δnef, HIV-1NA7 nef, or HIV-2BEN nef) (61), encoding replication-competent viruses with a nef-IRES-GFP element, and the bicistronic plasmid encoding the SIVCol Nef allele (Col Nef) and yellow fluorescent protein (YFP) were kind gifts of Frank Kirchhoff (University of Ulm). The plasmids encoding a protein chimera consisting of β-lactamase linked to the N terminus of Vpr (BlaM-Vpr) and HIV-1NL4-3 GIA Env were kindly provided by Nathaniel Landau (Salk Institute) and Matthias Dittmar (University of Heidelberg), respectively.
Cells, stable transfectants, and SDF-1α treatment.
All cell lines were kept under standard tissue culture conditions in Dulbecco's modified Eagle medium (DMEM) or RPMI 1640 supplemented with 10% fetal bovine serum, 1% penicillin-streptomycin, and 1% l-glutamine (all from Invitrogen). The original sources of Sup-T1, HeLa, and HeLa-derived TZM cells, which stably express CD4, CXCR4, and an HIV long terminal repeat-driven β-galactosidase gene, have been reported previously (36, 49). Human HeLa Z24 cells were kindly provided by Olivier Schwartz (Institut Pasteur, Paris).
Chinese hamster ovary (CHO) cells, which constitutively express HIV-1 Tat (CHO Tat cells), have been described previously (49). CHO cells constitutively expressing human CXCR4 (hCXCR4) alone or hCXCR4 in combination with hCD4 were generated by transfection with the plasmid pFX4neo, followed by neomycin selection and fluorescence-activated cell sorting (FACS). To obtain hCXCR4/hCD4-positive CHO cells, clones expressing high levels of CXCR4 were subsequently transfected with pCD4hygro. Double-positive clones were obtained by selection with hygromycin and subsequent FACS. CHO cells stably expressing an hCXCR4 protein with a carboxy-terminal truncation (CXCR4 Δtail) were generated by transfection of parental CHO cells with pCXCR4 Δtail (43) (kindly provided by Stephen Peiper, Medical College of Georgia, Augusta), selection with neomycin, and subsequent FACS. Human peripheral blood lymphocytes (PBL) were isolated from buffy coats by using Ficoll gradients and activated with phytohemagglutinin (PHA-P) (Sigma) and human recombinant interleukin-2 (IL-2) (a gift of Chiron Co.) as described previously (35). The Amaxa nucleofection technology was used for high-level transfection of activated PBL (Human T Cell Nucleofector kit; 107 cells, 2 μg DNA; Nucleofector program T-23) as recommended by the manufacturer (Amaxa Biosystems). Standard lipofection reagents, including Lipofectamine 2000 (Invitrogen) and Metafectene (Biontex), were used according to the manufacturers' protocols for transfection of cell lines. TZM cells were transfected with the Amaxa nucleofection technology (Cell Line Nucleofector kit) as described by the manufacturer. For treatment with stromal cell-derived factor-1 alpha (SDF-1α), human PBL were incubated with SDF-1α (100 nM) (R&D Systems) in standard medium at 37°C for the indicated periods.
HIV infection of human PBL.
PHA-P/IL-2 activated PBL were inoculated with replication-competent VSV-G-pseudotyped HIV-1NL4-3 IRES-GFP viruses (∼1,500 ng p24/8 × 105 cells) carrying either a defective nef gene (Δnef), HIV-1NA7 nef, or HIV-2BEN nef. At day 4 postinfection, infected PBL were harvested, fixed for 1.5 h in 2% paraformaldehyde-phosphate-buffered saline (PBS), and subsequently stained for cell surface CXCR4 and MHC-I. Analysis was performed by flow cytometry.
Adenoviral vectors.
Recombinant adenoviruses encoding GFP or GFP fusion proteins of HIV-1SF2 wt Nef or the NefEDAA or NefAxxA mutant were produced in 293 cells with the pAdEasy adenoviral vector system (Stratagene), and titers were determined as described previously (49).
Flow cytometry.
Cells were stained in FACS medium (3% fetal bovine serum and 0.05% Na-azide in PBS) or in PBS with the following mouse monoclonal antibodies (MAbs): nonconjugated, allophycocyanin (APC)-conjugated, biotinylated, or phycoerythrin (PE)-conjugated anti-human CXCR4 MAb (clone 12G5; BD PharMingen); unconjugated anti-human CXCR4 MAb (clone 4G10, a kind gift of Christopher C. Broder, Uniformed Services University, Bethesda, Md.); unconjugated or APC-, PE-, or PE-Cy5-conjugated anti-human CD4 (clone RPA-T4; BD PharMingen); PE- or APC-conjugated anti-HLA-ABC MAb (clone W6/32; DakoCytomation); unconjugated anti-HLA-A2 (clone BB7.2; BD PharMingen); PE-conjugated anti-human CD3 (clone SK7; BD PharMingen); peridinin chlorophyll protein-conjugated anti-human CD8 MAb (clone SK1; BD PharMingen); and unconjugated anti-FLAG epitope MAb (clone M2; Stratagene). APC- and PE-conjugated goat anti-mouse antibodies (Jackson ImmunoResearch) and streptavidin Alexa Fluor 660 (Molecular Probes) were used as secondary reagents. A FACSCalibur with BD CellQuest Pro 4.0.2 software (BD PharMingen) was used for analysis.
Confocal immunofluorescence microscopy.
Transfected cells grown on coverslips were fixed with 4% paraformaldehyde and permeabilized for 25 min with 0.2% saponin in PBS. Cells were blocked for 1 h with 1% bovine serum albumin in PBS and stained with PE-conjugated anti-CXCR4 MAb (clone 12G5) and biotin-conjugated anti-HLA-ABC MAb (clone W6/32; Serotec) in combination with Alexa Fluor 660-conjugated streptavidin (Molecular Probes). Coverslips were mounted in Histoprime (Linaris) and analyzed with a Zeiss LSM510 confocal microscope with a 100× PLAN-APO objective lens. Images were recorded with the Zeiss proprietary software LSM5 and processed with Adobe Photoshop CS2.
HIV Env-plasma membrane fusion assay.
The HIV Env-plasma membrane fusion assay was performed basically as described previously (49). Briefly, CHO Tat cells were transiently transfected with expression plasmids for the indicated HIV-1 Envs, VSV-G, or the Marburg virus GP for 24 h. In parallel, TZM cells were transduced with recombinant adenoviruses encoding GFP alone or Nef.GFP fusion proteins at an multiplicity of infection of 3 for 10 to 14 h. Subsequently, transfected CHO Tat cells and transduced TZM cells were harvested and cocultured in 200 μl of a 1:1 mixture of complete RPMI and DMEM. After 6 h, the medium of cultures with VSV-G- or Marburg virus GP-expressing CHO Tat cells was replaced by 200 μl DMEM at pH 5.5 for 2 min or at pH 5.0 for 10 min, respectively. After cocultivation for 20 h, cells were washed once with PBS, and the β-galactosidase enzyme activity and protein concentration in cell lysates were determined with the luminometric Galakto-Star kit (Applied Biosystems) and the bicinchoninic acid protein assay kit (Pierce), respectively. Luminometric activity in the samples was analyzed with a Luminoskan Ascent luminometer (Thermo Labsystems) and the Ascent software 2.0.
HIV-1 virion fusion assay.
The HIV-1 virion fusion assay is based on the incorporation of BlaM-Vpr chimeric proteins into HIV-1 virions and their subsequent delivery into the cytoplasm of target cells as a result of virion fusion (12). Fusion events can be detected on a single-cell level by flow cytometric measurement of the CCF2 dye, which is loaded on the target cells after infection and cleaved by the β-lactamase in case of a fusion of the virion with the cell. HIV-1 virions containing the BlaM-Vpr chimera were produced by triple transfection of 293T cells with pNL4-3 proviral DNA (60 μg) and the pBlaM-Vpr (20 μg) and pAdVAntage (3 μg) vectors (Invitrogen) in 15-cm2 dishes, using calcium phosphate DNA precipitation. At 2 days posttransfection, the virus-containing supernatant was first concentrated using Centricon Plus-70 spin columns (Millipore) and then purified using a 20% sucrose cushion (44,000 rpm, 4°C, 60 min). The virion-enriched pellet was resuspended in DMEM for storage at −80°C.
Here, Jurkat T cells were electroporated using a standard protocol to coexpress either the SIVCol Nef allele (Col Nef) (73) from a bicistronic vector containing YFP or YFP alone together with monomeric red fluorescent protein (mRFP). At 48 h posttransfection, cells were either challenged with HIV-1NL4-3 BlaM-Vpr virions (600 ng p24/5 × 106 cells) or stained for cell surface levels of CXCR4 and CD4. Each infection was performed in triplicate. As a control, cells were pretreated with the CXCR4 antagonist AMD3100 (50 μM) (a kind gift of Jose Esté) 30 min prior to infection. At 6 h postinfection cells were washed in PBS and stained with the β-lactamase loading solution containing the CCF2 dye (Invitrogen) overnight in a moist chamber at room temperature in the dark. Cells were then washed again in PBS and fixed for 1.5 h in 2% paraformaldehyde-PBS prior to FACS analysis. Transfected cells were detected as mRFP-positive cells, and infected cells were detected as cells positive for cleaved CCF2 dye, which has a higher emission wavelength than the uncleaved substrate dye (12). For the quantification of cell surface receptor levels of CD4 and CXCR4 by flow cytometry, cells were stained with unlabeled MAbs to CD4 (clone RPA-T4) or CXCR4 (clone 12G5) followed by the monoclonal APC-conjugated mouse anti-goat antibody (Jackson ImmunoResearch).
RESULTS
HIV downregulates CXCR4 from the surfaces of primary CD4 T cells in a Nef-dependent manner.
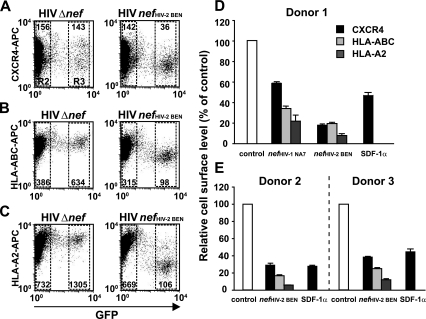
HIV and SIV entry into target cells critically depends on the levels of human CD4 and a suitable chemokine coreceptor on the surface. Here, we investigated whether Nef affects cell surface levels of the major HIV coreceptor CXCR4. This chemokine coreceptor is utilized by many HIV-1 strains, particularly those prevalent in the late phase of disease (18). Infections with replication-competent HIV-1NL4-3-based viruses carrying either a nef IRES GFP element or a Δnef IRES GFP element allow for a quantitative assessment of Nef-dependent changes in receptor cell surface levels (61). Here, GFP serves as a general marker for productive HIV infection and is a quantitative marker for Nef expression in cells infected with viruses containing an intact nef open reading frame. Expression levels of the Nef protein are comparable in T cells infected with these GFP reporter viruses or with wt viruses (Frank Kirchhoff, personal communication). As a means of quantifying the Nef-induced reduction of receptor surface levels, the mean fluorescence intensity (MFI) of receptor staining on viable, GFP-negative, uninfected cells (Fig. 1A to C, gate R2) was compared to the MFI on infected cells with medium to high GFP expression levels (Fig. 1A to C, gate R3) within the same sample. Following infection, a strong, Nef-mediated downregulation of CXCR4 was observed in primary CD4 T cells (Fig. 1), as well as in the human T-cell lines Jurkat and SupT-1 (data not shown) for the HIV-1NA7 nef allele and, more pronounced, for the HIV-2BEN allele. Experiments performed with PBL from three different donors showed a Nef-mediated downregulation of CXCR4 ranging from 27 to 42% for the nef allele from HIV-1NA7 and from 62 to 82% for the HIV2BEN allele. The extent of CXCR4 downregulation was well within the range induced by physiological concentrations of the natural ligand SDF-1α on CD4 T cells from the same donors. Furthermore, a side-by-side comparison of the Nef-mediated effects on cell surface levels of CXCR4 and MHC-I revealed a comparable (HLA-ABC) or slightly higher (HLA-A2) degree of downmodulation for the immune receptor. Collectively, HIV infection induces a marked and comparable Nef-dependent downregulation of CXCR4 and MHC-I in HIV-infected primary CD4 T cells, and the degree of CXCR4 downregulation lies within the range induced by treatment with the potent CXCR4 ligand SDF-1α.
FIG. 1.
CXCR4 is downregulated by Nef in HIV-infected primary human T lymphocytes. PHA-P/IL-2-activated PBL from three donors were challenged with replication-competent VSV-G-pseudotyped HIV-1NL4-3 nefHIV-2 BEN IRES GFP, HIV-1NL4-3 nefHIV-1 NA7 IRES GFP, or HIV-1NL4-3 Δnef IRES GFP viruses. At 4 days postinfection cells were fixed, stained with either an anti-CXCR4 MAb (clone 12G5), an anti-MHC-I MAb (clone W6/32, detecting HLA-ABC), or the HLA-A2-specific MAb BB7.2 and analyzed by flow cytometry. Independently, PBL from the same donors were also treated with SDF-1α (100 nM) for 1 h and subsequently analyzed for cell surface CXCR4 levels. FACS dot plots of cell surface levels of (A) CXCR4, (B) HLA-ABC, and (C) HLA-A2 (on the y axis) relative to GFP (on the x axis), which serves as a quantitative marker for Nef expression, are shown. The MFI for cell surface-exposed CXCR4 and MHC-I was quantified on cells with medium to high GFP expression within the R3 gate (see panels A to C for gating) relative to the MFI of GFP-negative cells in the R2 gate. This R3/R2 ratio takes into account staining variations that exist between individual samples in an experiment and most accurately reflects the degree of Nef-mediated downregulation of the analyzed receptor. For SDF-1α-treated cells, the MFI of treated cells relative to untreated cells was quantified. Ratios obtained for cells expressing GFP in the context of the HIV Δnef infection (control) were set to 100% (D and E); their standard deviations ranged from 2 to 8% (CXCR4), 1 to 9% (HLA-ABC), and 4 to 10% (HLA-A2). Histograms show the arithmetic means of triplicates + standard deviations from three independent donors.
CXCR4 downmodulation by Nef is ubiquitous.
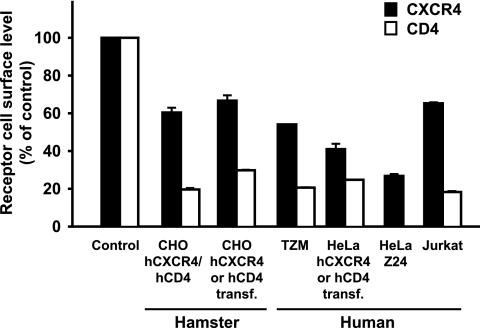
The effect of transiently expressed HIV-1SF2 Nef on the steady-state cell surface levels of CXCR4 and CD4 was examined in various cell types. We found that besides infected human CD4 T cells (Fig. 1), the HIV-1 Nef-induced downmodulation of CXCR4 was also supported by several human and rodent fibroblast and T-cell lines in the transfection context (Fig. 2). Transient expression of Nef induced a reduction in the surface levels of endogenous CXCR4 by 46 to 73% in HeLa-derived TZM and HeLa Z24 cells. CHO hCXCR4/hCD4 cells, which stably express high levels of both human receptors, displayed a marked and concentration-dependent downregulation of CXCR4 and CD4 upon expression of a Nef.GFP fusion protein, which was absent in cells expressing GFP alone (data not shown). Notably, CXCR4 was downmodulated with similar efficiency from the surfaces of parental HeLa and CHO cells lacking CD4 (Fig. 2, hCXCR4 or hCD4 transfected). This indicated that the decrease in CXCR4 levels was not merely a consequence of a co-downmodulation with CD4 mediated by the preassociation of both molecules at the plasma membrane (48). In summary, Nef reduces CXCR4 surface levels independent of an association of CXCR4 with CD4, and the cellular machinery that Nef exploits for this activity appears to be ubiquitous.
FIG. 2.
Nef reduces cell surface levels of CXCR4 in several mammalian cell lines. Human and hamster cell lines, which either constitutively coexpress human CXCR4 and CD4 or were transiently transfected with either CXCR4 or CD4 expression plasmids (hCXCR4 or hCD4 transf.), were transfected with expression constructs encoding for Nef.GFP or GFP alone. HeLa Z24 cells were transduced with adenoviral vectors expressing Nef.GFP or GFP. At 2 days posttransfection or posttransduction, relative cell surface levels of CXCR4 and CD4 were assessed by flow cytometry and quantified as described in the legend to Fig. 1. Values obtained for cells expressing GFP alone were set to 100%. Values are the arithmetic means of triplicates + standard deviations and are representative results from two to eight independent experiments.
Downmodulation of CXCR4 in primary T cells is a conserved function of lentiviral Nef proteins, and HIV-1SF2 Nef employs distinct signature motifs to reduce levels of surface-exposed CXCR4 and CD4.
Next, we compared the relative abilities of Nef proteins from HIV-1 (SF2, NL4-3, and NA7), HIV-2 (NEP), and SIV (Mac239) to downmodulate CXCR4 and CD4 from the surfaces of primary CD4 T cells (Fig. 3A). PHA-P/IL-2-activated PBL were transfected by Amaxa nucleofection (34) with bicistronic GFP vectors and analyzed for surface exposure of CXCR4, MHC-I, and CD4 by flow cytometry 1 day later. CD4 T lymphocytes were identified as the CD3+/CD8− population and subsequently plotted for receptor expression relative to GFP expression. Expression of Nef proteins from HIV-1, HIV-2, or SIV reduced steady-state cell surface levels of CXCR4 on primary CD4 T cells with comparable efficiencies. On cells from the same donor, the degree of downregulation of the chemokine receptor (26 to 49%) was within the range of or slightly lower than that observed for MHC-I (35 to 80%). As seen for transfected cell lines, CD4 downregulation was more pronounced for all lentiviral Nef alleles analyzed in PBL, ranging from 75 to 88%. Thus, downregulation of CXCR4 from the surfaces of primary HIV target cells is a conserved function of lentiviral Nef proteins.
FIG. 3.
CXCR4 downregulation by Nef in primary CD4 T lymphocytes is a conserved function and requires the same Nef motifs as downmodulation of MHC-I. PHA-P/IL-2-activated PBL from two donors were transfected with bicistronic GFP vectors encoding the various Nef proteins. One day later, CD4 T lymphocytes were identified as CD3+/CD8− cells, and cell surface levels of CXCR4, MHC-I, and CD4 were quantified by flow cytometry (A and B). The GFP control was set to 100%, and values are the arithmetic means + standard deviations from two donors. Two independent experiments, each comprising two donors, were performed.
We next sought to identify the signature motifs in Nef that are responsible for the reduction of CXCR4 cell surface levels by analyzing the effects of several well-characterized HIV-1SF2 Nef mutants (21, 24). PHA-P/IL-2-activated PBL were transfected by Amaxa nucleofection with bicistronic GFP vectors, and cell surface levels of CXCR4, MHC-I, and CD4 were analyzed by flow cytometry 1 day later. The flexible loop mutants disrupting Nef's interaction with the vacuolar ATPase H subunit, NefEDAA, had no activity for CD4 downregulation, while wt Nef (Nef.GFP) reduced CD4 levels by 91% (Fig. 3B). As expected, mutations in the binding site of Nef for SH3 domains (NefAxxA) or in the acidic cluster of Nef (NefE4A) did not significantly affect the Nef-induced reduction of CD4 surface levels. In sharp contrast, the reduction of cell surface-exposed CXCR4 was mediated at least as potently by NefEDAA as by the wt Nef protein (Fig. 3B). Furthermore, the binding site for SH3 domains and the acidic cluster motif were essential for the Nef-mediated regulation of CXCR4 cell surface levels, since the respective mutants, NefAxxA and NefE4A, displayed no downmodulating activity. In summary, downmodulation of CXCR4 and CD4 in primary T cells is mediated by genetically separable functions of HIV-1 Nef and thus likely depends on the interaction of Nef with distinct cellular ligands and machineries. In contrast, the identical motifs were required for CXCR4 and MHC-I downregulation.
CXCR4 and MHC-I accumulate in a perinuclear compartment in HIV-1 Nef-expressing cells.
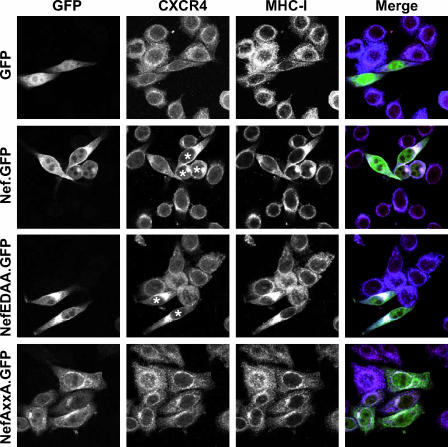
Based on the observation that Nef triggers a reduction of cell surface-exposed CXCR4, we explored the subcellular localization of CXCR4 in Nef-expressing cells by confocal immunofluorescence microscopy (Fig. 4). In human TZM cells expressing GFP or the NefAxxA.GFP mutant, CXCR4 localized to the cell surface and was associated with tubular structures throughout the cytoplasm. Occasionally, a weak perinuclear accentuation was observed in these or untransfected cells. Conversely, Nef.GFP- and NefEDAA.GFP-expressing cells displayed a reduced level of CXCR4 at the surface and, importantly, prominent CXCR4-containing clusters in the perinuclear region. In Nef.GFP- and NefEDAA.GFP-expressing cells, MHC-I and CXCR4 colocalized to a significant degree in these perinuclear clusters. Notably, Nef.GFP and NefEDAA.GFP also colocalized within these clusters, leading to a white signal in merged three-color images (Fig. 4, right panels). In summary, mediated by the identical motifs, Nef targets CXCR4 and MHC-I to perinuclear compartments where all three molecules partially colocalize.
FIG. 4.
Nef induces the accumulation of CXCR4 in a perinuclear compartment, where both molecules colocalize with MHC-I. Subcellular localization of CXCR4 and MHC-I in human TZM cells expressing the indicated Nef.GFP fusion proteins or GFP alone is shown. Shown are individual confocal sections from the centers of representative cells. GFP (green), CXCR4 (red), MHC-I (blue) are shown in merged images (right panels). White asterisks indicate prominent perinuclear accumulation of CXCR4 in Nef.GFP- or NefEDAA.GFP-expressing cells.
CXCR4 downmodulation by Nef is independent of the carboxy terminus of the chemokine receptor.
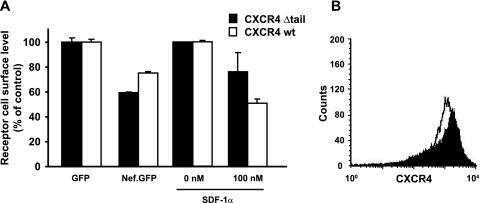
Since the carboxy-terminal tail of CXCR4 contains receptor elements which are important for basal and ligand-induced internalization (43, 51), we hypothesized that this part of the receptor may also be critical for the Nef-mediated downregulation. We studied a CXCR4 mutant that lacks all carboxy-terminal amino acids after A316 and has T311 and S312 replaced by alanine (CXCR4 Δtail) (43) and therefore is devoid of all potential serine/threonine phosphorylation sites and the alternative I328L329 endocytosis motif in the carboxy terminus (64). First, we generated CHO cells stably expressing either this carboxy-terminally truncated CXCR4 Δtail receptor or the CXCR4 wt receptor. The overall surface levels of the mutant and wt receptors were quite similar (Fig. 5B). Next, these stable cell lines were transfected with plasmids expressing either GFP alone or a Nef.GFP fusion protein. Remarkably, the CXCR4 Δtail mutant was downmodulated by Nef at least as efficiently as wt CXCR4. Consistent with a previous report (28), the SDF-1α-mediated internalization of the truncated receptor was partially abrogated (Fig. 5A). Thus, the carboxy-terminal tail of CXCR4, although involved in the SDF-1α-mediated internalization, is completely dispensable for the Nef-mediated downregulation of the receptor.
FIG. 5.
Downregulation of CXCR4 by Nef does not require motifs in the cytoplasmic tail of the receptor. CHO cells stably expressing the carboxy-terminal truncation mutant CXCR4 Δtail or wt CXCR4 were transfected with expression constructs encoding Nef.GFP of GFP alone. (A) At 1 day posttransfection, cell surface levels of CXCR4 were analyzed by flow cytometry. In parallel, untransfected cells were treated with SDF-1α (100 nM) for 1 h prior to flow cytometric analysis. (B) FACS histogram depicting CXCR4 expression levels of both stable CHO cell lines.
Genetically separable Nef motifs for reduction of CXCR4 and CD4 cell surface levels independently and synergistically inhibit fusion of the viral envelope with the plasma membrane.
To investigate whether Nef expression affects the earliest events in the infection process, i.e., the interaction of X4 HIV Env with the receptor complex and subsequent membrane fusion, we first performed a cell-to-cell fusion assay (49, 60). CHO cells stably expressing HIV-1 Tat (CHO Tat cells) were transiently transfected with expression constructs encoding various Env proteins and then mixed with TZM target cells, which stably express CD4 and CXCR4 and also contain a long terminal repeat-driven β-galactosidase gene (Fig. 6A). To assay the functional consequences of Nef expression, these target cells were, 1 day prior to mixing, transduced with adenoviruses encoding GFP fusion proteins of wt Nef, NefEDAA, or NefAxxA or GFP alone (49). In principle, fusion of both cell types results in a Tat-mediated expression of the β-galactosidase reporter, the relative enzymatic activity of which can be quantified. The validity and specificity of the assay were confirmed with antireceptor antibodies, the coreceptor antagonist AMD3100, SDF-1α, and the fusion inhibitor T-20 (data not shown).
FIG. 6.
Simultaneous downregulation of CXCR4 and CD4 by Nef correlates with maximum inhibition of X4 HIV Env-mediated fusion at the plasma membrane. (A) Assay used for quantification of Env-mediated cell-cell fusion. (B, C, and D) Fusion efficiencies of TZM target cells expressing the indicated GFP or Nef.GFP proteins after they were mixed with CHO Tat cells expressing the indicated HIV-1 Env proteins (HXB-2, NL4-3GIA, and NL4-3) were determined via β-galactosidase enzyme activity in relation to the protein concentration. (E and F) Marburg virus GP and VSV-G served as control envelopes that fuse independently of CD4 and CXCR4. The relative fusion efficiencies of GFP-expressing cells were set to 100%, and the arithmetic means + standard deviations (n = 8) are given. The experiment shown is representative of three independent experiments. *, P < 0.05 by Student's t test.
Expression of Nef.GFP in TZM target cells drastically reduced the cell-to-cell fusion efficiency for all three X4 HIV-1 Envs tested (74 to 87% reduction) (Fig. 6B to D). Expression of the discriminatory NefEDAA or NefAxxA mutants, which are deficient in the downmodulation of CD4 or CXCR4, respectively, also markedly interfered with fusion, but with slightly reduced efficiency relative to Nef.GFP. This observation is consistent with a synergistic effect on membrane fusion when both CD4 and CXCR4 are downmodulated by Nef. As specificity controls, Env proteins derived from unrelated viruses, which do not require CD4 or the CXCR4 coreceptor for fusion, were used in this assay. Cell-cell fusion mediated by Marburg virus GP (Fig. 6E) or VSV-G (Fig. 6F) was not significantly affected by the presence of Nef. Together, these results demonstrate that Nef expression drastically and specifically interferes with HIV Env-mediated cell fusion. Our data suggest that Nef mediates this efficient protection from X4 HIV Env fusion by simultaneous removal of both components of the entry receptor-coreceptor complex from the cell surface.
Expression of a naturally occurring Nef allele that downregulates CXCR4, but not CD4, inhibits fusion of HIV-1 virions with T cells.
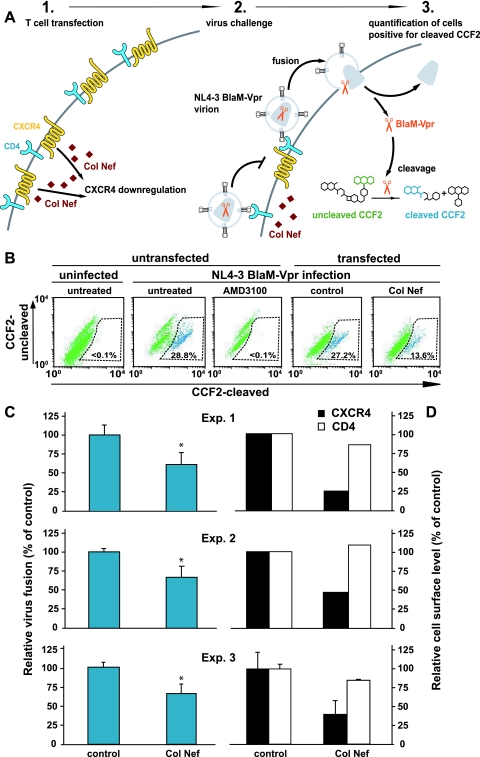
To corroborate our finding that the Nef-mediated downregulation of the HIV coreceptor CXCR4 alone can interfere with HIV Env-mediated fusion under more natural conditions, we next adapted a recently developed flow cytometry-based virion fusion assay (12) to study directly the efficiency of viral entry in Nef-expressing target T cells (Fig. 7A). This assay system is based on the incorporation of BlaM-Vpr chimeric proteins into replication-competent HIV-1 virions and their subsequent delivery into the cytoplasm of the target T cells as a result of virion fusion. Cleavage of a fluorescent dye (CCF2) which is loaded into target cells allows for detection of fusion events by flow cytometry. In order to exclusively analyze the effect of Nef-mediated CXCR4 downregulation on virion fusion, we expressed Col Nef, which potently reduces cell surface levels of CXCR4, but not CD4, on human T cells (73) (Fig. 7D).
FIG. 7.
Fusion of HIV-1NL4-3 virions is significantly inhibited in T cells expressing Col Nef, which downregulates CXCR4 but not CD4, from the surface. (A) Experimental setup. Jurkat T cells were transfected with a plasmid encoding Col Nef or a control plasmid together with a plasmid for mRFP. At 48 h posttransfection, cells were either infected with HIV-1NL4-3 BlaM-Vpr virions or stained for cell surface levels of CXCR4 and CD4. As a specificity control, cells were treated with the CXCR4 antagonist AMD3100 (50 μM) 30 min prior to infection. (B) Representative dot plots of the flow cytometric detection of the CCF2 substrate cleavage after virion fusion. (C) Cleaved CCF2-positive cells among Col Nef-expressing cells were quantified and calculated as a percentage of cleaved-CCF2-positve cells relative to control-transfected cells. (D) Respective cell surface levels of CXCR4 and CD4 on Jurkat T cells in each experiment. Values represent the arithmetic means + standard deviations of triplicates; results of three independent experiments (Exp.1, 2, and 3) are shown. *, P < 0.05 by Student's t test.
Here, Jurkat T cells were first transfected with either a Col Nef expression plasmid or a control plasmid together with an expression plasmid encoding for mRFP as a subsequent reporter for transfected cells. At 2 days posttransfection, cells were challenged with HIV-1NL4-3 BlaM-Vpr virions for 6 h and subsequently stained with CCF2 overnight. In Col Nef-expressing cells CXCR4 was reduced by 55 to 80%, while CD4 surface levels were not significantly affected relative to control cells (Fig. 7D). Expression of Col Nef moderately (34 to 39%) but significantly reduced HIV-1NL4-3 virion fusion in three independent experiments (Student's t test, P < 0.05) (Fig. 7C). As a specificity control, AMD3100 treatment completely abrogated fusion events following viral challenge (Fig. 7B). In summary, this virion fusion assay independently shows that the Nef-mediated downregulation of the CXCR4 coreceptor can contribute to the ability of productively infected cells to resist superinfection by additional viruses.
DISCUSSION
This study demonstrates the ability of the accessory protein Nef to efficiently downregulate the chemokine receptor CXCR4 from the surfaces of HIV-infected primary CD4 T cells and a variety of cell lines transiently expressing lentiviral Nef proteins. This finding extends our recent observation of a Nef-induced reduction of cell surface-exposed CCR5 (49) to the second major HIV coreceptor. In infected primary CD4 T cells, the magnitude of the Nef-mediated CXCR4 downregulation was within the range of that observed for the Nef-susceptible MHC-I haplotype HLA-A2 or that induced by treatment with SDF-1α. Functionally, the Nef-mediated downregulation of CXCR4 from the surfaces of target T cells was sufficient to significantly diminish cells' susceptibility to incoming X4 HIV-1 virions at the entry step. This underscores the potential importance of Nef's strategy to synergistically target the binding receptor CD4 and the major entry coreceptors to accomplish maximum interference with superinfection (this study and reference 49).
Downregulation of CXCR4 and MHC-I molecules, as expected, required signature motifs in HIV-1SF2 Nef that have been reported to mediate the interaction of the viral protein with phosphofurin acidic cluster sorting protein 1 as well as several cellular proteins harboring SH3 domains. On the other hand, motifs in the carboxy-terminal flexible loop of Nef that link the early viral gene product to proteins, including AP-2, for clathrin-dependent endocytosis and vesicle trafficking were completely dispensable for CXCR4 downregulation, although these motifs are required for CD4 internalization. Interestingly, for Nef from SIVMac239 a recent study showed that motifs which connect the viral protein to the AP-2 clathrin adapter and which are required for CD4 downregulation were essential for CXCR4 downregulation by this allele (30). Thus, Nef proteins from HIV-1 and SIV both downregulate CXCR4 from the cell surface; however, they have apparently evolved fundamentally different and genetically distinct strategies for this activity. Notably, Hrecka et al. (30) observed a markedly higher degree of CXCR4 downregulation for Nef proteins from SIV and HIV-2, compared to HIV-1, in an infected T-cell line. For the lentiviral Nef alleles that we have analyzed in our transfection studies in primary CD4 T cells and CHO hCD4/hCXCR4 cells (data not shown), including that from SIVMac239, we did not note such a difference.
Based on the identical Nef motif requirement, it is tempting to speculate that the downmodulation of CXCR4 occurs via a mechanism similar to that used for the reduction of MHC-I and CCR5 cell surface levels. This would predict that Nef leads to an acceleration of the endocytosis rate of CXCR4, which conceivably contributes to its reduced steady-state levels on the surfaces of Nef-expressing cells (1, 49, 63). Unfortunately, this could not be assessed, since direct labeling of the receptor (49, 63) in untransfected control cells with anti-CXCR4 MAb 12G5 or 4G10 per se either drastically accelerated (4G10) or inhibited (12G5) the basal endocytosis rate of CXCR4 (data not shown). Similarly, Western blot or immunoprecipitation detection of endogenous CXCR4 is notoriously difficult (reference 13 and data not shown), which precluded alternative experimental approaches to assess receptor endocytosis. As an additional strategy, Nef may also disrupt the anterograde transport or recycling of receptors, as has been reported for MHC-I (6, 58).
Regardless of the mechanism employed by Nef to intercept the trafficking of CXCR4, both the CCR5 and CXCR4 coreceptors as well as MHC-I are targeted by Nef via the same signature motifs to the perinuclear region, in which all molecules accumulate and, to a significant extent, colocalize (6, 49, 56; this report). For MHC-I, two studies suggested the compartment to be the trans-Golgi network (6, 56). Careful ultrastructural and mechanistic studies of these compartments will be needed to unambiguously define their identity and the exact pathways employed by Nef to target different cell surface receptors, some of which are structurally completely unrelated, to these compartments via identical signature motifs in the viral protein.
On the receptor side, the carboxy terminus of CXCR4 is critical for SDF-1α-induced enhancement of receptor endocytosis and signaling (51, 71). Interestingly, a previously described carboxy-terminal truncation mutant (CXCR4 Δtail) (43) and wt CXCR4 were downregulated by Nef with comparable efficiencies. This demonstrates that motifs in the carboxy terminus of CXCR4 are not required for the Nef-induced reduction of surface-exposed coreceptor levels and suggests fundamentally different mechanisms by which the natural ligand and Nef remove CXCR4 from the cell surface. This is also in line with our recent data indicating that the Nef-mediated downregulation of the entire family of G protein-coupled seven-transmembrane chemokine receptors occurs via a novel mechanism and may involve the highly conserved DRY box in the second intracellular loop (50).
We pursued two experimental strategies to assess the functional significance of the Nef-mediated reduction in CXCR4 cell surface exposure in the context of superinfection interference. To explore a scenario in which expression of the early gene product prevents infection by additional virions at the entry step, we first used a cell-cell fusion assay (49, 60). Transient expression of wt Nef in TZM target cells drastically and specifically interfered with X4 HIV Env-mediated cell fusion. Expression of discriminatory Nef mutants suggested that the viral protein achieved maximum protection by simultaneous removal of both components of the entry receptor complex, CD4 and CXCR4, from the cell surface.
Second, to corroborate our finding that the Nef-mediated reduction of CXCR4 surface levels, in the absence of CD4 downregulation, can interfere with HIV Env-mediated fusion under more natural conditions, we adapted a recently described flow cytometry-based virion fusion assay (12). Here, we exploited the ability of the Col Nef allele to potently reduce surface levels of CXCR4 but not of CD4 (73). Expression of Col Nef in human Jurkat T cells moderately but significantly inhibited X4 HIV-1 virion fusion. Thus, both the cell- and virion-based fusion assays suggest that Nef-mediated downregulation of CXCR4 alone can impair the ability of the HIV receptor complex to allow fusion and thus enhances resistance to superinfection. Not surprisingly, maximum interference of Nef with superinfection at the entry step appeared to require both CD4 and CXCR4 downmodulation.
These results are consistent with data from other laboratories that have used knock-down approaches to investigate the functional importance of altered CXCR4 levels in HIV fusion and infection. A recent study reported a marked inhibition of X4 HIV Env fusion efficiency (65 to 75% reduction) in HOS CD4/CXCR4 cells, in which CXCR4 levels had been decreased by 80% by delivery of small interfering RNA (siRNA) (76). Another study employing short hairpin RNA against CXCR4 and CCR5 in MAGI-CXCR4 and GHOST-CCR5 cells showed that a ∼70% downregulation of the coreceptors resulted in an over 10-fold reduction in viral antigen levels in culture supernatants (3). These findings are also in line with a third study, which demonstrated that siRNA-mediated suppression of CXCR4 (63% reduction) and CCR5 (48% reduction) in U87 cell lines resulted in strongly impaired productive infections by X4 HIV-1NL4-3 (75 to 82% reduction) and by R5 HIV-1Ba-L (64 to 85% reduction), respectively (47). Thus, Nef and selective siRNAs can reduce cell surface levels of CXCR4 to similar degrees, and data from several experimental systems support the notion that this degree of reduction of coreceptor levels can have a marked effect on HIV (super-)infection at the entry step. However, our studies cannot exclude the possibility that Nef, besides downmodulating the entry receptor complex, also employs strategies at a postentry level to achieve maximum interference with superinfection.
What implications of the Nef-mediated CXCR4 downmodulation can be envisioned for HIV infections in vivo? The signature motifs in Nef that we identified as critical for CXCR4 downregulation have previously been implicated in activities of Nef in the context of HIV-1 replication, such as enhancement of virion infectivity and virus spread (reviewed in reference 24). Interestingly, both the proline-rich stretch and the acidic cluster motif in Nef are required for full pathogenicity of HIV and SIV in vivo (8-10, 27). So far, this correlation had been interpreted as a reflection of Nef's role in immune evasion by MHC-I downmodulation (17). Our results on HIV-1 Nef-mediated downmodulation of CXCR4 (this paper) and CCR5 (49) show the requirement for these protein interaction sites for maximum protection from HIV Env-plasma membrane fusion and HIV superinfection, suggesting that this Nef activity may be important in vivo. Interference with superinfection may protect the infected cell from accumulation of unintegrated viral genomes that are cytotoxic and may thus impede virus propagation (53, 57). By rendering cells refractory to superinfection, Nef also facilitates viral spread to uninfected cells by interfering with the loss of newly generated virus particles to cells with an established infection. Notably, the frequency of CXCR4 coreceptor usage is high among HIV-1 isolates, lower among HIV-2 isolates, and absent for SIV strains (5), while in our hands Nef proteins from all three primate lentiviruses downregulated CXCR4 with comparable efficiencies. This suggests that additional functions other than interference with superinfection may have supported the evolutionary conservation of the Nef activities that modulate surface exposure of CXCR4.
Nef may also facilitate proper morphogenesis and thus higher infectivity of viral progeny by preventing premature interactions of CXCR4 with the viral Env. In support of this model, Lallos et al. have reported a virtual exclusion of coreceptors from HIV particles derived from cell lines expressing CXCR4, CCR5, and CCR3 (41). Furthermore, X4 HIV-1 Env and SDF-1α can signal through CXCR4 and induce apoptosis (11, 29, 38). The Nef-mediated reduction of the CXCR4 surface density could protect the productively infected cell from these undesired consequences. As an additional viral evasion function, CXCR4 downmodulation could inhibit the migration of infected CD4 T cells towards SDF-1α-secreting cytotoxic T lymphocytes within lymphoid tissue (16, 75). Indeed, it has been shown that the SIVMac239 Nef protein inhibits the migration of lymphocytes to SDF-1α, in part, by downregulating CXCR4 (30). In this context, two studies have also reported that HIV-1 Nef inhibits SDF-1α-induced chemotaxis of T lymphocytes by affecting downstream signaling effectors (15, 31). Similarly, Nef also downregulates chemokine receptors that do not serve as coreceptors for HIV entry (50).
In conclusion, our findings establish a conserved ability of lentiviral Nef proteins to potently reduce the surface exposure of the chemokine coreceptor CXCR4 on primary target cells of HIV infection and highlight the importance of this Nef function to maximize interference with superinfection by additional virions already at the entry step.
ADDENDUM
Following submission of this paper, Wildum et al. (73) reported that expression of Nef proteins interferes with X4 HIV superinfection and suggested that maximal protection from superinfection involved mechanisms at the level of the receptor-coreceptor complex and by an as-yet-unknown mechanism independent of CD4 and coreceptor downregulation. Notably, this study provided direct experimental evidence that superinfected cells are indeed more prone to premature apoptotic cell death, as previously suggested (53, 57).
Acknowledgments
We thank Hans-Georg Kräusslich for support and stimulating discussions. We thank Christopher Aiken, Christopher Broder, Jane Burns, Matthias Dittmar, Jose Esté, Frank Kirchhoff, Nathaniel Landau, Jan Münch, Stephen Peiper, Jacek Skowronski and Stephan Urban, and Simone Giese for kindly providing reagents. We thank Dieter Stefan for FACS and the Gladstone Graphics Department for assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft to O.T.K. (Ke 742/2) and to O.T.F. (SFB 638 project A11 and TR13 project C4), by a fellowship from the C.H.S. Stiftung to O.T.F., and by subcontract R0051-B from the J. David Gladstone Institutes to O.T.K.
Footnotes
Published ahead of print on 23 August 2006.
REFERENCES
- 1.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 2.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson, J., and R. Akkina. 2005. HIV-1 resistance conferred by siRNA cosuppression of CXCR4 and CCR5 coreceptors by a bispecific lentiviral vector. AIDS Res. Ther. 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arora, V. K., B. L. Fredericksen, and J. V. Garcia. 2002. Nef: agent of cell subversion. Microbes Infect. 4:189-199. [DOI] [PubMed] [Google Scholar]
- 5.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 6.Blagoveshchenskaya, A. D., L. Thomas, S. F. Feliciangeli, C. H. Hung, and G. Thomas. 2002. HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111:853-866. [DOI] [PubMed] [Google Scholar]
- 7.Breiner, K. M., S. Urban, B. Glass, and H. Schaller. 2001. Envelope protein-mediated down-regulation of hepatitis B virus receptor in infected hepatocytes. J. Virol. 75:143-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carl, S., T. C. Greenough, M. Krumbiegel, M. Greenberg, J. Skowronski, J. L. Sullivan, and F. Kirchhoff. 2001. Modulation of different human immunodeficiency virus type 1 Nef functions during progression to AIDS. J. Virol. 75:3657-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carl, S., A. J. Iafrate, S. M. Lang, C. Stahl-Hennig, E. M. Kuhn, D. Fuchs, K. Matz-Rensing, P. ten Haaft, J. L. Heeney, J. Skowronski, and F. Kirchhoff. 1999. The acidic region and conserved putative protein kinase C phosphorylation site in Nef are important for SIV replication in rhesus macaques. Virology 257:138-155. [DOI] [PubMed] [Google Scholar]
- 10.Casartelli, N., G. Di Matteo, C. Argentini, C. Cancrini, S. Bernardi, G. Castelli, G. Scarlatti, A. Plebani, P. Rossi, and M. Doria. 2003. Structural defects and variations in the HIV-1 nef gene from rapid, slow and non-progressor children. AIDS 17:1291-1301. [DOI] [PubMed] [Google Scholar]
- 11.Castedo, M., K. F. Ferri, J. Blanco, T. Roumier, N. Larochette, J. Barretina, A. Amendola, R. Nardacci, D. Metivier, J. A. Este, M. Piacentini, and G. Kroemer. 2001. Human immunodeficiency virus 1 envelope glycoprotein complex-induced apoptosis involves mammalian target of rapamycin/FKBP12-rapamycin-associated protein-mediated p53 phosphorylation. J. Exp. Med. 194:1097-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cavrois, M., C. De Noronha, and W. C. Greene. 2002. A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat. Biotechnol. 20:1151-1154. [DOI] [PubMed] [Google Scholar]
- 13.Chabot, D. J., H. Chen, D. S. Dimitrov, and C. C. Broder. 2000. N-linked glycosylation of CXCR4 masks coreceptor function for CCR5-dependent human immunodeficiency virus type 1 isolates. J. Virol. 74:4404-4413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaudhry, A., S. R. Das, A. Hussain, S. Mayor, A. George, V. Bal, S. Jameel, and S. Rath. 2005. The Nef protein of HIV-1 induces loss of cell surface costimulatory molecules CD80 and CD86 in APCs. J. Immunol. 175:4566-4574. [DOI] [PubMed] [Google Scholar]
- 15.Choe, E. Y., E. S. Schoenberger, J. E. Groopman, and I. W. Park. 2002. HIV Nef inhibits T cell migration. J. Biol. Chem. 277:46079-46084. [DOI] [PubMed] [Google Scholar]
- 16.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 17.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 18.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Craig, H. M., M. W. Pandori, and J. C. Guatelli. 1998. Interaction of HIV-1 Nef with the cellular dileucine-based sorting pathway is required for CD4 down-regulation and optimal viral infectivity. Proc. Natl. Acad. Sci. USA 95:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 21.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 22.Fackler, O. T., and A. S. Baur. 2002. Live and let die: Nef functions beyond HIV replication. Immunity 16:493-497. [DOI] [PubMed] [Google Scholar]
- 23.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 24.Geyer, M., O. T. Fackler, and B. M. Peterlin. 2001. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg, M., L. DeTulleo, I. Rapoport, J. Skowronski, and T. Kirchhausen. 1998. A dileucine motif in HIV-1 Nef is essential for sorting into clathrin-coated pits and for downregulation of CD4. Curr. Biol. 8:1239-1242. [DOI] [PubMed] [Google Scholar]
- 26.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanna, Z., X. Weng, D. G. Kay, J. Poudrier, C. Lowell, and P. Jolicoeur. 2001. The pathogenicity of human immunodeficiency virus (HIV) type 1 Nef in CD4C/HIV transgenic mice is abolished by mutation of its SH3-binding domain, and disease development is delayed in the absence of Hck. J. Virol. 75:9378-9392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haribabu, B., R. M. Richardson, I. Fisher, S. Sozzani, S. C. Peiper, R. Horuk, H. Ali, and R. Snyderman. 1997. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. J. Biol. Chem. 272:28726-28731. [DOI] [PubMed] [Google Scholar]
- 29.Holm, G. H., C. Zhang, P. R. Gorry, K. Peden, D. Schols, E. De Clercq, and D. Gabuzda. 2004. Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J. Virol. 78:4541-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hrecka, K., T. Swigut, M. Schindler, F. Kirchhoff, and J. Skowronski. 2005. Nef proteins from diverse groups of primate lentiviruses downmodulate CXCR4 to inhibit migration to the chemokine stromal derived factor 1. J. Virol. 79:10650-10659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janardhan, A., T. Swigut, B. Hill, M. P. Myers, and J. Skowronski. 2004. HIV-1 Nef binds the DOCK2-ELMO1 complex to activate Rac and inhibit lymphocyte chemotaxis. PLoS Biol. 2:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayakumar, P., I. Berger, F. Autschbach, M. Weinstein, B. Funke, E. Verdin, M. A. Goldsmith, and O. T. Keppler. 2005. Tissue-resident macrophages are productively infected ex vivo by primary X4 isolates of human immunodeficiency virus type 1. J. Virol. 79:5220-5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kasper, M. R., and K. L. Collins. 2003. Nef-mediated disruption of HLA-A2 transport to the cell surface in T cells. J. Virol. 77:3041-3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keppler, O. T., N. Tibroni, S. Venzke, S. Rauch, and O. T. Fackler. 2006. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein of HIV-1. J. Leukoc Biol. 79:616-627. [DOI] [PubMed] [Google Scholar]
- 35.Keppler, O. T., F. J. Welte, T. A. Ngo, P. S. Chin, K. S. Patton, C. L. Tsou, N. W. Abbey, M. E. Sharkey, R. M. Grant, Y. You, J. D. Scarborough, W. Ellmeier, D. R. Littman, M. Stevenson, I. F. Charo, B. G. Herndier, R. F. Speck, and M. A. Goldsmith. 2002. Progress toward a human CD4/CCR5 transgenic rat model for de novo infection by human immunodeficiency virus type 1. J Exp. Med. 195:719-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keppler, O. T., W. Yonemoto, F. J. Welte, K. S. Patton, D. Iacovides, R. E. Atchison, T. Ngo, D. L. Hirschberg, R. F. Speck, and M. A. Goldsmith. 2001. Susceptibility of rat-derived cells to replication by human immunodeficiency virus type 1. J. Virol. 75:8063-8073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kestler, H. W., and K. T. Jeang. 1995. Attenuated retrovirus vaccines and AIDS. Science 270:1219-1222. [PubMed] [Google Scholar]
- 38.Khan, M. Z., R. Brandimarti, J. P. Patel, N. Huynh, J. Wang, Z. Huang, A. Fatatis, and O. Meucci. 2004. Apoptotic and antiapoptotic effects of CXCR4: is it a matter of intrinsic efficacy? Implications for HIV neuropathogenesis. AIDS Res. Hum. Retrovir. 20:1063-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirchhoff, F., T. C. Greenough, D. B. Brettler, J. L. Sullivan, and R. C. Desrosiers. 1995. Absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 332:228-232. [DOI] [PubMed] [Google Scholar]
- 40.Krautkramer, E., S. I. Giese, J. E. Gasteier, W. Muranyi, and O. T. Fackler. 2004. Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts. J. Virol. 78:4085-4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lallos, L. B., S. Laal, J. A. Hoxie, S. Zolla-Pazner, and J. C. Bandres. 1999. Exclusion of HIV coreceptors CXCR4, CCR5, and CCR3 from the HIV envelope. AIDS Res. Hum. Retrovir. 15:895-897. [DOI] [PubMed] [Google Scholar]
- 42.Lama, J. 2003. The physiological relevance of CD4 receptor down-modulation during HIV infection. Curr. HIV Res. 1:167-184. [DOI] [PubMed] [Google Scholar]
- 43.Lee, B., B. J. Doranz, M. Z. Ratajczak, and R. W. Doms. 1998. An intricate web: chemokine receptors, HIV-1 and hematopoiesis. Stem Cells 16:79-88. [DOI] [PubMed] [Google Scholar]
- 44.Levesque, K., A. Finzi, J. Binette, and E. A. Cohen. 2004. Role of CD4 receptor down-regulation during HIV-1 infection. Curr. HIV Res. 2:51-59. [DOI] [PubMed] [Google Scholar]
- 45.Lu, X., H. Yu, S. H. Liu, F. M. Brodsky, and B. M. Peterlin. 1998. Interactions between HIV1 Nef and vacuolar ATPase facilitate the internalization of CD4. Immunity 8:647-656. [DOI] [PubMed] [Google Scholar]
- 46.Mangasarian, A., V. Piguet, J. K. Wang, Y. L. Chen, and D. Trono. 1999. Nef-induced CD4 and major histocompatibility complex class I (MHC-I) down-regulation are governed by distinct determinants: N-terminal alpha helix and proline repeat of Nef selectively regulate MHC-I trafficking. J. Virol. 73:1964-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martinez, M. A., A. Gutierrez, M. Armand-Ugon, J. Blanco, M. Parera, J. Gomez, B. Clotet, and J. A. Este. 2002. Suppression of chemokine receptor expression by RNA interference allows for inhibition of HIV-1 replication. AIDS 16:2385-2390. [DOI] [PubMed] [Google Scholar]
- 48.Mbemba, E., L. Saffar, and L. Gattegno. 2002. Role of N-glycans and SDF-1alpha on the coassociation of CD4 with CXCR4 at the plasma membrane of monocytic cells and blood lymphocytes. FEBS Lett. 514:209-213. [DOI] [PubMed] [Google Scholar]
- 49.Michel, N., I. Allespach, S. Venzke, O. T. Fackler, and O. T. Keppler. 2005. The Nef protein of human immunodeficiency virus establishes superinfection immunity by a dual strategy to downregulate cell surface CCR5 and CD4. Curr. Biol. 15:714-723. [DOI] [PubMed] [Google Scholar]
- 50.Michel, N., K. Ganter, S. Venzke, J. Bitzegeio, O. T. Fackler, and O. T. Keppler. 2006. The Nef Protein of human immunodeficiency virus is a broad-spectrum modulator of chemokine receptor cell surface levels that acts independently of classical motifs for receptor endocytosis and Gαi-signaling. Mol. Biol. Cell 14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Neel, N. F., E. Schutyser, J. Sai, G. H. Fan, and A. Richmond. 2005. Chemokine receptor internalization and intracellular trafficking. Cytokine Growth Factor Rev. 16:637-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nokta, M. A., X. D. Li, J. Nichols, M. Mallen, A. Pou, D. Asmuth, and R. B. Pollard. 2001. Chemokine/CD4 receptor density ratios correlate with HIV replication in lymph node and peripheral blood of HIV-infected individuals. AIDS 15:161-169. [DOI] [PubMed] [Google Scholar]
- 53.Pauza, C. D., J. E. Galindo, and D. D. Richman. 1990. Reinfection results in accumulation of unintegrated viral DNA in cytopathic and persistent human immunodeficiency virus type 1 infection of CEM cells. J. Exp. Med. 172:1035-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paxton, W. A., and S. Kang. 1998. Chemokine receptor allelic polymorphisms: relationships to HIV resistance and disease progression. Semin. Immunol. 10:187-194. [DOI] [PubMed] [Google Scholar]
- 55.Philpott, S. M. 2003. HIV-1 coreceptor usage, transmission, and disease progression. Curr. HIV Res. 1:217-227. [DOI] [PubMed] [Google Scholar]
- 56.Piguet, V., L. Wan, C. Borel, A. Mangasarian, N. Demaurex, G. Thomas, and D. Trono. 2000. HIV-1 Nef protein binds to the cellular protein PACS-1 to downregulate class I major histocompatibility complexes. Nat. Cell Biol. 2:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson, H. L., and D. M. Zinkus. 1990. Accumulation of human immunodeficiency virus type 1 DNA in T cells: results of multiple infection events. J. Virol. 64:4836-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roeth, J. F., M. Williams, M. R. Kasper, T. M. Filzen, and K. L. Collins. 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J. Cell Biol. 167:903-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rose, J. J., K. Janvier, S. Chandrasekhar, R. P. Sekaly, J. S. Bonifacino, and S. Venkatesan. 2005. CD4 down-regulation by HIV-1 and simian immunodeficiency virus (SIV) Nef proteins involves both internalization and intracellular retention mechanisms. J. Biol. Chem. 280:7413-7426. [DOI] [PubMed] [Google Scholar]
- 60.Rucker, E., J. Munch, S. Wildum, M. Brenner, J. Eisemann, L. Margolis, and F. Kirchhoff. 2004. A naturally occurring variation in the proline-rich region does not attenuate human immunodeficiency virus type 1 Nef function. J. Virol. 78:10197-10201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schindler, M., S. Wurfl, P. Benaroch, T. C. Greenough, R. Daniels, P. Easterbrook, M. Brenner, J. Munch, and F. Kirchhoff. 2003. Down-modulation of mature major histocompatibility complex class II and up-regulation of invariant chain cell surface expression are well-conserved functions of human and simian immunodeficiency virus nef alleles. J. Virol. 77:10548-10556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 96:8167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 64.Signoret, N., M. M. Rosenkilde, P. J. Klasse, T. W. Schwartz, M. H. Malim, J. A. Hoxie, and M. Marsh. 1998. Differential regulation of CXCR4 and CCR5 endocytosis. J. Cell Sci. 111:2819-2830. [DOI] [PubMed] [Google Scholar]
- 65.Sol-Foulon, N., A. Moris, C. Nobile, C. Boccaccio, A. Engering, J. P. Abastado, J. M. Heard, Y. van Kooyk, and O. Schwartz. 2002. HIV-1 Nef-induced upregulation of DC-SIGN in dendritic cells promotes lymphocyte clustering and viral spread. Immunity 16:145-155. [DOI] [PubMed] [Google Scholar]
- 66.Stove, V., I. Van de Walle, E. Naessens, E. Coene, C. Stove, J. Plum, and B. Verhasselt. 2005. Human immunodeficiency virus Nef induces rapid internalization of the T-cell coreceptor CD8αβ. J. Virol. 79:11422-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stumptner-Cuvelette, P., S. Morchoisne, M. Dugast, S. Le Gall, G. Raposo, O. Schwartz, and P. Benaroch. 2001. HIV-1 Nef impairs MHC class II antigen presentation and surface expression. Proc. Natl. Acad. Sci. USA 98:12144-12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Thoulouze, M. I., N. Sol-Foulon, F. Blanchet, A. Dautry-Varsat, O. Schwartz, and A. Alcover. 2006. Human immunodeficiency virus type-1 infection impairs the formation of the immunological synapse. Immunity 24:547-561. [DOI] [PubMed] [Google Scholar]
- 69.Tolstrup, M., L. Ostergaard, A. L. Laursen, S. F. Pedersen, and M. Duch. 2004. HIV/SIV escape from immune surveillance: focus on Nef. Curr. HIV Res. 2:141-151. [DOI] [PubMed] [Google Scholar]
- 70.Tuttle, D. L., J. K. Harrison, C. Anders, J. W. Sleasman, and M. M. Goodenow. 1998. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. J. Virol. 72:4962-4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Verani, A., and P. Lusso. 2002. Chemokines as natural HIV antagonists. Curr. Mol. Med. 2:691-702. [DOI] [PubMed] [Google Scholar]
- 72.Wang, J. K., E. Kiyokawa, E. Verdin, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA 97:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wildum, S., M. Schindler, J. Münch, and F. Kirchhoff. 2006. Contribution of Vpu, Env, and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infectd T cells to superinfection. J. Virol. 80:8047-8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams, M., J. F. Roeth, M. R. Kasper, T. M. Filzen, and K. L. Collins. 2005. Human immunodeficiency virus type 1 Nef domains required for disruption of major histocompatibility complex class I trafficking are also necessary for coprecipitation of Nef with HLA-A2. J. Virol. 79:632-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaitseva, M., K. Peden, and H. Golding. 2003. HIV coreceptors: role of structure, posttranslational modifications, and internalization in viral-cell fusion and as targets for entry inhibitors. Biochim. Biophys. Acta 1614:51-61. [DOI] [PubMed] [Google Scholar]
- 76.Zhou, N., J. Fang, M. Mukhtar, E. Acheampong, and R. J. Pomerantz. 2004. Inhibition of HIV-1 fusion with small interfering RNAs targeting the chemokine coreceptor CXCR4. Gene Ther. 11:1703-1712. [DOI] [PubMed] [Google Scholar]