Abstract
The mechanisms of CD4+ T-cell depletion during human immunodeficiency virus type 1 (HIV-1) infection remain incompletely characterized. Of particular importance is how CD4+ T cells are depleted within the lymphoid organs, including the lymph nodes and thymus. Herein we characterize the pathogenic mechanisms of an envelope from a rapid progressor (R3A Env) in the NL4-3 backbone (NL4-R3A) which is able to efficiently replicate and deplete CD4+ thymocytes in the human fetal-thymus organ culture (HF-TOC). We demonstrate that uninterrupted replication is required for continual thymocyte depletion. During depletion, NL4-R3A induces an increase in thymocytes which uptake 7AAD, a marker of cell death, and which express active caspase-3, a marker of apoptosis. While 7AAD uptake is observed predominantly in uninfected thymocytes (p24−), active caspase-3 is expressed in both infected (p24+) and uninfected thymocytes (p24−). When added to HF-TOC with ongoing infection, the protease inhibitor saquinavir efficiently suppresses NL4-R3A replication. In contrast, the fusion inhibitors T20 and C34 allow for sustained HIV-1 production. Interestingly, T20 and C34 effectively prevent thymocyte depletion in spite of this sustained replication. Apoptosis of both p24− and p24+ thymocytes appears to be envelope fusion dependent, as T20, but not saquinavir, is capable of reducing thymocyte apoptosis. Together, our data support a model whereby pathogenic envelope-dependent fusion contributes to thymocyte depletion in HIV-1-infected thymus, correlated with induction of apoptosis in both p24+ and p24− thymocytes.
Infection with human immunodeficiency virus type 1 (HIV-1) is characterized by progressive depletion of CD4+ T cells and eventual progression to AIDS. The mechanisms responsible for CD4+ T-cell depletion are still not fully understood. While it was initially thought that direct infection of target cells was responsible for T-cell depletion (26, 55), subsequent observations suggested a contribution of indirect or bystander killing of uninfected cells (reviewed in reference 24). Throughout infection, less than 1% of peripheral target cells are infected (8, 11), while most apoptotic T cells in lymphoid organs of infected children and simian immunodeficiency virus (SIV)-infected macaques are not productively infected (1, 19). Increased bystander cell death during chronic infection may represent activation-induced cell death consistent with an immune response to a chronic pathogen (24, 42). Because lack of immune activation in conjunction with high viral loads is observed in sooty mangabees that do not develop disease (9, 32, 43), bystander activation likely plays a role in human progression to AIDS.
In contrast to chronic infection, acute infection is characterized by massive and rapid depletion of CD4+ memory T cells, particularly in the gut-associated lymphoid tissue, that is thought to occur primarily through direct viral infection and lysis (7, 23, 25, 51, 52). Greater understanding of the mechanisms by which transmitted viruses mediate T-cell depletion during acute infection will improve our understanding of HIV-1 pathogenesis. In particular, the dynamics and mechanisms of cell depletion in solid lymphoid organs, including the gut, lymph nodes, spleen, and thymus, require further elucidation.
A number of in vivo and ex vivo organ systems have been developed as models to study HIV-1-induced CD4+ T-cell depletion. These peripheral blood lymphocyte include the SCID-hu, SCID-hu thymus/liver,lymph node organ culture (or tonsil histoculture) and the human fetal thymus-organ culture (HF-TOC). All offer primary cell microenvironments that do not require exogenous stimulation for replication of primary HIV-1 isolates (18, 21, 22) and in some cases are refractory to replication by tissue culture-adapted isolates (40, 49). These systems differ from human infection in that they cannot support an adaptive immune response against HIV. Rather, they serve as models for what might happen in lymphoid organs in vivo if innate immunity was the lone defense against viral replication, such as during acute infection. Evidence from these models has indicated a prominent role for bystander apoptosis (31, 41) and direct viral lysis (22, 33) as mechanisms of T-cell depletion.
The thymus is an apoptotic factory designed to produce new naïve T cells and eliminate auto- or nonreactive T cells by apoptosis. It is a target for HIV-1 infection, and its disruption has been correlated with disease progression in pediatric patients (13, 34, 53). Furthermore, recovery of thymic function after highly active antiretroviral therapy has been correlated with immune recovery (15-17, 36). Thymic sections from HIV-1-infected humans or SIV/SHIV-infected macaques show increased apoptosis, suggesting that HIV-1 can either directly or indirectly hasten thymocyte depletion (28, 29, 45, 47, 56). A number of studies addressing mechanisms of CD4+ thymocyte death in the thymus organ have indicated that both direct viral lysis and bystander apoptosis occur during thymocyte depletion (5, 6, 30, 48). Whether bystander apoptosis is specifically induced by HIV-1 or occurs nonspecifically after the bulk of lysis-induced thymocyte depletion remains a subject of ongoing debate.
Herein we characterize the pathogenic mechanisms of an envelope from a rapid progressor (R3A Env) in the NL4-3 backbone (NL4-R3A) which is able to mediate efficient replication and depletion of CD4+ thymocytes in the human fetal-thymus organ culture (HF-TOC). Notably, the R3A Env is capable of using both CCR5 and CXCR4 as entry coreceptors (37, 38). We demonstrate that uninterrupted replication is required for continual thymocyte depletion. During depletion, NL4-R3A induces an increase in thymocytes which uptake 7AAD, a marker of cell death, and express active caspase-3, a marker of apoptosis. While 7AAD is observed predominantly in uninfected thymocytes (p24−), active caspase-3 is expressed in both infected (p24+) and uninfected thymocytes (p24−). While the anti-HIV drug saquinavir efficiently suppresses ongoing NL4-R3A replication, the fusion inhibitors T20 and C34 allow for sustained HIV-1 production. Interestingly, T20 and C34 effectively prevent thymocyte depletion in spite of this sustained replication. Apoptosis of both p24− and p24+ thymocytes appears to be envelope fusion dependent, as the fusion inhibitors T20 and C34, but not the protease inhibitor saquinavir, are capable of reducing thymocyte apoptosis. These data are the first to describe Env-specific and fusion-dependent induction of apoptosis in a relevant lymphoid organ model.
MATERIALS AND METHODS
Viral isolates and drugs.
The NL4-R3A and NL4-R3B viruses have been previously described (38). Saquinavir (National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]) was dissolved in dimethyl sulfoxide at a 10 mM concentration and was used in HF-TOC at a 1 μM concentration. Peptides T20 and C34 were synthesized by a standard solid-phase 9-fluorenylmethoxy carbonyl method at the MicroChemistry Laboratory of the New York Blood Center. The peptides were purified to homogeneity (>95% purity) by high-performance liquid chromatography and identified by laser desorption mass spectrometry (PerSeptive Biosystems, Framingham, MA). T20 was reconstituted at a stock concentration of 0.5 mg/ml in 50% ethanol and was used at a concentration of 10 to 50 μg/ml in HF-TOC. C34 was reconstituted at a stock concentration of 1 mg/ml in phosphate-buffered saline and was used at a concentration of 10 μg/ml in HF-TOC.
Fluorescent-activated cell sorter (FACS) analysis.
CD4-PE and CD8-TC (Caltag) were used for surface staining of thymocytes. 7AAD was used to stain dead thymocytes prior to intracellular staining. The Cytofix/Cytoperm kit (BD Biosciences) was used for intracellular staining with active caspase-3-phycoerythrin (BD Biosciences) and anti-p24 KC57-fluorescein isothiocyanate (FITC) (Beckman Coulter).
Viral quantitation.
A p24 enzyme-linked immunosorbent assay (ELISA) kit (Perkin-Elmer or AIDS Vaccine Program, NIH) was used to detect Gag present in the HF-TOC supernatant.
Human fetal-thymus organ culture.
The procedure for HF-TOC has been previously described (6, 38, 40). Briefly, human fetal thymuses (19 to 24 gestational weeks) were dissected into ∼2-mm3 fragments using a dissecting microscope. Five to six fragments were placed on organotypic culture membranes (Millipore) underlaid by media (RPMI with 10% fetal bovine serum, 50 μg of streptomycin/ml, 50 U of penicillin G/ml, 1× minimal essential medium vitamin solution [Gibco-BRL], 1× insulin-transferrin-sodium selenite medium supplement [Sigma], and beta-mercaptoethanol) in 6-well tissue culture plates. An equal amount of virus (100 to 800 IU) in 15 μl of supernatant from infected phytohemagglutinin-stimulated peripheral blood mononuclear cells (PBMCs) or mock supernatant was applied to each fragment. Viral and mock supernatants produced from the same PBMC donor were used within each experiment. Fragments were cultured at 37°C in 5% CO2 for up to 12 days with daily changes of culture media. Thymocytes were teased out of the fragments using pestles (Bellco Co.) and were stained as described above.
Immunofluorescence.
Thymus fragments were fixed in formaldehyde and embedded in paraffin. Sections were probed with rabbit anti-active caspase-3 (Promega, Madison, WI) and/or HIV p24 monoclonal antibody (AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH), followed by the secondary antibodies Fluor 546-conjugated goat anti-rabbit immunoglobulin G (IgG) and/or Fluor 488-conjugated goat anti-mouse IgG (Invitrogen, Carlsbad, CA). Stained slides were analyzed by confocal microscopy.
Statistical analysis.
Trend line significance was tested with simple linear regression. A P value of less than 0.05 was considered significant. Differences in “mock/no drug versus treatment” trends were tested by the significance of the regression coefficient of the interaction term between the “mock/no drug and treatment” variables. A P value of less than 0.05 was considered significant. All analyses were performed using SAS statistical software (version 9.1; SAS Institute, Inc.).
RESULTS
Depletion of thymocytes requires ongoing HIV-1 replication.
To assess mechanisms of HIV-1-induced thymocyte depletion, we analyzed a pathogenic virus (NL4-R3A) which efficiently depletes CD4+ thymocytes in the HF-TOC. For comparison, we also studied a related virus (NL4-R3B) which is less pathogenic (37, 38). The NL4-R3A and NL4-R3B viruses contain env genes transmitted to a rapid progressor cloned into the NL4-3 backbone, but they lack nef (37, 38). Both R3A and R3B Env are capable of using CCR5 and CXCR4 as entry coreceptors. During a typical infection with NL4-R3A, there is a reduction in the percentage of live cells gated by forward and side scatter light profiles and the percentage of live thymocytes which are CD4+ (Fig. 1A and B). This loss of thymocytes typically occurs rapidly around 8 to 10 days postinfection (dpi) at the peak of viral infection, likely after a threshold of viral replication is reached (10, 38). In contrast, live cell and CD4+ thymocyte depletion during infection with NL4-R3B occur at a much slower rate (Fig. 1A and B).
FIG. 1.
CD4+ thymocyte depletion is dependent on sustained viral replication. (A and B) Thymocytes from mock-, NL4-R3A-, and NL4-R3B-infected HF-TOC were analyzed using flow cytometry for forward and side scatter (% gated live) (A) and expression of CD4 on cells which were gated live (B). Shown are data from at least seven independent experiments. (*, P < 0.05 for the NL4-R3A trend line relative to mock and NL4-R3B.) (C and D) Saquinavir was added to NL4-R3A-infected HF-TOC 6 (C) or 8 (D) days postinfection and each day thereafter, with the first day of drug addition indicated by the arrow. CD4+ thymocyte depletion was assessed at the indicated times. Error bars are from quadruplicate samples (*, P < 0.05 by the student's t test for NL4-R3A with drug relative to NL4-R3A without drug).
To understand whether thymocyte depletion during infection is dependent upon continual viral replication, we added the antiviral drug saquinavir during infection, which blocks HIV-1 protease function and virion maturation. Notably, the dose used was sufficient to completely inhibit NL4-R3A infection of HF-TOC when added at the time of initial infection (data not shown).
Daily addition of saquinavir starting at 6 dpi, before the peak in viral replication and depletion, resulted in prevention of CD4+ thymocyte depletion observed at 10 dpi (Fig. 1C). When saquinavir was added at 8 dpi at the peak of viral replication and depletion, there was still a drop in CD4+ percentage at 10 dpi, but this depletion was attenuated relative to no drug (Fig. 1D). Furthermore, there was no subsequent depletion of CD4+ thymocytes at 12 dpi. Together, these data suggest that cell death continues in part after the addition of saquinavir, followed by complete inhibition of subsequent CD4+ thymocyte depletion. Inhibition of replication, even during the peak of depletion, is sufficient to rescue at least some remaining thymocytes from cell death, indicating that continual viral replication is necessary for continued thymocyte depletion.
Depletion of CD4+ thymocytes is accompanied by an increase in the frequency of 7AAD+ cells, the majority of which are not productively infected.
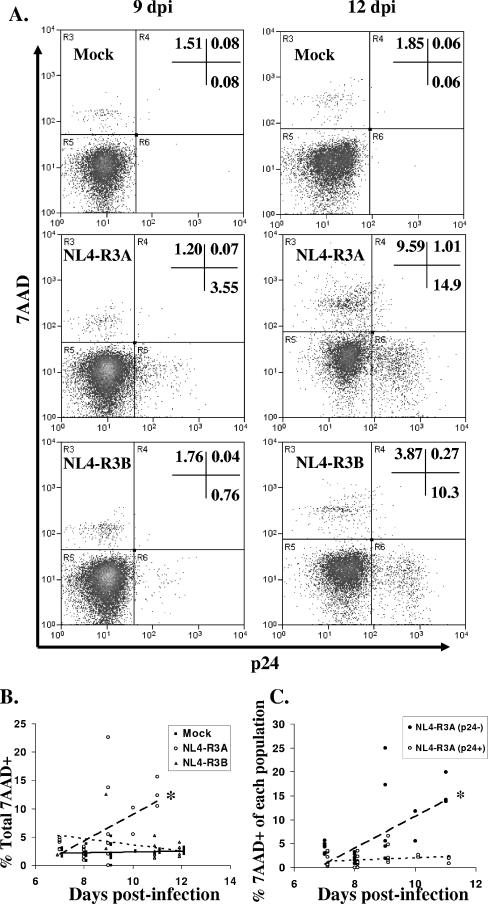
We next determined the frequency of dead cells in the scatter-defined gate using 7AAD, a dye that stains cells with permeable membranes. We additionally stained for intracellular p24 to delineate thymocytes which are productively infected. Figure 2A shows a representative plot at 9 and 12 dpi. Infection with NL4-R3A is accompanied by an increase in 7AAD+ thymocytes and a large number of infected cells but little overlap between the two populations. An analysis of multiple HF-TOC assays with multiple donor thymuses indicates that the frequency of 7AAD+ cells increases around 9 to 10 dpi at the time of maximal thymocyte depletion but occurs predominantly in NL4-R3A-infected HF-TOC, not NL4-R3B-infected HF-TOC (Fig. 2B). Furthermore, the increase in 7AAD positivity occurs predominantly in p24− thymocytes (Fig. 2C). Even at 11 to 12 dpi, when many cells are infected and dying, there is no significant detection of p24+ 7AAD+ thymocytes. Together, these data indicate that infection with NL4-R3A increases the frequency of 7AAD+ thymocytes only in the p24− population. Infected thymocytes appear to die in a way that does not involve or does not allow for detection of 7AAD+ p24+ cells.
FIG. 2.
Increase in 7AAD+ thymocytes in NL4-R3A-infected HF-TOC is predominantly in the p24− population. (A) Cells in the scatter-defined live cell gate were stained for 7AAD to measure dead cells and p24 to measure productively infected cells. Shown are representative plots for mock-, NL4-R3A-, and NL4-R3B-infected thymus at 9 and 12 dpi. (B) The percentage of 7AAD+ thymocytes in the live gate for mock-, NL4-R3A-, and NL4-R3B-infected thymus over time. (*, P < 0.05 for NL4-R3A trend line relative to mock and NL4-R3B.) (C) The increase in 7AAD+ cells occurs predominantly in the uninfected (p24−) population of the NL4-R3A-infected thymus. The proportion of uninfected thymocytes (p24− thymocytes) and infected thymocytes (p24+ thymocytes) which are 7AAD+ is shown. (B and C) Data are from seven independent experiments. (*, P < 0.05 for p24− trend line relative to p24+.)
Infection with NL4-R3A increases apoptosis of p24+ and p24− thymocytes.
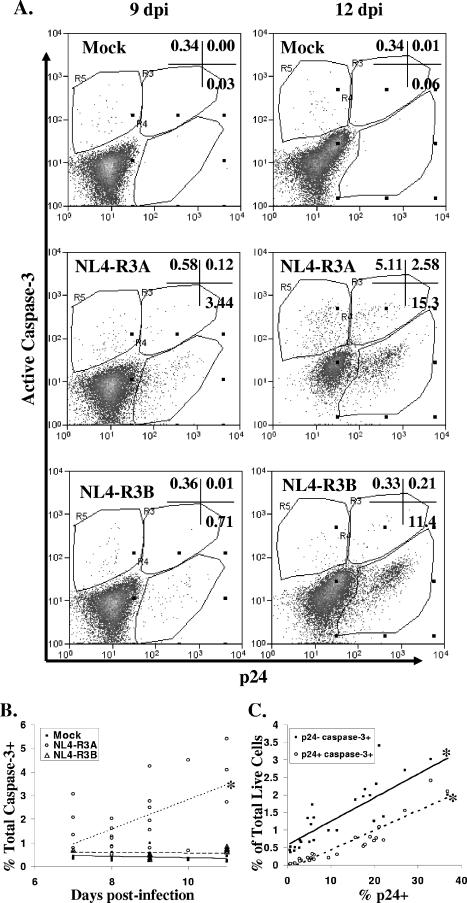
We next determined whether infected or uninfected thymocytes in NL4-R3A-infected HF-TOC were dying by apoptosis. To measure apoptosis, we analyzed active caspase-3 expression, a downstream effecter caspase of both the intrinsic and extrinsic apoptosis pathways that is activated in HIV-1-infected patients (14) and upon exposure of PBMCs and cell lines to HIV-1 Env (4, 12, 44). For these analyses, only 7AAD-negative cells in the live cell gate were considered. In mock-infected thymus, ∼0.3% of cells express active caspase-3 throughout HF-TOC culture (Fig. 3A). In NL4-R3A-infected thymus, there is a clear detection of cells which costain for active caspase-3 and p24, indicating that a fraction of infected cells are likely dying by apoptosis. Additionally, we observed the significant induction of bystander apoptosis (7AAD−, p24−, active caspase-3+) in NL4-R3A- but not NL4-R3B-infected thymus.
FIG. 3.
Infection with NL4-R3A increases the frequency of thymocytes expressing active caspase-3. (A) 7AAD-negative live cells were stained for p24 and active caspase-3. Shown is a representative of seven independent experiments from 9 and 12 dpi for mock-, NL4-R3A-, and NL4-R3B-infected thymus. (B) NL4-R3A increases the frequency of total thymocytes with active caspase-3 expression (*, P < 0.05 for NL4-R3A trend line relative to mock and NL4-R3B.) (C) The increase in active caspase-3+ cells in NL4-R3A-infected HF-TOC occurs in both uninfected (p24−) and infected (p24+) thymocytes in proportion to the level of NL4-R3A replication. (*, P < 0.05 for the strength of significance for each trend line.) (B and C) Data are from seven independent experiments.
An analysis of the total percentage of thymocytes expressing active caspase-3 over time indicates that apoptosis is specifically induced in NL4-R3A-infected thymus relative to either mock- or NL4-R3B-infected thymus (Fig. 3B). Furthermore, this increase is observable as early as 7 dpi, before significant thymocyte depletion typically occurs (Fig. 1). The increase in active caspase-3 expression observed in both uninfected and infected thymocytes correlates with the extent of NL4-R3A replication, as measured by FACS detection of intracellular p24, with a greater number of bystander apoptotic cells than infected apoptotic cells at all levels of infection (Fig. 3C). Using annexin-V as an additional marker for the detection of apoptotic cells, we confirmed the presence of apoptosis in both bystander and infected apoptotic cells in NL4-R3A-infected thymus relative to mock-infected thymus (data not shown).
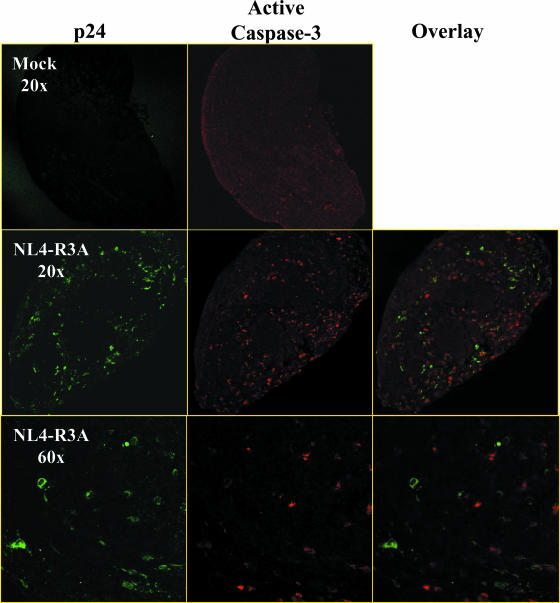
Immunofluorescent staining of mock- and NL4-R3A-infected thymus at 12 dpi confirmed the increase in active caspase-3+ thymocytes in infected thymus (Fig. 4). Furthermore, the majority of apoptotic thymocytes do not costain for p24, suggesting they are not productively infected. Together, these data implicate apoptosis of infected and uninfected thymocytes as contributors to the rapid thymocyte depletion induced by NL4-R3A.
FIG. 4.
Most caspase-3+ thymocytes in NL4-R3A-infected HF-TOC do not express p24. Fragments from mock- and NL4-R3A-infected thymus at 12 dpi were stained for p24 (green; Fluor 546) and active caspase-3 (red; Fluor 488). Shown is a representative of two independent experiments. Low (×20) and high (×60) magnifications are shown. Isotype control antibodies showed no specific signals (data not shown).
T20 and C34 fail to efficiently inhibit ongoing viral replication but efficiently prevent thymocyte depletion.
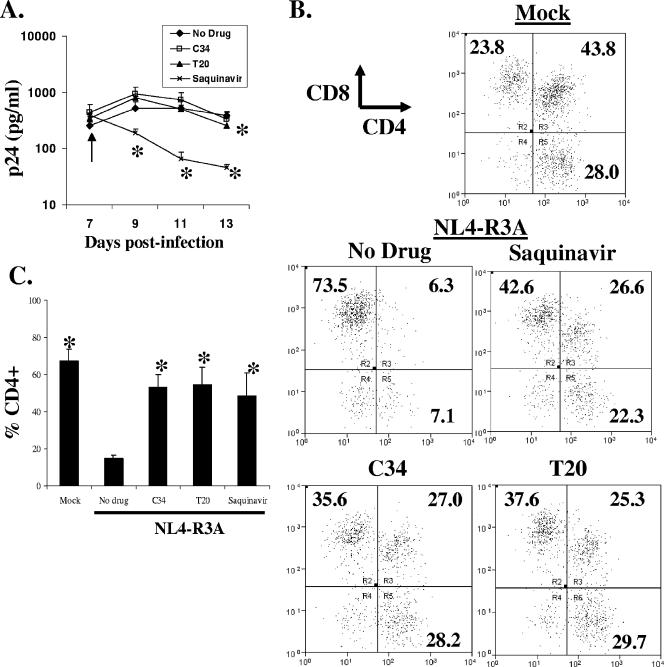
We next assessed whether kinetics of viral replication and depletion are altered by inhibition with saquinavir or the envelope fusion inhibitors T20 and C34. Drugs were added starting at 7 dpi and daily thereafter. The doses used for T20, C34, and saquinavir were sufficient to completely inhibit NL4-R3A infection of HF-TOC when added at the time of initial infection (data not shown). In saquinavir-treated HF-TOC, viral replication was inhibited very rapidly after addition of drug, as assessed by p24 ELISA (Fig. 5A). To address whether unprocessed gag-pol may limit detection of p24 by ELISA, we tested HIV-1 mutants with defective gag processing treated with two different detergents and demonstrated that unprocessed gag was detected as efficiently as processed gag in our ELISA (data not shown), as previously described (46), suggesting these results were not a detection bias.
FIG. 5.
Prevention of thymocyte depletion in T20- and C34-treated HF-TOC in spite of sustained replication. (A to C) NL4-R3A-infected HF-TOC was treated with C34, T20, or saquinavir from 7 dpi for 6 days. Arrows indicate the day of drug addition. (A) Viral load was quantitated by ELISA detection of Gag antigen in the HF-TOC supernatant on the indicated days. Error bars are derived from triplicate samples. (*, P < 0.05 by the student's t test for drug treatment relative to no drug.) (B and C) Similar percentages of CD4+ thymocyte protection after T20, C34, and saquinavir treatment were detected 6 days after drug treatment by CD4 and CD8 staining. Error bars are derived from triplicate samples. Shown is a representative of three independent experiments. (*, P < 0.05 by the student's t test relative to no drug.)
In T20- and C34-treated samples, however, p24 production from NL4-R3A infection was not significantly inhibited. In spite of this difference in viral replication, similar rescue of CD4+ thymocytes was observed for all three drugs assessed at 13 dpi, 6 days after the addition of drug (Fig. 5B and C). Because T20 and C34 do not efficiently inhibit viral replication but preserve CD4+ thymocytes to a level similar to that of saquinavir, we conclude that fusion-dependent thymocyte depletion plays a major role in NL4-R3A pathogenesis in the thymus.
Together, these data suggest that HIV-infected thymocytes die rapidly after de novo infection is inhibited by saquinavir. In contrast, although T20 and C34 should inhibit de novo infection of thymocytes, viral production is relatively spared through extended periods of culture. This suggests that T20 and C34 either incompletely inhibit viral spread in HF-TOC during ongoing infection or that inhibition of fusion preserves viral production from an infected cellular reservoir.
Apoptosis of both bystander and infected cells is inhibited by T20.
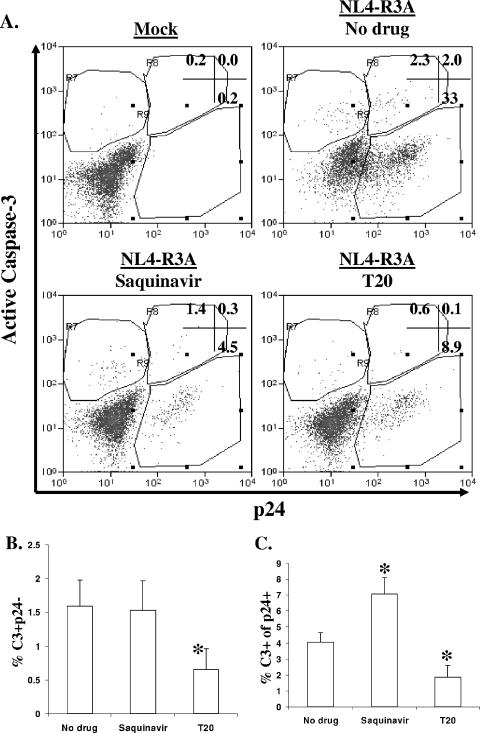
We next assessed whether saquinavir or T20 is capable of modulating the level of apoptosis observed during infection with NL4-R3A. Neither drug affected the level of apoptosis in mock-infected HF-TOC (data not shown). Interestingly, while viral replication was inhibited more efficiently by saquinavir than by T20, only T20 reduced apoptosis of both bystander and HIV-1-infected thymocytes (Fig. 6A). When observed over multiple experiments with multiple donor thymus tissues, the frequency of caspase-3+ p24− cells was not significantly changed for saquinavir-treated samples but was notably reduced for T20-treated samples relative to no-drug controls (Fig. 6B). When this analysis was extended to HIV-1-infected (p24+) cells, saquinavir was found to cause a significant increase and T20 a significant decrease in apoptosis relative to no-drug controls at comparable levels of replication (Fig. 6C). Since T20 has been shown to inhibit HIV-1 fusion by targeting both gp41 and gp120 (3, 57), another HIV-1 fusion inhibitor, C34, which only interacts with gp41, was used to confirm this finding. Addition of C34 to HIV-1-infected HF-TOC also resulted in a reduction of apoptosis in both p24+ and p24− thymocytes (data not shown).
FIG. 6.
Apoptosis in NL4-R3A-infected HF-TOC is inhibited by T20. (A) NL4-R3A-infected HF-TOC was treated with saquinavir or T20 at 6 dpi for up to 4 days. Thymocytes were stained with 7AAD, p24, and active caspase-3. Shown is a representative of five experiments for 7AAD− live cells 3 days after drug addition. (B) The frequency of bystander apoptosis (percent caspase-3+ p24−) was determined for each experimental treatment. The combined data for all time points from five independent experiments are shown with standard error bars. (mean percentage p24+ of 14% for no drug, 4.5% for saquinavir, and 5.7% for T20). (C) Saquinavir increased, but T20 decreased, apoptosis of infected thymocytes. To compare HF-TOC with similar levels of replication, only samples with less than 7% p24+ were considered for “no drug” treatment (mean percent p24+ of 4.4% for no drug, 4.5% for saquinavir, and 5.7% for T20). (*, P < 0.05 by the student's t test relative to no drug for panels B and C.)
In multiple experiments we observed similar p24 production in the presence of T20 or C34 compared to no drug treatment assessed by p24 ELISA, as depicted in Fig. 5A, but a reduced percentage of p24+ cells measured by FACS analysis, as depicted in Fig. 6A. This is likely explained by the massive depletion in no-drug-treated thymus, leading to observed increases in the relative percentage of p24+ thymocytes but an overall equal amount of p24 production compared to T20-treated samples which have CD4 preservation. Thus, similar numbers of productively infected (p24+) thymocytes per HF-TOC are detected in T20-treated and no-drug-treated HF-TOC samples (data not shown). Together, these data suggest that Env-mediated fusion plays a role in the induction of apoptosis of both bystander and infected thymocytes in the NL4-R3A-infected thymus.
DISCUSSION
In this study, we characterize the depletion of CD4+ thymocytes in the intact thymus by a highly pathogenic envelope obtained from a rapid progressor at the time of transmission. Our goal is to understand the mechanisms of thymocyte death in the HIV-infected thymus organ. We observed a replication- and Env-dependent depletion of CD4+ thymocytes (Fig. 1). During depletion with NL4-R3A, the percentage of thymocytes which stain with 7AAD increases, predominantly in the uninfected (p24−) population (Fig. 2). Prior to and concurrent with this depletion, we detected a significant increase in the frequency of thymocytes expressing both active caspase-3 and annexin-V, suggesting that they are undergoing apoptosis (Fig. 3 and 4; data not shown). Notably, this apoptosis is detected in productively infected (p24+) thymocytes as well as in uninfected (p24−) thymocytes. We show that induction of apoptosis is preferentially observed in the NL4-R3A-infected thymus but not in the less pathogenic NL4-R3B-infected thymus (Fig. 3). Although T20- and C34-treated HF-TOC during ongoing infection showed higher HIV-1 replication levels than saquinavir-treated HF-TOC, T20 and C34 were noticeably better at blocking the frequency of apoptosis in both uninfected and infected thymocytes and efficiently protected total CD4+ thymocyte depletion in spite of high levels of HIV-1 (Fig. 5 and 6). In sum, these data implicate envelope-mediated fusion in the induction of apoptosis and thymocyte depletion during infection of HF-TOC with the pathogenic NL4-R3A virus. These data are the first to describe envelope-specific and fusion-dependent induction of apoptosis in a relevant lymphoid organ model.
From previous studies it is still unclear whether HIV-1 infection in lymphoid organs depletes only infected cells or both infected and uninfected bystander cells (6, 30, 39, 48). Our data suggest that direct depletion of infected thymocytes is clearly involved, as inhibition of HIV-1 infection during its peak levels of replication and depletion halts further thymocyte depletion (Fig. 1). The mechanism of this “lytic” depletion remains unclear, but it does not appear to involve a cell which stains for both p24 and 7AAD (Fig. 2). Notably, we also observe significant induction of apoptosis in “bystander” p24− thymocytes and in p24+ thymocytes (Fig. 3). Together, our data suggest a model for thymocyte depletion induced by highly pathogenic HIV-1 isolates such as NL4-R3A. During NL4-R3A replication, Env expressed on virus or infected cells is likely capable of binding and fusing uninfected cells. Bystander cells which encounter the R3A Env are triggered to express active caspase-3 and eventually die by apoptosis (Fig. 3), consistent with findings from in vitro studies showing Env can trigger caspase-3-dependent cell death (4, 12, 44). Infected cells are also observed to express active caspase-3 (Fig. 3), suggesting apoptosis as one means of infected cell death. Intriguingly, T20 reduces apoptosis of infected cells (Fig. 5 and 6), suggesting autologous Env fusion may contribute to pathogenesis. Less fusogenic Env proteins, such as R3B, are less capable of inducing apoptosis (Fig. 3), perhaps helping to explain their lower level of activity in thymocyte depletion.
Advantageously, our study does not involve prolonged culture of isolated thymocytes outside of the thymic organ before analysis, which could enhance their susceptibility to death. However, the major mechanistic limitation to this study is the inability to precisely determine the relative life span and eventual fate of individual cells. For example, it is difficult to ascertain how long an infected or an apoptotic cell resides as a single cell in the thymus before engulfment or disintegration, even though estimates from other studies suggest a half-life of 12 to 36 h for apoptotic cells (2). Rather, our study relies on a series of snapshots over time. This limitation prevents us from attributing a contributory or a predominant role to apoptosis, relative to other cell death pathways, in the context of overall thymocyte depletion.
When added prior to or together with HIV-1 infection in HF-TOC, saquinavir, T20, and C34 all efficiently prevent infection (E. Meissner, L. Zhang, and L. Su, unpublished results). Interestingly, viral production in HF-TOC with ongoing infection was efficiently suppressed by saquinavir but not by T20 or C34 (Fig. 5 and 6). In the presence of high levels of HIV-1 replication, T20 and C34 both efficiently prevent HIV-1-induced thymocyte depletion, suggesting a protective effect of T20 and C34 on a cellular reservoir remaining in the thymic fragment that has yet to be characterized. This higher level of replication may explain the observation that although it is more efficient at blocking bystander and infected cell apoptosis (Fig. 6), T20 is not noticeably better than saquinavir at blocking overall depletion of CD4+ thymocytes (Fig. 5). One possible explanation is that while T20 reduces the level of apoptosis, it may allow for prolonged survival of infected cells, leading to elevated HIV-1+ cells and virions, which may contribute to elevated levels of fusion-independent cell killing. Together, these data strongly suggest the contribution of envelope-induced apoptosis to the depletion of infected thymocytes.
How exactly does the R3A Env mediate thymocyte depletion in the thymus? It is likely that the high levels of replication supported by the R3A Env leads to thymocyte depletion through a combination of direct and indirect effects, including but not limited to the direct and bystander killing discussed above. Interestingly, at levels of infection that were comparable to those of NL4-R3A, we did not detect an increase in apoptosis by NL4-R3B, suggesting that the depletion of thymocytes is specifically mediated in part by the R3A Env protein, which shows enhanced affinity for CXCR4 and cytopathicity for T cells in vitro (37, 38). Whether CXCR4 affinity is linked to the induction of apoptosis, as has been previously observed (27, 54), remains to be elucidated. Interestingly, when 200 nM AMD3100 was added to HF-TOC with ongoing R3A HIV-1 infection, we observed little or no inhibition of HIV replication or pathogenesis (data not shown). This may be due to the increased resistance of R3A to AMD3100 or to the fact that R3A can use CCR5 as well as CXCR4 in HF-TOC (37). Future experiments will focus on possible inhibition of apoptosis by blockade of CXCR4-Env interactions in NL4-R3A-infected HF-TOC.
Because addition of AT-2 inactivated virions and transfer of supernatant from NL4-R3A-infected thymus to uninfected thymus in the presence of HIV-1 inhibitors is unable to recapitulate thymic pathogenesis (E. Meissner and L. Su, unpublished results), productive infection and cell-associated Env is likely essential for pathogenesis (1, 20, 50). Alternatively and additionally, other HIV-1 or host factors induced during HIV-1 infection may contribute to Env-mediated thymocyte depletion. Further study of NL4-R3A in HF-TOC should help elucidate viral and cellular mechanisms that result in rapid depletion of thymocytes.
The HF-TOC thymus model, an intact human lymphoid organ with multiple cell types in physiological orientation, is an ideal model for investigating acute HIV-1 infection in lymphoid organs. Our data here contrast with studies in cell lines in vitro which, like our study, show that cytopathicity is dependent on fusion of envelope but, unlike our study, do not detect any bystander cell death (35). These disparities highlight the differences that likely exist between mechanisms of death in single-cell cultures and in complex, tightly knit lymphoid organs that contain a variety of interacting cell types. The fact that T20 reduces apoptosis of cells productively infected with HIV-1 raises a number of potential clinical implications. Encouragingly, these data do indicate that C34 and T20 are capable of blocking most CD4+ T-cell depletion in an intact lymphoid organ. However, protection of HIV-1-infected cells from apoptosis and depletion, even transiently, may lead to enhanced HIV-1 latency and/or a viral reservoir in fusion inhibitor-treated patients. It will be of importance to investigate the effect of T20 on the survival and persistence of HIV-1+ cells in these patients.
Acknowledgments
We thank Sunil Suchindran for help with statistical analysis. We thank Dedeke Brouwer and Hua Su for technical support. We thank the UNC flow cytometry and confocal cores. Saquinavir was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH.
We also thank the UNC Center for AIDS Research, NIAID, DHHS, for institutional support. This work was supported by NIH grants AI041356 and AI53804. E.M. was supported in part by the NIH training grant T32-AI07419.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Ahr, B., V. Robert-Hebmann, C. Devaux, and M. Biard-Piechaczyk. 2004. Apoptosis of uninfected cells induced by HIV envelope glycoproteins. Retrovirology 1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alimonti, J. B., T. B. Ball, and K. R. Fowke. 2003. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J. Gen. Virol. 84:1649-1661. [DOI] [PubMed] [Google Scholar]
- 3.Bar, S., and M. Alizon. 2004. Role of the ectodomain of the gp41 transmembrane envelope protein of human immunodeficiency virus type 1 in late steps of the membrane fusion process. J. Virol. 78:811-820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Biard-Piechaczyk, M., V. Robert-Hebmann, V. Richard, J. Roland, R. A. Hipskind, and C. Devaux. 2000. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120). Virology 268:329-344. [DOI] [PubMed] [Google Scholar]
- 5.Bonyhadi, M. L., L. Rabin, S. Salimi, D. A. Brown, J. Kosek, J. M. McCune, and H. Kaneshima. 1993. HIV induces thymus depletion in vivo. Nature 363:728-732. [DOI] [PubMed] [Google Scholar]
- 6.Bonyhadi, M. L., L. Su, J. Auten, J. M. McCune, and H. Kaneshima. 1995. Development of a human thymic organ culture model for the study of HIV pathogenesis. AIDS Res. Hum. Retrovir. 11:1073-1080. [DOI] [PubMed] [Google Scholar]
- 7.Brenchley, J. M., T. W. Schacker, L. E. Ruff, D. A. Price, J. H. Taylor, G. J. Beilman, P. L. Nguyen, A. Khoruts, M. Larson, A. T. Haase, and D. C. Douek. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749-759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinchmann, J. E., J. Albert, and F. Vartdal. 1991. Few infected CD4+ T cells but a high proportion of replication-competent provirus copies in asymptomatic human immunodeficiency virus type 1 infection. J. Virol. 65:2019-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broussard, S. R., S. I. Staprans, R. White, E. M. Whitehead, M. B. Feinberg, and J. S. Allan. 2001. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J. Virol. 75:2262-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camerini, D., H. P. Su, G. Gamez-Torre, M. L. Johnson, J. A. Zack, and I. S. Chen. 2000. Human immunodeficiency virus type 1 pathogenesis in SCID-hu mice correlates with syncytium-inducing phenotype and viral replication. J. Virol. 74:3196-3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun, T. W., L. Carruth, D. Finzi, X. Shen, J. A. DiGiuseppe, H. Taylor, M. Hermankova, K. Chadwick, J. Margolick, T. C. Quinn, Y. H. Kuo, R. Brookmeyer, M. A. Zeiger, P. Barditch-Crovo, and R. F. Siliciano. 1997. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature 387:183-188. [DOI] [PubMed] [Google Scholar]
- 12.Cicala, C., J. Arthos, A. Rubbert, S. Selig, K. Wildt, O. J. Cohen, and A. S. Fauci. 2000. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4(+) T cells. Proc. Natl. Acad. Sci. USA 97:1178-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correa, R., and M. A. Munoz-Fernandez. 2001. Viral phenotype affects the thymic production of new T cells in HIV-1-infected children. AIDS 15:1959-1963. [DOI] [PubMed] [Google Scholar]
- 14.de Oliveira Pintoqq, L. M., S. Garcia, H. Lecoeur, C. Rapp, and M. L. Gougeon. 2002. Increased sensitivity of T lymphocytes to tumor necrosis factor receptor 1 (TNFR1)- and TNFR2-mediated apoptosis in HIV infection: relation to expression of Bcl-2 and active caspase-8 and caspase-3. Blood 99:1666-1675. [DOI] [PubMed] [Google Scholar]
- 15.Dion, M. L., J. F. Poulin, R. Bordi, M. Sylvestre, R. Corsini, N. Kettaf, A. Dalloul, M. R. Boulassel, P. Debre, J. P. Routy, Z. Grossman, R. P. Sekaly, and R. Cheynier. 2004. HIV infection rapidly induces and maintains a substantial suppression of thymocyte proliferation. Immunity 21:757-768. [DOI] [PubMed] [Google Scholar]
- 16.Douek, D. C., R. A. Koup, R. D. McFarland, J. L. Sullivan, and K. Luzuriaga.2000. Effect of HIV on thymic function before and after antiretroviral therapy in children. J. Infect. Dis. 181:1479-1482. [DOI] [PubMed] [Google Scholar]
- 17.Douek, D. C., R. D. McFarland, P. H. Keiser, E. A. Gage, J. M. Massey, B. F. Haynes, M. A. Polis, A. T. Haase, M. B. Feinberg, J. L. Sullivan, B. D. Jamieson, J. A. Zack, L. J. Picker, and R. A. Koup. 1998. Changes in thymic function with age and during the treatment of HIV infection. Nature 396:690-695. [DOI] [PubMed] [Google Scholar]
- 18.Eckstein, D. A., M. L. Penn, Y. D. Korin, D. D. Scripture-Adams, J. A. Zack, J. F. Kreisberg, M. Roederer, M. P. Sherman, P. S. Chin, and M. A. Goldsmith. 2001. HIV-1 actively replicates in naive CD4(+) T cells residing within human lymphoid tissues. Immunity 15:671-682. [DOI] [PubMed] [Google Scholar]
- 19.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 20.Fitzgerald, W., A. W. Sylwester, J. C. Grivel, J. D. Lifson, and L. B. Margolis. 2004. Noninfectious X4 but not R5 human immunodeficiency virus type 1 virions inhibit humoral immune responses in human lymphoid tissue ex vivo. J. Virol. 78:7061-7068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glushakova, S., B. Baibakov, J. Zimmerberg, and L. B. Margolis. 1997. Experimental HIV infection of human lymphoid tissue: correlation of CD4+ T cell depletion and virus syncytium-inducing/non-syncytium-inducing phenotype in histocultures inoculated with laboratory strains and patient isolates of HIV type 1. AIDS Res. Hum. Retrovir. 13:461-471. [DOI] [PubMed] [Google Scholar]
- 22.Grivel, J. C., A. Biancotto, Y. Ito, R. G. Lima, and L. B. Margolis. 2003. Bystander CD4+ T lymphocytes survive in HIV-infected human lymphoid tissue. AIDS Res. Hum. Retrovir. 19:211-216. [DOI] [PubMed] [Google Scholar]
- 23.Grossman, Z., M. Meier-Schellersheim, W. E. Paul, and L. J. Picker. 2006. Pathogenesis of HIV infection: what the virus spares is as important as what it destroys. Nat. Med. 12:289-295. [DOI] [PubMed] [Google Scholar]
- 24.Grossman, Z., M. Meier-Schellersheim, A. E. Sousa, R. M. Victorino, and W. E. Paul. 2002. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat. Med. 8:319-323. [DOI] [PubMed] [Google Scholar]
- 25.Guadalupe, M., E. Reay, S. Sankaran, T. Prindiville, J. Flamm, A. McNeil, and S. Dandekar. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708-11717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 27.Holm, G. H., C. Zhang, P. R. Gorry, K. Peden, D. Schols, E. De Clercq, and D. Gabuzda. 2004. Apoptosis of bystander T cells induced by human immunodeficiency virus type 1 with increased envelope/receptor affinity and coreceptor binding site exposure. J. Virol. 78:4541-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Igarashi, T., C. R. Brown, R. A. Byrum, Y. Nishimura, Y. Endo, R. J. Plishka, C. Buckler, A. Buckler-White, G. Miller, V. M. Hirsch, and M. A. Martin. 2002. Rapid and irreversible CD4+ T-cell depletion induced by the highly pathogenic simian/human immunodeficiency virus SHIV(DH12R) is systemic and synchronous. J. Virol. 76:379-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iida, T., H. Ichimura, T. Shimada, K. Ibuki, M. Ui, K. Tamaru, T. Kuwata, S. Yonehara, J. Imanishi, and M. Hayami. 2000. Role of apoptosis induction in both peripheral lymph nodes and thymus in progressive loss of CD4+ cells in SHIV-infected macaques. AIDS Res. Hum. Retrovir. 16:9-18. [DOI] [PubMed] [Google Scholar]
- 30.Jamieson, B. D., C. H. Uittenbogaart, I. Schmid, and J. A. Zack. 1997. High viral burden and rapid CD4+ cell depletion in human immunodeficiency virus type 1-infected SCID-hu mice suggest direct viral killing of thymocytes in vivo. J. Virol. 71:8245-8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jekle, A., O. T. Keppler, E. De Clercq, D. Schols, M. Weinstein, and M. A. Goldsmith. 2003. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J. Virol. 77:5846-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaur, A., R. M. Grant, R. E. Means, H. McClure, M. Feinberg, and R. P. Johnson. 1998. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J. Virol. 72:9597-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kiselyeva, Y., Y. Ito, R. G. Lima, J. C. Grivel, A. T. Das, B. Berkhout, and L. B. Margolis. 2004. Depletion of CD4 T lymphocytes in human lymphoid tissue infected ex vivo with doxycycline-dependent HIV-1. Virology 328:1-6. [DOI] [PubMed] [Google Scholar]
- 34.Kourtis, A. P., C. Ibegbu, A. J. Nahmias, F. K. Lee, W. S. Clark, M. K. Sawyer, and S. Nesheim. 1996. Early progression of disease in HIV-infected infants with thymus dysfunction. N. Engl. J. Med. 335:1431-1436. [DOI] [PubMed] [Google Scholar]
- 35.LaBonte, J. A., T. Patel, W. Hofmann, and J. Sodroski. 2000. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J. Virol. 74:10690-10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCune, J. M., R. Loftus, D. K. Schmidt, P. Carroll, D. Webster, L. B. Swor-Yim, I. R. Francis, B. H. Gross, and R. M. Grant. 1998. High prevalence of thymic tissue in adults with human immunodeficiency virus-1 infection. J. Clin. Investig. 101:2301-2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meissner, E. G., V. M. Coffield, and L. Su. 2005. Thymic pathogenicity of an HIV-1 envelope is associated with increased CXCR4 binding efficiency and V5-gp41-dependent activity, but not V1/V2-associated CD4 binding efficiency and viral entry. Virology 336:184-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meissner, E. G., K. M. Duus, F. Gao, X. F. Yu, and L. Su. 2004. Characterization of a thymus-tropic HIV-1 isolate from a rapid progressor: role of the envelope. Virology 328:74-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Meissner, E. G., K. M. Duus, R. Loomis, R. D'Agostin, and L. Su. 2003. HIV-1 replication and pathogenesis in the human thymus. Curr. HIV Res. 1:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, E. D., K. M. Duus, M. Townsend, Y. Yi, R. Collman, M. Reitz, and L. Su. 2001. Human immunodeficiency virus type 1 IIIB selected for replication in vivo exhibits increased envelope glycoproteins in virions without alteration in coreceptor usage: separation of in vivo replication from macrophage tropism. J. Virol. 75:8498-8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miura, Y., N. Misawa, N. Maeda, Y. Inagaki, Y. Tanaka, M. Ito, N. Kayagaki, N. Yamamoto, H. Yagita, H. Mizusawa, and Y. Koyanagi. 2001. Critical contribution of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) to apoptosis of human CD4+ T cells in HIV-1-infected hu-PBL-NOD-SCID mice. J. Exp. Med. 193:651-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muro-Cacho, C. A., G. Pantaleo, and A. S. Fauci. 1995. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 154:5555-5566. [PubMed] [Google Scholar]
- 43.Rey-Cuille, M. A., J. L. Berthier, M. C. Bomsel-Demontoy, Y. Chaduc, L. Montagnier, A. G. Hovanessian, and L. A. Chakrabarti. 1998. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J. Virol. 72:3872-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roggero, R., V. Robert-Hebmann, S. Harrington, J. Roland, L. Vergne, S. Jaleco, C. Devaux, and M. Biard-Piechaczyk. 2001. Binding of human immunodeficiency virus type 1 gp120 to CXCR4 induces mitochondrial transmembrane depolarization and cytochrome c-mediated apoptosis independently of Fas signaling. J. Virol. 75:7637-7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenzweig, M., M. Connole, A. Forand-Barabasz, M. P. Tremblay, R. P. Johnson, and A. A. Lackner. 2000. Mechanisms associated with thymocyte apoptosis induced by simian immunodeficiency virus. J. Immunol. 165:3461-3468. [DOI] [PubMed] [Google Scholar]
- 46.Ryzhova, E. V., R. M. Vos, A. V. Albright, A. V. Harrist, T. Harvey, and F. Gonzalez-Scarano. 2006. Annexin 2: a novel human immunodeficiency virus type 1 Gag binding protein involved in replication in monocyte-derived macrophages. J. Virol. 80:2694-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sodora, D. L., J. M. Milush, F. Ware, A. Wozniakowski, L. Montgomery, H. M. McClure, A. A. Lackner, M. Marthas, V. Hirsch, R. P. Johnson, D. C. Douek, and R. A. Koup. 2002. Decreased levels of recent thymic emigrants in peripheral blood of simian immunodeficiency virus-infected macaques correlate with alterations within the thymus. J. Virol. 76:9981-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su, L. 1997. HIV-1 pathogenesis and therapeutic intervention in the SCID-hu Thy/Liv mouse: a model for primary HIV-1 infection in the human thymus. Rev. Med. Virol. 7:157-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su, L., H. Kaneshima, M. L. Bonyhadi, R. Lee, J. Auten, A. Wolf, B. Du, L. Rabin, B. H. Hahn, E. Terwilliger, and J. M. McCune. 1997. Identification of HIV-1 determinants for replication in vivo. Virology 227:45-52. [DOI] [PubMed] [Google Scholar]
- 50.Sylwester, A. W., J. C. Grivel, W. Fitzgerald, J. L. Rossio, J. D. Lifson, and L. B. Margolis. 1998. CD4(+) T-lymphocyte depletion in human lymphoid tissue ex vivo is not induced by noninfectious human immunodeficiency virus type 1 virions. J. Virol. 72:9345-9347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 52.Veazey, R. S., P. A. Marx, and A. A. Lackner. 2001. The mucosal immune system: primary target for HIV infection and AIDS. Trends Immunol. 22:626-633.11698224 [Google Scholar]
- 53.Vigano, A., S. Vella, N. Principi, D. Bricalli, N. Sala, A. Salvaggio, M. Saresella, A. Vanzulli, and M. Clerici. 1999. Thymus volume correlates with the progression of vertical HIV infection. AIDS 13:F29-F34. [DOI] [PubMed] [Google Scholar]
- 54.Vlahakis, S. R., A. Algeciras-Schimnich, G. Bou, C. J. Heppelmann, A. Villasis-Keever, R. C. Collman, and C. V. Paya. 2001. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J. Clin. Investig. 107:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 56.Wykrzykowska, J. J., M. Rosenzweig, R. S. Veazey, M. A. Simon, K. Halvorsen, R. C. Desrosiers, R. P. Johnson, and A. A. Lackner. 1998. Early regeneration of thymic progenitors in rhesus macaques infected with simian immunodeficiency virus. J. Exp. Med. 187:1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang, X., L. Florin, M. Farzan, P. Kolchinsky, P. D. Kwong, J. Sodroski, and R. Wyatt. 2000. Modifications that stabilize human immunodeficiency virus envelope glycoprotein trimers in solution. J. Virol. 74:4746-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]