Abstract
Electron micrographic studies of neuronal axons have produced contradictory conclusions on how alphaherpesviruses are transported from neuron cell bodies to axon termini. Some reports have described unenveloped capsids transported on axonal microtubules with separate transport of viral glycoproteins within membrane vesicles. Others have observed enveloped virions in proximal and distal axons. We characterized transport of herpes simplex virus (HSV) in human and rat neurons by staining permeabilized neurons with capsid- and glycoprotein-specific antibodies. Deconvolution microscopy was used to view 200-nm sections of axons. HSV glycoproteins were very rarely associated with capsids (3 to 5%) and vice versa. Instances of glycoprotein/capsid overlap frequently involved nonconcentric puncta and regions of axons with dense viral protein concentrations. Similarly, HSV capsids expressing a VP26-green fluorescent protein fusion protein (VP26/GFP) did not stain with antiglycoprotein antibodies. Live-cell imaging experiments with VP26/GFP-labeled capsids demonstrated that capsids moved in a saltatory fashion, and very few stalled for more than 1 to 2 min. To determine if capsids could be transported down axons without glycoproteins, neurons were treated with brefeldin A (BFA). However, BFA blocked both capsid and glycoprotein transport. Glycoproteins were transported into and down axons normally when neurons were infected with an HSV mutant that produces immature capsids that are retained in the nucleus. We concluded that HSV capsids are transported in axons without an envelope containing viral glycoproteins, with glycoproteins transported separately and assembling with capsids at axon termini.
Herpes simplex virus (HSV) and other alphaherpesviruses infect mucosal tissues and spread rapidly between epithelial cells. Infection of sensory neurons is followed by retrograde traffic of capsids to nerve cell bodies in ganglia where latency is established. Periodic reactivation and virus replication produce virus particles that are transported in the anterograde direction in axons leading to reinfection of epithelial tissues. In order to accomplish this round trip from epithelium to ganglia and back to epithelium, alphaherpesviruses have evolved specialized mechanisms that promote their rapid and directed transport in neuronal axons. Fast axon microtubule transport moves virus in both directions (22, 39, 40), and thus, kinesin motors are likely involved in anterograde transport (from cell bodies to axon termini). There is also the suggestion that HSV is directed specifically into sensory axons and less frequently into dendrites because HSV more rarely infects the central nervous system (42). By analogy with all other cells tested, HSV spread from an infected neuron to epithelial cells is likely to be dependent upon four membrane glycoproteins: gB, gD, gE/gI, and gH/gL (1, 10, 14, 23, 36, 38). It is likely that HSV and other alphaherpesviruses are transferred across cell junctions formed between neurons and epithelial cells, rather than moving as extracellular virions (2, 5, 19). Consequently, it is essential that enveloped virions containing gB, gD, gE/gI, and gH/gL are transported to, or assembled at, axon termini.
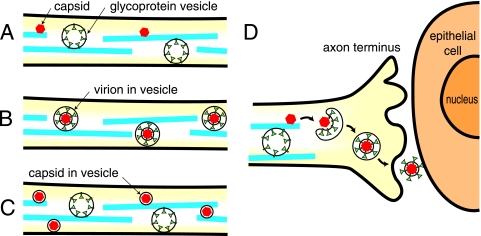
Neuronal cell bodies produce enveloped virions that reach the plasma membrane of cell bodies and can spread to other neurons in ganglia (22, 26, 32). However, it is presently controversial as to how alphaherpesviruses travel down axons to reach mucosal surfaces, whether as enveloped virions or as unenveloped capsids. Early electron microscopy (EM) studies of HSV and the pig pseudorabies virus (PRV) showed enveloped alphaherpesvirus particles within membrane vesicles being transported in the anterograde direction along axonal microtubules (2, 25, 27). These data were consistent with transport of infectious, enveloped virions within vesicles derived from cell membranes (see Fig. 1B). Presumably, on reaching axon termini, the outer membranes surrounding these virions can fuse with the plasma membrane delivering virions outside cells, onto sites of contact between neurons and epithelial cells (Fig. 1D).
FIG. 1.
Models for anterograde transport of HSV capsids and glycoproteins in axons and for the assembly at axonal termini. (A) Capsids (red) are transported on axonal microtubules (blue) separately from vesicles containing HSV glycoproteins (green). Subsets of the tegument proteins (not shown) are likely associated with capsid surfaces, while other tegument proteins associate with glycoproteins. (B) HSV virions composed of capsids, tegument proteins, and an envelope including viral glycoproteins are transported within vesicles that are bound onto microtubules. (C) HSV capsids surrounded by a lipid membrane that lacks the viral glycoproteins are transported separately from vesicles containing the viral glycoproteins. (D) At axon termini, capsids (coated with tegument proteins) assemble by budding into vesicles containing viral glycoproteins, producing a virion within a lipid vesicle. Fusion of the lipid vesicle with the plasma membrane releases virions (onto the surface of the neuron) that can then infect adjacent epithelial cells.
Penfold and Cunningham (34) proposed a different model for axonal transport in which capsids and envelope glycoproteins are transported separately (depicted in Fig. 1A). This “separate transport” model was initially based on EM studies of axons that were separated from neuronal cell bodies by using a two-chamber system and showing unenveloped HSV capsids moving in axons. These authors were able to discern hundreds of unenveloped capsids in both proximal and distal regions of axons, often in close apposition to microtubules. Subsequent immunogold labeling studies provided evidence that HSV glycoproteins were transported in axons separately from capsids and tegument proteins (18, 33). In these studies, it was clear that capsid-specific antibodies labeled electron-dense capsids, although it was more difficult to discern whether glycoprotein-specific antibodies decorated vesicles. Moreover, enveloped capsids can be observed at axonal varicosities (regions with bulged membranes derived from either branching of growth cones where axons cross one another or elsewhere along axonal membranes) (5, 37). A significant fraction of HSV particles observed in axons in subsequent studies were enveloped (2 enveloped particles in a total of 15 particles). However, EM studies can be difficult to interpret due to the relatively low numbers of capsids in distal axons as well as difficulties in recognizing whether the structures observed are in axons, varicosities, or growth cones.
Additional support for the separate transport model involved immunofluorescence studies showing PRV capsids as discrete puncta in axons that were distant from puncta stained with gB-specific antibodies (13, 39). Similarly, HSV gC and capsids were stained in axons and found to be at separate locations (32). Accumulation of PRV and HSV capsids, glycoproteins, and enveloped virions was observed at axon termini and varicosities (13, 37, 39) consistent with assembly at those sites. Some of the strongest support for separate transport came from experiments in which neurons were treated with brefeldin A (BFA), which disrupts the Golgi apparatus. BFA caused glycoproteins to be retained in cell bodies, but capsids were transported down axons, suggesting that capsids could be transported without an envelope (32). A fourth line of support for the separate model came from the characterization of a PRV US9− mutant that displayed reduced glycoprotein transport into axons without an effect on capsid movement (12, 42).
Recent reports from Enquist and colleagues have questioned the separate transport model and suggested that alphaherpesviruses are transported in axons as fully assembled enveloped virions, assembly occurring in neuronal cell bodies. This model, denoted the “married model,” is depicted in Fig. 1B. del Rio et al. (8) observed that the PRV VP22 tegument protein fused to green fluorescent protein (VP22/GFP) was found in similar quantities both in purified virions and associated with capsids moving in axons. Luxton et al. (24) made similar observations with five different PRV tegument proteins. These results suggested that tegument proteins may be packaged into enveloped virions for transport. Alternatively, tegument proteins could be bound onto the surfaces of capsids in transit by the separate model. del Rio et al. (8) also challenged the notion that BFA can be used to separate capsid and glycoprotein transport, observing that BFA blocked both PRV capsid and glycoprotein transport. Studies of the PRV gE membrane protein, which was required for both capsid and glycoprotein transport, implicated membranes in this process (4). Ch'ng et al. (5) characterized distal segments of rat axons by EM and document unmistakable images of PRV enveloped virions. They concluded that capsids enter axons in cellular membranes that are “most likely mature virions.” It is also formally possible that these capsids were surrounded by a single, cellular membrane lacking the viral glycoproteins (Fig. 1C), based on previous observations of PRV gB being separate from capsids (13, 39).
Whether alphaherpesviruses are transported toward axon termini in the form of unenveloped or enveloped capsids is a fundamentally important question and is clearly not yet resolved. We presume that axonal transport of HSV, PRV, and varicella-zoster virus will not differ substantially in its basic mechanism in different neurons, but this also remains to be determined. Here, we characterized whether several HSV glycoproteins colocalize with capsids in human and rat neuronal axons using detailed deconvolution microscopy. Hundreds of tiny puncta of fluorescence representing capsids or glycoproteins were counted. Only very rarely was there any overlap between capsid and glycoprotein signals, and this overlap was primarily observed in regions of axons with relatively dense concentrations of capsids and glycoproteins. Similarly, antiglycoprotein antibodies did not colocalize with capsids containing a VP26/GFP fusion protein. Live-cell imaging of axons showed that a substantial fraction of capsids moved rapidly in axons, while others were stalled or moved over short distances. Both capsids and glycoproteins accumulated in the cell bodies of neurons treated with BFA, indicating that this drug was not useful in characterizing transport. However, glycoprotein and capsid transport could be separated using a temperature-sensitive HSV mutant that accumulated capsids in the nucleus, with normal amounts of glycoproteins transported into axons. We concluded that HSV capsids are transported separately from membrane glycoproteins in rat and human neurons.
MATERIALS AND METHODS
Virus strains.
Wild-type HSV-1 strains F and KOS and F-VP26/GFP were propagated and their titers were determined on Vero cells grown in Dulbecco's modified Eagle's medium containing 7% fetal bovine serum (FBS). The protease mutant TS-Prot.A (15) was propagated on complementing BMS-MG22 cells at 31°C as described previously (15). F-VP26/GFP, a recombinant HSV-1 strain expressing a VP26/GFP fusion protein, was constructed by transfecting Vero cells with HSV-1 (F) DNA and a plasmid containing the VP26/GFP gene (12) provided by Prashant Desai (Johns Hopkins School of Medicine). GFP-expressing viruses underwent four rounds of plaque purification.
Antibodies.
Rat antiserum specific for gE/gI, diluted 1:1,000, was produced by immunizing rats with a soluble form of gE/gI (3) and has been described elsewhere (28). Rabbit polyclonal antiserum specific for gD (rabbit 45) (11) was kindly provided by Gary Cohen and Roselyn Eisenberg (University of Pennsylvania, Philadelphia) and diluted 1:1,000. Rabbit polyclonal antiserum specific for gB (rabbit 63), produced using purified gB extracted from virions, was similar to rabbit 67 serum (20) and was kindly provided by Pat Spear (Northwestern Medical School, Chicago, Ill.) and diluted 1:1,000. Rabbit polyclonal antiserum specific for gE and gI was produced by infecting rabbits with replication-competent (E1+) adenovirus vectors expressing gE and gI proteins (17) and diluted 1:1,000. Rabbit polyclonal anti-NC-1 antiserum specific for HSV-1 VP5 (6) obtained from Gary Cohen and Roselyn Eisenberg was absorbed overnight on undifferentiated SK-N-SH cells at a dilution of 1:100, and the absorbed antibody was used at a final dilution of 1:1,500. Mouse monoclonal antibodies (MAbs) ICP5 specific for capsid protein VP5 (Virusys, North Berwick, ME) and H1.4 specific for VP5 (Biodesign International, Sato, ME) were mixed together and diluted 1:500. Mouse monoclonal antibodies specific for the KSP repeats of the phosphorylated forms of neurofilament M (NF-M) and neurofilament H (NF-H) axonal proteins (Chemicon, Temecula, CA) were diluted 1:1,000.
Human neurons.
Human SK-N-SH neuroblastoma cells were purchased from the American Type Culture Collection (Manassas, VA) and propagated in growth medium: minimal essential medium with Earle's salts (Invitrogen) containing 2 mM l-glutamine, 1.0 mM sodium pyruvate, 0.1 mM nonessential amino acids, 1.5 g/liter sodium bicarbonate, and 10% FBS (HyClone, Logan, UT). Cells were removed from dishes with sodium citrate buffer (134 mm KCl, 15 mM Na citrate, pH 7.3 to 7.4), washed with phosphate-buffered saline (PBS), and counted, and 1 × 105 SK-N-SH cells were plated on 12-mm glass coverslips or Lab-TEK Permanox chambered slides (Nalge-Nunc) coated with poly-d-lysine (30 μg/ml; Sigma) and laminin (2 μg/ml; Sigma) or rat tail collagen (5 μg/ml; Roche). One day after plating, the medium was changed to 50% growth medium and 50% differentiation medium: 2 parts Dulbecco's modified Eagle's medium-nutrient mix F-12 (Invitrogen)-1 part medium 199 with Earle's salts and l-glutamine (Cellgro), 0.66 mg/ml bovine serum albumin (BSA), 0.66× insulin-transferrin-sodium selenite (ITS [100×; Cellgro]), 10 μM all-trans retinoic acid (RA) (Fluka). After 2 days, the medium was changed to 100% differentiation medium, and thereafter this medium was changed every other day for 10 to 14 days, at which time >70% of the cells exhibited a differentiated morphology (ovoid cell body and neurite outgrowths).
Rat neurons.
To produce trigeminal (TG) ganglion sensory neurons, 5-day-old rat pups were euthanized, TG ganglia were removed, and tissues were washed in Leibovit's L-15 medium and then resuspended in Leibovit's L-15 medium (Invitrogen) containing papain (20 U/ml [Sigma]) and incubated for 20 min at 37°C. Extracts were centrifuged at 800 × g, and pellets were resuspended in 0.3% collagenase-0.4% dispase (both from Sigma) and incubated for 20 min at 37°C. Samples were centrifuged at 800 × g and pellets resuspended in Leibovit's L-15 complete medium (10% FBS, penicillin-streptomycin, 5 mM HEPES) and triturated before being layered onto 30%/60% Percoll gradients which were centrifuged at 1,800 × g for 10 min. The 30% Percoll layer and the 30%/60% interface which contained the trigeminal ganglion neurons were harvested, diluted with Leibovit's L-15 medium, and centrifuged at 1,800 × g for 5 min. Pellets containing neurons were resuspended in Biowhittaker Ham's F-12 medium (Cambrex) with 50 μg/ml of nerve growth factor 7S (NGF 7S; Invitrogen), and 1 × 105 cells were plated onto glass coverslips coated with poly-d-lysine and laminin or glass CC2-coated slides (Nunc). One day after plating, 1 μM cytosine 1 β-d-arabinofuranoside (AraC; Fluka) was added to the cells to eliminate nonneuronal cells. Three days after AraC addition, the medium was changed to F-12 medium with fresh NGF 7S but lacking AraC. Thereafter, medium was changed with addition of fresh NGF every 3 days for 5 to 21 days. Rat dorsal root ganglia (DRG) were obtained from Cambrex and grown and propagated in neurobasal medium (Invitrogen) with B27 supplement according to the manufacturer's protocols for 5 to 14 days.
Immunofluorescence microscopy of infected neurons.
Neurons were infected with HSV-1 at 1 to 2 PFU/cell and incubated for 15 to 24 h at 37°C in growth medium. Infections with TsProt.A and KOS also involved 1 to 2 PFU/ml for 90 min at 37°C, and then neurons were shifted to 39.5°C for an additional 16.5 h. Neurons were fixed with 4% paraformaldehyde in PBS for 30 min, washed with PBS, permeabilized with 0.2% Triton X-100 in PBS for 15 min, washed in PBS containing 0.1% Tween 20 (PBS/Tween), and incubated in 2% goat serum in PBS-Tween or 2% donkey serum in PBS-Tween for 1 to 2 h. Cells were incubated with the various anti-HSV mouse, rat, or rabbit antibodies diluted in PBS-Tween for 1 h and then washed five times with PBS-Tween. The cells were incubated with secondary antibodies: Alexa 488-conjugated goat anti-rat immunoglobulin G (IgG), Alexa 594-conjugated goat anti-mouse IgG, and Alexa 488-conjugated goat anti-rabbit IgG (Molecular Probes, Eugene, Oreg.) diluted 1:2,000. Alternatively, Texas red-conjugated donkey anti-mouse IgG, fluorescein isothiocyanate (FITC)-conjugated donkey anti-rabbit IgG, Cy5-conjugated donkey anti-mouse IgG, and Texas red-conjugated donkey anti-rabbit IgG from Jackson Immunoresearch Labs (West Grove, Pa.) were used as secondary antibodies at 1:500. Cells were washed five times in PBS-Tween and mounted on glass slides using Fluormount G (Southern Biotech, Birmingham, AL). Immunofluorescence microscopy followed by deconvolution was performed in the OHSU-MMI Research Core Facility. Images were acquired on the Nikon TE 200-based Applied Precision Instruments (API) Deltavision image restoration system (includes a precision motorized XYZ stage, halogen illumination with the API light homogenizer, a CH350L camera, and DeltaVision software) using a ×60 1.4 NA oil objective (1.512 refractive index matched to the objective and specimen). Eleven 0.2-μm XY sections were obtained in two- or three-color images and deconvolved in nine iterations with the constrained algorithm of Sedat and Agard on an SGI Octane workstation using the SoftWoRx program (API, Issaquah, WA).
Purification and immunostaining of F-VP26/GFP virions.
Extracellular virus particles were purified from Vero culture supernatants collected at 19 h postinfection (p.i.). Virions were centrifuged through a 30% sucrose gradient cushion at 82,000 × g for 90 min and resuspended in PBS containing 5% FBS by sonication. Glass microscope slides were coated with 200 μg/ml of rat collagen, and the slides were air-dried for 1 h, washed twice with H2O, and dried for 2 h. Purified virus was added to coated slides for 15 min, the slides were washed once with PBS, and virions were stained with either rabbit polyclonal anti-gD or rabbit polyclonal anti-gE/gI antibody and Texas red-conjugated anti-mouse IgG secondary antibodies as described above and then mounted with Fluormount G.
Live-cell imaging.
SK-N-SH neurons were infected with HSV VP26/GFP using 1 PFU/cell for 24 h, and live-cell images were acquired from nine planes along the z axis every 0.2 μm in two colors every 5 s or every 20 s over 5 to 12 min. GFP was acquired at 488 nm excitation/528 nm emission with a 0.16-s exposure and differential interference contrast with a 0.1-s exposure and 68% neutral density filter. These images were acquired with the Olympus-based DVRT (Delta Vision Real Time; API) with a Solent Scientific Instruments (United Kingdom) environment chamber to maintain 37°C. Due to the short acquisition time, CO2 was deemed unnecessary to maintain culture medium pH. Stacked images (composed of the nine acquired planes for each image) with the corresponding differential interference contrast image were converted to a time-lapse Quick Time movie in SoftWorx (see video S1 in the supplemental material).
Treatment with BFA.
HSV-1 (F)-infected SK-N-SH neurons were treated with BFA (from a stock solution of 2 mg/ml in ethanol) to a final concentration of 2 μg/ml beginning 3 h after infection. The neurons were incubated for an additional 12 h at 37°C prior to fixation and immunostaining. In other experiments, BFA was added after 9 or 12 h until 15 h.
Single-step growth curve of F-VP26/GFP on differentiated SK-N-SH cells.
SK-N-SH cells (plated at 2 × 105 cells/well in 12-well dishes) were grown to confluence and differentiated with retinoic acid for 7 to 10 days. Cells from one well were counted, and the rest of the wells were infected in triplicate with either F-VP26/GFP or HSV-1 (F) at 5 PFU/cell. Cells and supernatants were combined and harvested at 2, 8, 12, 16, 18, and 24 h. Triplicate samples at each time point were sonicated (20 to 30 s) and serially diluted, and the titers of infectious virus were determined using Vero cells.
RESULTS
HSV capsid and glycoproteins do not colocalize in axons of human SK-N-SH cells.
The models depicted in Fig. 1 differ with respect to whether HSV capsids are transported in axons with or without an envelope that contains the viral glycoproteins necessary for spread to other cells. To address whether HSV capsids are enveloped, we performed detailed deconvolution microscopy experiments to capture 200-nm sections of neuronal axons simultaneously stained with anticapsid and antiglycoprotein antibodies. Human SK-N-SH neuroblastoma cells have been used previously as a model system for viral infection (16, 44). These cells were incubated in serum-free medium for 7 days and induced to differentiate with the addition of RA to produce axons. Over 70% of cells developed long, unbranched neurites that stained with antibodies specific for phosphorylated NF-H, an axonal marker (not shown), and thus, we concluded that these neurites were axons. Given that these cells could be plated more sparsely than in previous studies of human neurons (18, 37), axons were frequently isolated from one another and the number of axonal varicosities was reduced. Varicosities constitute sites of virus assembly and, therefore, complicate interpretation of the results (37).
Previous studies by Miranda-Saksena et al. (32) using cultured dissociated human fetal DRG neurons and rat DRG neurons demonstrated that capsids first appeared in axons after 15 to 17 h p.i., while glycoproteins appeared in 60% of axons as early as 13 h. In these studies, some infected neurons did not display capsids in axons until after 18 to 20 h. We repeated these kinetics studies with differentiated SK-N-SH cells staining for HSV-1 capsid protein VP5 and glycoproteins gB and gD at 15, 16, 17, and 18 h p.i. Glycoproteins and capsids could be detected in axons no earlier than 17 h p.i. It appeared that a bolus of HSV structural proteins and capsids accumulated in neuronal cell bodies near the axon hillock until these relatively late times followed by a burst of transport into axons.
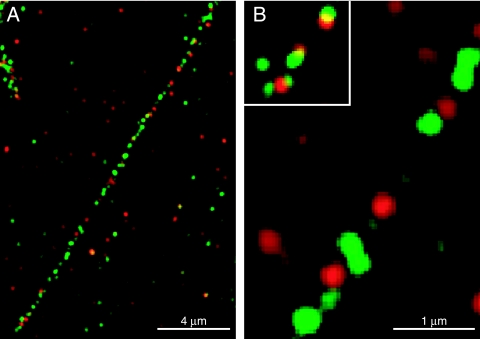
Neurons were infected with wild-type HSV-1 strain F for 18 h and then fixed, permeabilized, and stained with a combination of two mouse MAbs specific for capsid protein VP5 and, simultaneously, with rabbit polyclonal anti-gD antibodies. Individual VP5-specific MAbs gave identical results to pooled anti-VP5 MAbs, but the signal was marginally higher with pooled antibodies (not shown). Discrete puncta representing gD (green) and VP5 (red) were detected in axons, with very little colocalization of the two proteins (yellow) observed (Fig. 2A). At higher magnifications (Fig. 2B), it was clear that green and red puncta originated from individual spots that appeared 250 to 400 nm in size. Based on previous electron microscopic and immunofluorescence analyses of axons (8, 13, 18, 24, 34, 37, 39), these puncta represent individual capsids and vesicles containing viral glycoproteins. Although capsids are 125 nm and below the resolution of light microscopy, diffractive ballooning of fluorescent images has been noted (39). Likewise, vesicles containing HSV glycoproteins with sizes similar to capsids were described (18). Ten axons were further characterized, and 351 gD (green) and 257 VP5 (red) puncta (Table 1) were counted. Only 3.8% of these puncta exhibited overlapping (yellow) signals, defined by the merging of 25% or more of the areas of any red and green puncta. Individual sections of 200 nm were captured, and, thus, it was possible, but perhaps not likely, that a fraction of capsids were directly on top of vesicles containing glycoproteins within the section. It is important to note that most of the puncta with overlapping red and green signals were in regions of axons with relatively dense capsids and glycoproteins. Moreover, the majority of green and red puncta exhibited only partial overlap, i.e., nonconcentric green and red images (see inset in Fig. 2B), suggesting that capsids were adjacent to glycoprotein vesicles, rather than representing enveloped capsids.
FIG. 2.
HSV-1 capsids and glycoprotein gD do not colocalize in SK-N-SH neurons. Human SK-N-SH neurons were infected with HSV-1 (F) for 18 h, fixed with 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100. (A) The neurons were stained with a combination of two mouse MAbs specific for VP5 (red) and, simultaneously, with rabbit polyclonal antibodies specific for gD (green), followed by Alexa 594-conjugated goat anti-mouse IgG and Alexa 488-conjugated goat anti-rabbit IgG secondary antibodies. (B) Higher magnification of panel A showing individual VP5 (red) and gD (green) puncta. The inset in panel B represents rare examples of overlapping VP5 (red) and gD puncta (green). Single sections of 200 nm are shown. Scale bars are 4 μm in panel A and 1 μm in panel B.
TABLE 1.
Quantitation of glycoproteins and nucleocapsids in axons of SK-N-SH neuronsa
| Membrane glycoprotein | No. of punctab
|
||
|---|---|---|---|
| Glycoproteins | Nucleocapsids (VP5) | Overlapc | |
| gD | 351 | 257 | 23 |
| gB | 302 | 215 | 32 |
| gE/gI | 303 | 308 | 21 |
SK-N-SH neurons were infected with HSV-1 strain F (wild type) as described in Materials and Methods.
Individual glycoprotein (green) and nucleocapsid (red) puncta were counted in 10 axons from 10 independent neurons.
Overlap between glycoprotein and nucleocapsid puncta was defined as merging of 25% or more of the areas of any red and green puncta.
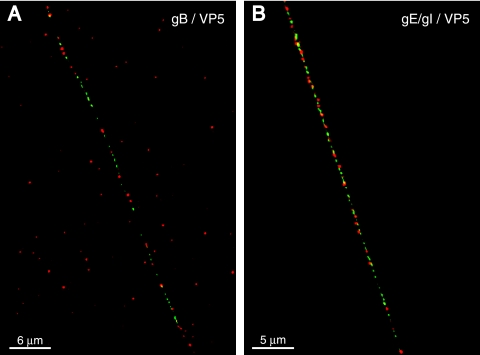
We extended these analyses to two other HSV-1 glycoproteins: gB and gE/gI, proteins that are also necessary in the virion envelope for efficient virus spread. SK-N-SH neurons were infected with HSV-1 and stained with pooled anti-VP5 MAb and, simultaneously, with rabbit anti-gB or rat gE/gI polyclonal antibodies. Again, discrete puncta of green, representing gB or gE/gI, and red, representing VP5, were observed (Fig. 3A and B). Occasionally a yellow punctum was observed, but these were rare and often nonconcentric (Table 1). Overall, only 3 to 6% of capsids displayed as much as 25% overlap with gB, gD, or gE/gI, and these tended to be in regions of axons with dense concentrations of capsids and glycoproteins. In other studies, axons were stained with mouse anti-gE/gI antibodies and, simultaneously, with rabbit anti-gD antibodies, and both glycoproteins were found in the majority of vesicles (not shown), consistent with the notion that vesicles contain a full complement of virion glycoproteins. We concluded that the vast majority of HSV capsids were transported in axons without a glycoprotein coat.
FIG. 3.
HSV-1 capsids do not colocalize with gB or gE/gI in human neurons. SK-N-SH neurons were infected with HSV-1 (F) for 18 h, fixed, permeabilized, and stained simultaneously with two mouse MAbs specific for VP5 (red) and rabbit polyclonal antibodies specific for gB (green) in panel A or rabbit polyclonal antibodies specific for gE/gI (green) in panel B followed by Alexa 594-conjugated goat anti-mouse IgG and Alexa 488-conjugated goat anti-rabbit IgG. Scale bars are 6 μm in panel A and 5 μm in panel B.
Glycoproteins move separately from VP26/GFP-labeled capsids that rarely stalled.
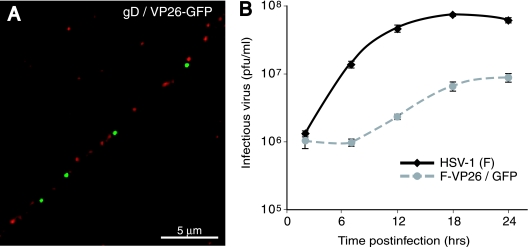
Previously, Desai and Person (9) constructed a recombinant HSV-1 KOS strain expressing GFP fused to the VP26 capsid protein. In order to study the colocalization of capsids and glycoproteins by another approach, we constructed a recombinant HSV-1 strain F expressing this VP26/GFP. SK-N-SH neurons were infected with F-VP26/GFP for 24 h, and the cells were fixed, permeabilized, and stained with rabbit anti-gD polyclonal antibodies. Again, discrete puncta of green, representing VP26, and red, representing gD, were observed in axons, with very little colocalization between the two proteins (Fig. 4A). Other experiments showed very little colocalization of VP26/GFP and gB or gE/gI (not shown). These results supported our conclusions that HSV capsids and glycoproteins travel in axons as separate units.
FIG. 4.
Localization of capsids and glycoproteins in human SK-N-SH neurons infected with HSV-1 F-VP26-GFP. (A) SK-N-SH neurons were infected with F-VP26-GFP for 24 h, fixed, permeabilized, and stained with rabbit polyclonal anti-gD antibodies followed by Texas red-conjugated donkey anti-rabbit IgG. Capsids are depicted in green, and gD is depicted in red. Scale bar, 5 μm. (B) Growth of F-VP26/GFP on differentiated SK-N-SH cells. SK-N-SH neurons were infected with either VP26/GFP or wild-type HSV strain F using 5 PFU/cell, and the combined cells and cell culture supernatants were collected at 2, 8, 12, 18, and 24 h. The titer of infectious virus was determined on Vero cells.
There have been observations that a fraction of PRV capsids stall during relatively short periods of observation (≈2 min) in live-cell imaging experiments (13, 39). Thus, it was possible that there were two populations of HSV capsids in our experiments: those that move progressively along the axon and others that were stalled either transiently or permanently. If this was the case, it was conceivable that unenveloped capsids (without gB and gD) were not moving and a second population of relatively rare, enveloped capsids (if these exist) were transported and constituted the biologically important fraction of particles in axons. To investigate this possibility, live-cell imaging was performed with F-VP26/GFP-infected SK-N-SH neurons for 24 h. Images were taken either every 20 s for 8 to 10 min or every 5 s for 5 min. The majority of capsids exhibited some form of movement throughout the time of recording. A fraction of capsids (30 to 40%) traveled at speeds we estimated at approximately 0.2 to 1 μm/s, and other, more rare particles (10%) moved very quickly (>1 μm/s) in an uninterrupted path in the axon (see video S1 in the supplemental material). The rapidly advancing particles moved in a saltatory fashion: moving first in an anterograde direction, then reversing direction for a time, followed by anterograde transport, as was described for PRV (24, 39). Other particles (≈15 to 25% of the total particles) moved over very short distances, vibrating or jittering back and forth, although many of these resumed more substantial movement over time. Another fraction of capsids (≈15 to 25%) were stationary for most of the measurement period. It is important to note that these studies were not designed to measure the speed of capsid transport, as was the case in previous experiments that involved shorter periods between image capture: i.e., 5 frames per second (13, 39). Fluorescence bleaching occurs in these analyses, limiting the total number of observations that can be made. We elected to obtain images over a more extended time frame. Our studies indicated some rapid capsid movement, consistent with fast axonal transport: there was some stalling and jiggling, but a substantial fraction of capsids moved during 5 to 10 min.
We found it necessary to study the movement of VP26/GFP capsids in axons after 24 to 28 h of infection of SK-N-SH neurons (Fig. 4A), rather than after 17 to 18 h with wild-type HSV-1 (Fig. 2 and 3) as capsids were not transported in axons earlier than 24 h. There were also fewer capsids detected in axons infected with F-VP26/GFP (Fig. 4A) compared with those produced by wild-type HSV (Fig. 2 and 3). We analyzed replication of this recombinant in SK-N-SH neurons. F-VP26/GFP produced approximately 10-fold fewer infectious virions than with wild-type HSV-1 (Fig. 4B). Based on observations that a PRV VP26/GFP recombinant incorporated variable amounts of VP26/GFP into capsids (13), it is likely that some HSV capsids may contain reduced quantities of VP26/GFP compared with wild-type VP26. However, our conclusions related to the separate transport model were not altered by these observations, as there remained significant numbers of GFP-labeled capsids that were transported into axons and these capsids were separate from virion glycoproteins.
Separate transport of HSV capsids and glycoproteins in rat neurons.
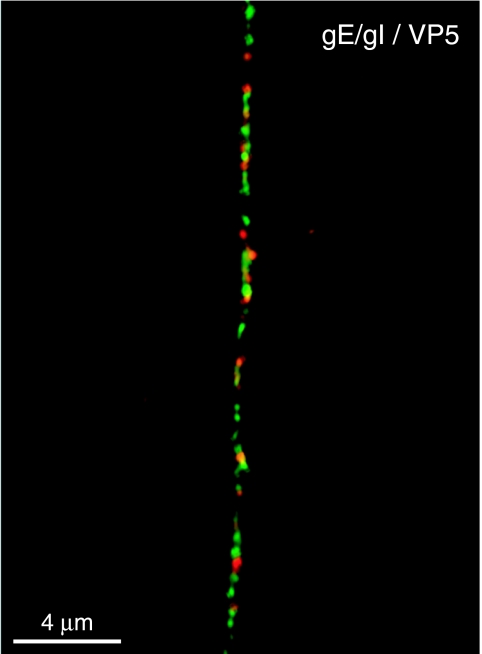
Our studies initially focused on human neuroblastoma cells differentiated to form axons. We presume that HSV capsid movement into axons is a very basic property that would not differ mechanistically in other neurons. To examine this point further, we characterized HSV capsids and glycoproteins in neurons derived from rat TG. Rat TG neurons were derived from newborn rat pups and stimulated with nerve growth factor to produce axons by procedures similar to those in our previous studies (10). As with SK-N-SH neurons, TG neurons produced long axons, although the rat neuronal axons were bipolar, extending away from one another, and unbranched. The rat TG neurons are sensory neurons and do not produce dendrites (21, 41). Rat TG neurons were infected with HSV-1 for 18 h, fixed, permeabilized, and stained with two anti-VP5 MAbs and rat polyclonal gE/gI-specific antibodies. Although glycoprotein and nucleocapsid puncta were more dense in rat TG neurons than in SK-N-SH neurons, glycoproteins, exemplified by gE/I (green), and capsids stained with anti-VP5 MAb (red) were found in distinct locations (Fig. 5). Those red and green puncta that were in close proximity to one another infrequently showed a concentric profile. Similar results were observed with rat DRG neurons infected with HSV-1 and stained with antibodies to VP5 and gE/gI (data not shown). Taken together, these results support separate transport of glycoproteins and capsids in axons of HSV-infected rat neurons.
FIG. 5.
HSV-1 glycoprotein gE/gI does not colocalize with capsids in axons of rat TG neurons. TG neurons were isolated from rat pups and cultured for 7 days in the presence of nerve growth factor. Cells were infected with HSV-1 (F) for 18 h, fixed, permeabilized, and stained with a combination of mouse MAb specific for VP5 (red) and rat polyclonal gE/gI-specific antibodies (green) followed by Alexa 594-conjugated goat anti-mouse IgG and Alexa 488-conjugated goat anti-rat IgG antibodies. (A) Scale bar, 4 μm.
Glycoproteins and nucleocapsids colocalize in intact virions.
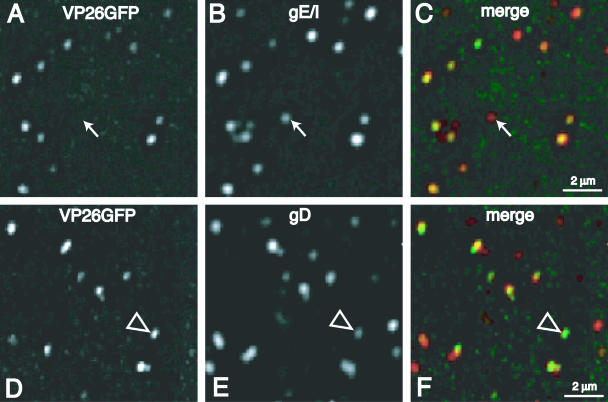
Our immunofluorescence studies demonstrated primarily separate localization of capsids and glycoproteins in axons. To confirm that glycoprotein-specific antibodies can stain fully assembled extracellular virions, we purified F-VP26/GFP particles from Vero cell culture supernatants. Virions were immobilized on collagen-coated glass slides and immunostained with rat polyclonal anti-gE/gI antibody or rat polyclonal anti-gD antibodies. The vast majority of VP26/GFP capsids (95% of 717 VP26/GFP puncta) were stained with anti-gE/gI antibodies (Fig. 6A to C), and 96% of 551 VP26/GFP puncta were stained with anti-gD antibodies (Fig. 6D to F). We did observe some red glycoprotein puncta with relatively low levels of green nucleocapsid signal that may represent light or L particles or could represent virions with low levels of VP26/GFP (see Fig. 6A to C, filled arrows). In addition, there was a smaller fraction of particles that displayed stronger green capsid signal accompanied by weaker or nonexistent red glycoprotein signal (Fig. 6D to F, empty arrowheads). Particles without any detectable glycoproteins did not represent more than 5% of the total sample. However, the outcome of relatively low green or red signals in some cases did not appear yellow in the merge panels (Fig. 6C and F). However, we counted each particle carefully in the red and green channels to reduce the effects of this variability. Therefore, we concluded that most extracellular virions with VP26/GFP fluorescence could be stained with antiglycoprotein antibodies. This further supports our observations that capsids in axons are not coated with an envelope containing viral glycoproteins.
FIG. 6.
Nucleocapsids and glycoproteins colocalize in extracellular purified virions. HSV-1 F-VP26/GFP virions were purified from Vero cell culture supernatants, immobilized on collagen-coated glass coverslips, and stained with mouse anti-gE/gI-specific MAb (A to C) or rabbit polyclonal gD-specific antibodies (D to F) followed by FITC-conjugated anti-mouse IgG or Texas red-conjugated anti-rabbit IgG. Filled arrows in panels A to C represent a particle that contained glycoproteins and little or no capsid signal. Empty arrowheads in panels D to F represent a particle with high levels of capsid fluorescence compared with anti-gD staining.
Brefeldin A retards capsid and glycoprotein transport in axons.
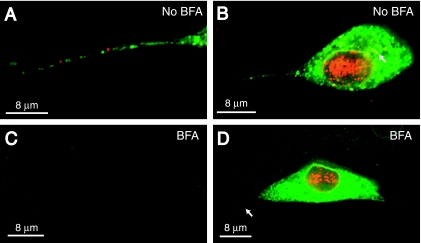
Miranda-Saksena et al. (32) reported that HSV capsids were transported into and along axons when neurons were treated with BFA, but glycoprotein transport was blocked. In contrast, del Rio et al. (8) observed that axonal transport of both PRV capsids and glycoproteins was inhibited by BFA. To study this issue, SK-N-SH neurons were infected with HSV-1 and then treated with 2 μg/ml of BFA from 3 h until 15 h p.i. Some neurons treated with BFA exhibited axonal retraction and neuronal detachment from the substrate near the end of this treatment. However, the majority of BFA-treated neurons remained anchored, exhibited long, unbranched axons, and were morphologically indistinguishable from untreated neurons. Neurons were fixed and stained simultaneously with pooled anti-VP5 MAb and rat polyclonal anti-gE/gI antibodies. As in previous experiments, neurons not treated with BFA exhibited multiple red (VP5) and green (gE/gI) puncta in axons (Fig. 7A). The cell bodies of these untreated neurons displayed extensive VP5 staining in the nucleus, as well as discrete puncta and larger accumulations of capsids in the cytoplasm (Fig. 7B, see arrow in cytoplasm). gE/gI (green) was found in the cytoplasm and nuclear envelope of untreated neurons (Fig. 7B). Cell bodies of neurons treated with BFA exhibited capsids in the nucleus, but not in the cytoplasm (Fig. 7D). Little or no VP5 (red) and gE/gI (green) puncta were observed in axons of BFA-treated neurons (Fig. 7C and D). Note that the image shown in Fig. 7C contains an axon crossing the panel from the upper left to lower right observed by phase contrast (not shown). SK-N-SH neurons treated with BFA from 3 to 15 h p.i. and stained with rabbit polyclonal anti-gB or anti-gD antibodies and, simultaneously, with mouse anti-VP5 MAb produced identical results: i.e., there were no glycoproteins or capsids in axons (data not shown). Treatment of neurons with lower concentrations of BFA or with 2 μg/ml BFA from 9 h until 15 h or from 12 h until 15 h produced less-pronounced inhibition of glycoprotein and capsid transport (not shown). Importantly, no conditions of drug dose or time of treatment allowed for transport of capsids while inhibiting transport of viral glycoproteins. Similar results were obtained with both rat TG neurons and DRG neurons treated with BFA and immunostained for VP5 and gE/gI proteins (data not shown). We concluded that BFA blocks the axonal transport of both HSV capsids and glycoproteins and cannot be used to distinguish between models of HSV axonal transport.
FIG. 7.
Effects of BFA on axonal transport of HSV capsids and glycoproteins. SK-N-SH neurons were infected with HSV-1 (F) and either not treated or treated with BFA (2 μg/ml) from 3 h until 15 h after infection. Neurons were fixed, permeabilized, and stained with a combination of mouse anti-VP5 MAb (red) and rabbit polyclonal gE/gI-specific antibodies (green) followed by Texas red-conjugated donkey anti-mouse IgG and FITC-conjugated donkey anti-rabbit IgG. (A) Axon of neuron not treated with BFA. (B) Cell body and axon of neuron not treated with BFA. The arrow points to anti-VP5 staining in the cytoplasm. (C) Axon of neuron treated with BFA. (D) Cell body and axon of neuron treated with BFA. The arrow points to an axon extending from the neuronal cell body. Scale bars, 8 μm.
Transport of glycoproteins in axons does not require capsids.
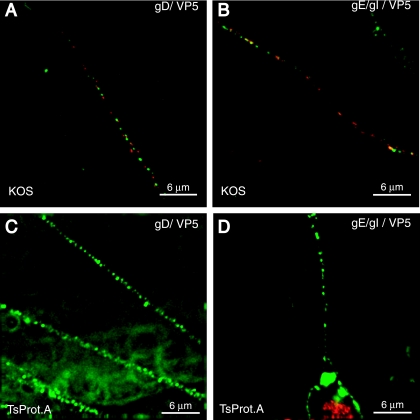
Taking another approach to study this question, we characterized transport of capsids and glycoproteins in neurons infected with HSV mutant TsProt.A, which expresses a temperature-sensitive viral protease (15, 35). At 39.5°C, viral nucleocapsids accumulate in the nucleus of TsProt.A-infected cells so that very few capsids reach the cytoplasm. For example, in our recent studies, TsProt.A produced only approximately 0.6% of the number of enveloped particles compared with wild-type HSV-1 (KOS) (43). SK-N-SH neurons were infected with either TsProt.A or wild-type HSV-1 strain KOS for 90 min at 37°C and then shifted to 39.5°C for an additional 16.5 h. Neurons were fixed and simultaneously stained with anti-VP5 monoclonal antibodies and rabbit polyclonal antibodies specific for either gD or gE/gI. Numerous gD and gE/gI (green) and VP5 (red) puncta were observed in axons of neurons infected with wild-type KOS (Fig. 8A and B). In contrast, axons of neurons infected with TsProt.A displayed very few or no capsids (red) in axons, although abundant staining for gD and gE/gI (green) was detected in these axons (Fig. 8C and D). Intense VP5 staining (red) was observed in the nuclei of TsProt.A-infected cells, but not in the cytoplasm (Fig. 8D), consistent with retention in the nucleus. Therefore, HSV glycoproteins can be efficiently transported into and down neuronal axons when capsids are not available.
FIG. 8.
HSV glycoproteins are transported normally into axons when capsids are retained in neuronal cell bodies. SK-N-SH neurons were infected with wild-type HSV-1 (KOS) (A and B) or TsProt.A (C and D) for 90 min at 37°C then shifted to 39.5°C for 16.5 h. Neurons were stained with a pool of anti-VP5 MAb (red) and rabbit polyclonal gD-specific antibodies (green) (A and C) or rabbit polyclonal gE/gI-specific antibodies (green) (B and D) followed by Alexa 594-conjugated anti-mouse IgG and Alexa 488-conjugated anti-rabbit IgG. Scale bars, 6 μm.
Anti-NC-1 polyclonal antibodies stain HSV viral proteins apart from capsids.
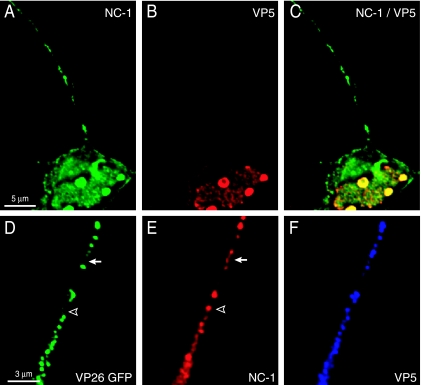
Earlier observations that HSV capsids were transported down axons without viral glycoproteins in BFA-treated neurons involved the use of the anti-VP5 rabbit polyclonal serum known as anti-NC-1 (32). Anti-NC-1 antibodies were produced by injecting rabbits with gel-purified VP5 protein, known then as NC-1 (6). During our studies of TsProt.A-infected neurons, where capsids were retained in the nucleus, we noticed that anti-NC-1 antibodies stained axons of HSV-infected neurons (Fig. 9A). This was in contrast to observations that two MAbs specific for VP5 did not stain these axons (Fig. 8C and D and Fig. 9B). Note that anti-NC-1 sera were adsorbed against permeabilized, uninfected SK-N-SH neurons and did not react detectably with uninfected cells by immunofluorescence (not shown). To characterize this further, we infected neurons with F-VP26/GFP and stained the cells with NC-1 antibodies and, simultaneously, with two VP5-specific MAbs. Anti-NC-1 antibodies generally stained regions of axons that contained VP26/GFP (Fig. 9D and E). However, in addition, anti-NC-1 stained separate regions of axons, sites that did not contain VP26/GFP-labeled capsids (see arrow and arrowhead in Fig. 9E). VP5-specific MAbs more extensively colocalized with VP26/GFP (Fig. 9F). Thus, it appears that polyclonal anti-NC-1 antibodies react with HSV proteins other than those that are strictly associated with HSV capsids.
FIG. 9.
Anti-NC-1 antibodies stain HSV proteins other than those in capsids. (A to C) SK-N-SH neurons were infected with HSV TsProt.A for 18 h, fixed, permeabilized, and stained with a combination of pooled mouse anti-VP5 MAb (red) and preabsorbed rabbit polyclonal anti-NC-1 antibodies (green) followed by Texas red-conjugated donkey anti-mouse IgG and FITC-conjugated donkey anti-rabbit IgG secondary antibodies. (D to F) SK-N-SH neurons infected with F-VP26/GFP for 24 h were fixed, permeabilized, and simultaneously stained with preabsorbed rabbit polyclonal anti-NC-1 antibodies and anti-VP5 mouse MAb and then with secondary antibodies: Texas red-conjugated donkey anti-mouse IgG and Cy5-conjugated donkey anti-rabbit IgG. VP26/GFP-labeled axons are shown in green, NC-1 is shown in red (pseudoimaged from Cy5 blue), and VP5 is shown in blue (pseudoimaged from Texas red). The arrow and arrowhead indicate examples of NC-1 staining which did not overlap with the VP26/GFP signal (green).
DISCUSSION
Egress of alphaherpesviruses from cells has been a controversial subject. EM studies of nonneuronal cells supported several different models for acquisition of the final virion envelope. Ultimately, deenvelopment at the outer nuclear enveloped followed by reenvelopment at the TGN was proven by biochemical and genetic studies (reviewed in references 19 and 29 to 31). Similarly, much of the support for how alphaherpesviruses move from neuronal cell bodies to axon termini has been based on EM studies (2, 8, 18, 25, 27, 32-34, 37). Interpretation of these results may be difficult because capsids are often rare in distal axons and it can be difficult to discern whether images were derived from axons versus varicosities. This problem may be more problematic with explanted neuronal tissues that produce dense networks of axons and more numerous varicosities.
Previous immunofluorescence microscopic studies showed that HSV gC (32) did not colocalize with capsids stained with anti-VP5 (NC-1) polyclonal antibodies and PRV gB did not overlap with VP26/GFP-labeled capsids (39). These light microscopic studies could more reliably discern sections of axons versus varicosities. Our studies involved a deconvolution microscopic examination of ultrathin sections and a more extensive panel of glycoproteins: HSV gB, gD, and gE/gI. We carefully quantified hundreds of puncta representing glycoproteins and capsids and concluded that glycoprotein puncta rarely overlap with any of these glycoproteins. Those rare puncta that stained with both antiglycoprotein and anti-VP5 antibodies were frequently in axonal regions with high concentrations of glycoprotein and capsids, and overlap was almost always nonconcentric. In addition, VP26/GFP-labeled capsids in axons were distributed at separate sites from glycoprotein puncta. In contrast, virions purified from Vero cell culture supernatants displayed extensive colocalization of VP26/GFP and glycoprotein staining. Live-cell analysis of neurons expressing VP26/GFP showed that only a small fraction of capsids remained stalled for the entire recording period of 5 to 10 min. Over half the capsids moved—some with the kinetics of fast axonal transport. These observations ruled out the possibility that a small subset of enveloped capsids were the relevant ones that moved. We observed defects in replication of F-VP26/GFP virus in SK-N-SH cells, and capsids moved into axons more slowly: i.e., at 24 to 28 h p.i. compared with 17 to 18 h of infection. However, numerous VP26/GFP-labeled capsids were transported into axons, and these capsids were uniformly separate from viral glycoproteins, supporting our observations with antibodies. We concluded that HSV capsids are transported in axons without an envelope that contains the viral glycoproteins.
Many of our studies involved differentiated human SK-N-SH neurons, and one might argue that these cells do not accurately represent the sensory neurons that HSV infects in vivo. SK-N-SH neurons expressed neurites that were morphologically similar to rat TG and DRG neuronal axons and expressed axonal markers, and HSV was efficiently transported in SK-N-SH neurites. SK-N-SH neurons have some advantages (over explanted human or rat tissues or neurons) in that these cells could be plated more sparsely, thereby avoiding the formation of numerous varicosities. The process by which alphaherpesviruses transport capsids would seem to be a very basic one, and it could be argued that this should not differ mechanistically in different neurons. Consistent with this, we found that HSV glycoproteins and capsids were also found in completely separate transport structures in axons of rat TG and DRG neurons.
One of the more compelling pieces of evidence for the separate transport model involved effects of BFA that blocked axonal transport of HSV glycoproteins, without affecting capsid transport (32). On the other hand, del Rio et al. (8) reported that both PRV capsids and glycoproteins accumulated in cell bodies of BFA-treated rat neurons. BFA can have pleiotropic effects beyond the ER and Golgi apparatus, especially in cells treated for longer periods. HSV capsids accumulate in the nucleus of nonneuronal cells treated with BFA, apparently through effects on the nuclear envelope (7). We found that both HSV capsids and glycoproteins accumulated in the cell bodies of BFA-treated human and rat neurons. There was evidence that capsids remained in the nucleus. We could find no conditions of drug concentration or times of treatment in which glycoprotein transport was blocked while capsids were transported. Therefore, in our hands, BFA cannot be used to support the separate transport model.
While performing experiments with TsProt.A-infected neurons at 39.5°C, we observed that anti-NC-1 polyclonal antibodies (adsorbed against uninfected neurons) stained neuronal axons, yet anti-VP5 antibodies did not. It is well established that TsProt.A accumulates capsids in the nucleus of nonneuronal cells at the nonpermissive temperature (15, 43), and our confocal data supported the conclusion that this was the case in neurons. Moreover, neurons infected with F-VP26/GFP displayed substantial anti-NC-1 staining of puncta (or other structures) in axons that were distinct from that of VP26/GFP-labeled capsids. Anti-NC-1 antibodies stained axons more diffusely and in a nonoverlapping fashion compared with anti-VP5 MAb. Thus, anti-NC-1 sera apparently reacts with not only VP5 but also some other HSV protein (e.g., a tegument protein) that is transported into axons separately from capsids. Less likely is the possibility that a fraction of VP5 is not associated with capsids and yet transported into axons, but this VP5 must not be recognized by two anti-VP5 MAbs. These results may explain observations of Miranda-Saksena et al. (32) who used anti-NC-1 antibodies in their BFA experiments. If NC-1 recognizes HSV proteins other than capsid proteins, the effects of BFA on capsid movement might have been misinterpreted.
Even though BFA experiments did not separate glycoprotein and capsid transport, our TsProt.A experiments produced evidence that HSV glycoproteins can be transported in axons when capsids were absent. Axonal transport of HSV glycoproteins appeared normal when capsids were retained in the cell body in TsProt.A-infected neurons. If a substantial fraction of glycoproteins normally gain access to axons in the form of enveloped virions, then one might expect reduced glycoprotein transport without cytoplasmic capsids. Nevertheless, these results do not exclude the possibility that enveloped virions are transported in axons of wild-type HSV-infected neurons. However, our deconvolution microscopy experiments strongly argue against the notion that there are significant quantities of HSV glycoproteins in membranes surrounding capsids in axons. Our results do not allow us to comment on whether capsids are transported in axons surrounded by a cellular membrane, one lacking viral glycoproteins (see Fig. 1C). Ch'ng et al. (5) recently described EM images of PRV capsids surrounded by either cellular or viral membranes in distal axons. There was the suggestion that the surrounding membrane might not include viral glycoproteins, especially if PRV gB and capsids do not colocalize (39). However, a model in which alphaherpesvirus capsids were transported in a single cellular membrane that lacks the viral glycoproteins is difficult to rationalize in terms of the final assembly and release of virions at axon termini. Unenveloped capsids transported as in Fig. 1A can bud into vesicles containing viral glycoproteins to produce infectious virions (Fig. 1D). Infectious virions transported as in Fig. 1B are already in this form. However, it is difficult to imagine how capsids surrounded by a single cellular membrane without glycoproteins (as in Fig. 1C) would acquire an envelope and exit cells.
In summary, our results show that HSV glycoproteins are transported in axons independently from capsids, supporting the separate transport model of Penfold and Cunningham (34).
Supplementary Material
Acknowledgments
We are most grateful to Aurelie Snyder of the Microbiology Core Facility for invaluable assistance and expertise with deconvolution microscopy and Tiffany Howard for assistance with graphics.
This work was supported by a grant from the National Institute of Allergy and Infectious Diseases (AI73996) and a training grant (2-T32-AI007472-11 to A.S.) titled “Interactions at the Microbe/Host Interface.”
Footnotes
Published ahead of print on 13 September 2006.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Cai, W., S. Person, S. C. Warner, J. Zhou, and N. A. DeLuca. 1987. Linker-insertion nonsense and restriction-site deletion mutations of the gB glycoprotein gene of herpes simplex virus type 1. J. Virol. 61:714-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Card, J. P., L. Rinaman, R. B. Lynn, B. H. Lee, R. P. Meade, R. R. Miselis, and L. W. Enquist. 1993. Pseudorabies virus infection of the rat central nervous system: ultrastructural characterization of viral replication, transport, and pathogenesis. J. Neurosci. 13:2515-2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman, T. L., I. You, I. M. Joseph, P. J. Bjorkman, S. L. Morrison, and M. Raghavan. 1999. Characterization of the interaction between the herpes simplex virus type I Fc receptor and immunoglobulin G. J. Biol. Chem. 274:6911-6919. [DOI] [PubMed] [Google Scholar]
- 4.Ch'ng, T. H., and L. W. Enquist. 2005. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. J. Virol. 79:8835-8846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 79:10875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, G. H., M. Ponce de Leon, H. Diggelmann, W. C. Lawrence, S. K. Vernon, and R. J. Eisenberg. 1980. Structural analysis of the capsid polypeptides of herpes simplex virus types 1 and 2. J. Virol. 34:521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dasgupta, A., and D. W. Wilson. 2001. Evaluation of the primary effect of brefeldin A treatment upon herpes simplex virus assembly. J. Gen. Virol. 82:1561-1567. [DOI] [PubMed] [Google Scholar]
- 8.del Rio, T., T. H. Ch'ng, E. A. Flood, S. P. Gross, and L. W. Enquist. 2005. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. J. Virol. 79:3903-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai, P., and S. Person. 1998. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J. Virol. 72:7563-7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dingwell, K. S., L. C. Doering, and D. C. Johnson. 1995. Glycoproteins E and I facilitate neuron-to-neuron spread of herpes simplex virus. J. Virol. 69:7087-7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg, R. J., D. Long, M. Ponce de Leon, J. T. Matthews, P. G. Spear, M. G. Gibson, L. A. Lasky, P. Berman, E. Golub, and G. H. Cohen. 1985. Localization of epitopes of herpes simplex virus type 1 glycoprotein D. J. Virol. 53:634-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enquist, L. W. 2002. Exploiting circuit-specific spread of pseudorabies virus in the central nervous system: insights to pathogenesis and circuit tracers. J. Infect. Dis. 186(Suppl. 2):S209-S214. [DOI] [PubMed] [Google Scholar]
- 13.Enquist, L. W., M. J. Tomishima, S. Gross, and G. A. Smith. 2002. Directional spread of an alpha-herpesvirus in the nervous system. Vet. Microbiol. 86:5-16. [DOI] [PubMed] [Google Scholar]
- 14.Forrester, A., H. Farrell, G. Wilkinson, J. Kaye, N. Davis-Poynter, and T. Minson. 1992. Construction and properties of a mutant of herpes simplex virus type 1 with glycoprotein H coding sequences deleted. J. Virol. 66:341-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao, M., L. Matusick-Kumar, W. Hurlburt, S. F. DiTusa, W. W. Newcomb, J. C. Brown, P. J. McCann III, I. Deckman, and R. J. Colonno. 1994. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J. Virol. 68:3702-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu, H., and B. Roizman. 2003. The degradation of promyelocytic leukemia and Sp100 proteins by herpes simplex virus 1 is mediated by the ubiquitin-conjugating enzyme UbcH5a. Proc. Natl. Acad. Sci. USA 100:8963-8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanke, T., F. L. Graham, V. Lulitanond, and D. C. Johnson. 1990. Herpes simplex virus IgG Fc receptors induced using recombinant adenovirus vectors expressing glycoproteins E and I. Virology 177:437-444. [DOI] [PubMed] [Google Scholar]
- 18.Holland, D. J., M. Miranda-Saksena, R. A. Boadle, P. Armati, and A. L. Cunningham. 1999. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. J. Virol. 73:8503-8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, D. C., and M. T. Huber. 2002. Directed egress of animal viruses promotes cell-to-cell spread. J. Virol. 76:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson, D. C., and P. G. Spear. 1982. Monensin inhibits the processing of herpes simplex virus glycoproteins, their transport to the cell surface, and the egress of virions from infected cells. J. Virol. 43:1102-1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kendel, E. R., J. H. Schwartz, and T. M. Jessell. 2000. Principles of neural science, 4th ed. McGraw-Hill Medical, New York, N.Y.
- 22.Kristensson, K., E. Lycke, M. Roytta, B. Svennerholm, and A. Vahlne. 1986. Neuritic transport of herpes simplex virus in rat sensory neurons in vitro. Effects of substances interacting with microtubular function and axonal flow [nocodazole, taxol and erythro-9-3-(2-hydroxynonyl)adenine]. J. Gen. Virol. 67:2023-2028. [DOI] [PubMed] [Google Scholar]
- 23.Ligas, M. W., and D. C. Johnson. 1988. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by β-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 62:1486-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luxton, G. W., S. Haverlock, K. E. Coller, S. E. Antinone, A. Pincetic, and G. A. Smith. 2005. Targeting of herpesvirus capsid transport in axons is coupled to association with specific sets of tegument proteins. Proc. Natl. Acad. Sci. USA 102:5832-5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lycke, E., B. Hamark, M. Johansson, A. Krotochwil, J. Lycke, and B. Svennerholm. 1988. Herpes simplex virus infection of the human sensory neuron. An electron microscopy study. Arch. Virol. 101:87-104. [DOI] [PubMed] [Google Scholar]
- 26.Lycke, E., K. Kristensson, B. Svennerholm, A. Vahlne, and R. Ziegler. 1984. Uptake and transport of herpes simplex virus in neurites of rat dorsal root ganglia cells in culture. J. Gen. Virol. 65:55-64. [DOI] [PubMed] [Google Scholar]
- 27.Marchand, C. F., and M. E. Schwab. 1986. Binding, uptake and retrograde axonal transport of herpes virus suis in sympathetic neurons. Brain Res. 383:262-270. [DOI] [PubMed] [Google Scholar]
- 28.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106:167-180. [DOI] [PubMed] [Google Scholar]
- 30.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 76:1537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mettenleiter, T. C., and T. Minson. 2006. Egress of alphaherpesviruses. J. Virol. 80:1610-1611. (Letter [author reply 80:1611-1612].) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda-Saksena, M., P. Armati, R. A. Boadle, D. J. Holland, and A. L. Cunningham. 2000. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. J. Virol. 74:1827-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miranda-Saksena, M., R. A. Boadle, P. Armati, and A. L. Cunningham. 2002. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. J. Virol. 76:9934-9951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penfold, M. E., P. Armati, and A. L. Cunningham. 1994. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proc. Natl. Acad. Sci. USA 91:6529-6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preston, V. G., J. A. V. Coates, and F. J. Rixon. 1983. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J. Virol. 45:1056-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roop, C., L. Hutchinson, and D. C. Johnson. 1993. A mutant herpes simplex virus type 1 unable to express glycoprotein L cannot enter cells, and its particles lack glycoprotein H. J. Virol. 67:2285-2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saksena, M. M., H. Wakisaka, B. Tijono, R. A. Boadle, F. Rixon, H. Takahashi, and A. L. Cunningham. 2006. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. J. Virol. 80:3592-3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson, S. A., M. D. Manchak, E. J. Hager, C. Krummenacher, J. C.Whitbeck, M. J. Levin, C. R. Freed, C. L. Wilcox, G. H. Cohen, R. J. Eisenberg, and L. I. Pizer. 2005. Nectin-1/HveC mediates herpes simplex virus type 1 entry into primary human sensory neurons and fibroblasts. J. Neurovirol. 11:208-218. [DOI] [PubMed] [Google Scholar]
- 39.Smith, G. A., S. P. Gross, and L. W. Enquist. 2001. Herpesviruses use bidirectional fast-axonal transport to spread in sensory neurons. Proc. Natl. Acad. Sci. USA 98:3466-3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith, G. A., L. Pomeranz, S. P. Gross, and L. W. Enquist. 2004. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proc. Natl. Acad. Sci. USA 101:16034-16039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Squire, L. R., F. E. Bloom, N. C. Spitzer, J. L. Roberts, M. J. Zigmond, and S. K. Mcconnell. 2002. Fundamental neuroscience, 2nd ed. Academic Press, San Diego, Calif.
- 42.Tomishima, M. J., G. A. Smith, and L. W. Enquist. 2001. Sorting and transport of alpha herpesviruses in axons. Traffic 2:429-436. [DOI] [PubMed] [Google Scholar]
- 43.Wisner, T. W., and D. C. Johnson. 2004. Redistribution of cellular and herpes simplex virus proteins from the trans-Golgi network to cell junctions without enveloped capsids. J. Virol. 78:11519-11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou, G., V. Galvan, G. Campadelli-Fiume, and B. Roizman. 2000. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J. Virol. 74:11782-11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.