Abstract
The Kaposi's sarcoma-associated herpesvirus (KSHV) envelope glycoprotein gpK8.1 contributes to cellular attachment through binding cell surface heparan sulfate proteoglycans. By using a soluble recombinant form of gpK8.1, we discovered that a consequence of gpK8.1 interaction with human fibroblasts is the induction of an antiviral response, as characterized by the activation of interferon regulatory factor 3 (IRF-3), production of interferon beta (IFN-β), and expression of interferon-stimulated antiviral genes. In contrast, neither IFN-β expression nor a functional antiviral response is observed in cells treated with KSHV virions. The interferon response induced by soluble gpK8.1 can be inhibited by simultaneous treatment with UV-inactivated virions, while the induction of an indicator inflammatory cytokine, interleukin-6, was readily evident in the response to both gpK8.1 and KSHV. In addition, KSHV virions abrogate gpK8.1-mediated activation of IRF-3, an early transcriptional regulator for cellular antiviral responses. Although innate immune responses are initiated during contact between gpK8.1 and cellular receptor(s), these results suggest that the virion contains one or more structural elements that selectively repress an effective antiviral response while allowing cellular responses favorable to the KSHV life cycle.
Epidemiological evidence indicates that Kaposi's sarcoma-associated herpesvirus (KSHV) is the causative agent of Kaposi's sarcoma (KS), a subset of multicentric Castleman's disease, and B-cell primary effusion lymphoma (PEL), also known as body cavity lymphoma (37). One of the hallmarks of KS is the abnormal modulation of cytokine levels. Cells associated with KS lesions produce elevated levels of inflammatory cytokines, including tumor necrosis factor alpha, gamma interferon (IFN-γ), interleukin-1β (IL-1β), interleukin-2, and interleukin-6 (reviewed in reference 17). In particular, IL-6 plays a significant role since the neoplasms associated with KSHV use IL-6 as a growth factor (3, 28, 33). The importance of cytokines to KSHV is highlighted by the fact that KSHV encodes homologues of cellular cytokines, including viral IL-6 (vIL-6) (open reading frame [ORF] K2), vCCL1 (ORF K6), vCCL2 (ORF K4), and vCCL3 (ORF K4.1) (11). These viral cytokines promote the proliferation of B cells, production of angiogenic factors, recruitment of infiltrating inflammatory cells, and activation of endothelial cells to acquire the spindle cell phenotype observed in vitro (16). KSHV reactivation from latency may also require the presence of a subset of inflammatory cytokines, but the molecular mechanisms underlying this process are unknown (36).
One group of cytokines critically important for combating viral infection are the type I interferons (IFN-α/β). These molecules represent the first line of defense by the innate immune system and prepare cells for invading pathogens by inducing the transcription of genes involved in cellular antiviral responses and the activation of innate and adaptive immune responses (21). IFN-α/β can be produced by nearly all cell types in response to viral infection; their expression is tightly controlled at the level of transcription by interferon regulatory factors (IRFs), primarily IRF-3 and IRF-7 (reviewed in reference 24). This regulation is biphasic, with the first phase resulting in the activation of cytoplasmic IRF-3 by the phosphorylation of its C terminus (62). The noncanonical IκB kinase homologues IKKɛ and TANK-binding kinase 1 are required for IRF-3 activation and can phosphorylate IRF-3 directly (18, 50). Phosphorylated IRF-3 homodimerizes and is rapidly translocated to the nucleus (31), where it complexes with CBP/p300 and acts directly as a transcriptional activator of both beta interferon (IFN-β) and a subset of interferon-stimulated genes (ISGs) (60). Recent work has identified IRF-7 as an essential factor in the initial production of IFN-β in response to viral infection (23), suggesting that IRF-7 heterodimerizes with IRF-3 to activate the IFN-β promoter during the initial phase of IFN-α/β production. In the second phase, nascent IFN-β feeds back in both autocrine and paracrine fashions to amplify IRF-7 expression, resulting in amplified IFN-β production, the upregulation of additional antiviral genes, including IFN-α subtypes, the induction of apoptosis in infected cells, and the priming of neighboring cells for IFN-α/β production in the event of further infection (reviewed in reference 49). In addition to their antiviral effects, IFN-α/β also influence the expression and activity of other cytokines, such as IFN-γ and IL-6, and play roles in the adaptive immune response through their effects on dendritic cells (27).
The mechanisms by which viruses activate the IFN pathway are becoming increasingly diverse, and a growing body of evidence suggests that viruses can initiate IFN responses during cell entry. Specifically, viral envelope glycoproteins that mediate viral attachment and/or entry have been demonstrated to elicit IFN-α/β responses from cells. For example, both glycoprotein 120 of human immunodeficiency virus and glycoprotein M of transmissible gastroenteritis virus induce IFN-α/β production in target cells (1, 7, 26). Similarly, studies using soluble versions of both glycoprotein B of human cytomegalovirus (HCMV) and glycoprotein D of herpes simplex virus type 1 (HSV-1) have demonstrated that these envelope components are important contributors to the IFN-α/β response (2, 4, 5, 10, 53).
Like many viruses, members of the family Herpesviridae are sensitive to the antiviral effects of IFN-α/β. For example, immediate early gene expression of both HCMV and HSV is restricted but not abolished by IFN-α/β (34, 44, 48, 55). To minimalize antiviral responses activated during entry, herpesviruses have evolved various mechanisms to counter the cellular response. Both HCMV and HSV carry genes that rapidly counter the cellular IFN-α/β response during infection. ICP0 of HSV-1 is an immediate early regulatory gene that inhibits the IFN pathway, as transcriptionally inactivated virions and viral mutants lacking ICP0 are hypersensitive to IFN-α/β in vitro (39). Similarly, the overexpression of the HCMV immediate early gene 2 in fibroblasts dramatically reduced amounts of IFN-β in response to infection by UV-inactivated HCMV, indicating that immediate early gene 2 contributes to inhibiting the HCMV-induced antiviral response (56). The HCMV tegument protein pp65 has been implicated as an additional mechanism by which HCMV can downregulate the IFN response since virions lacking pp65 induce a much stronger IFN-α/β response than do wild-type virions (6). However, wild-type virions still induce measurable IFN-α/β production during the first hours of infection (4), indicating that pp65 protein delivered to the cell during infection is unable to confer complete inhibition. More recently, it has been reported that rhesus cytomegalovirus (RhCMV) employs an alternative strategy to counter the cellular response as it fails to elicit a measurable antiviral response during infection, even in the absence of viral gene expression. Furthermore, RhCMV was able to inhibit antiviral responses mediated by HCMV, suggesting that RhCMV virions contain one or more factors that rapidly act to suppress the IFN-α/β response (15).
Microarray analysis performed during early KSHV infection revealed that several IFN-responsive genes were upregulated with rapid kinetics (41). Additionally, upon chemical induction of the KSHV lytic cycle, some of the same ISGs are upregulated in latently infected endothelial cells (45). However, the mechanisms by which KSHV initiates IFN-α/β responses are undefined. Since other viral glycoproteins are initiators of antiviral responses, we examined the role of KSHV envelope glycoprotein gpK8.1 in eliciting an IFN response from target cells. gpK8.1, positionally conserved with Epstein-Barr virus gp350/220 (43), is a type 1 membrane glycoprotein that accumulates on the plasma membrane during lytic replication and is incorporated into the viral envelope during KSHV egress (29). Recent studies demonstrate that gpK8.1 can induce specific host cell signaling pathways, rapidly activating extracellular signal-regulated kinase 1 and 2 during primary infection, but not phosphatidylinositol-3 kinase or focal adhesion kinase (51, 52). Recombinant gpK8.1 is strongly recognized by KS patient sera and may be used as a serological marker for KSHV infection (8, 29, 47), indicating that gpK8.1 is subject to immune surveillance. Recombinant gpK8.1 also binds cell surface heparan sulfate, consistent with envelope-associated gpK8.1 contributing to virus attachment to target cells (58), a role similar to those of other viral glycoproteins shown to activate IFN-α/β responses (4). We observed that cells respond to a soluble version of gpK8.1 by activating the IFN-α/β signaling pathway, including IRF-3 activation, ISG expression, IFN-β secretion, and the establishment of an antiviral state. In contrast, a functional antiviral response was not detected in cells challenged with KSHV virions, indicating that virions contain a component that dampens this response. Consistent with this hypothesis, virions were able to block gpK8.1-mediated induction of the IFN-α/β pathway. Unlike the IFN-α/β response, the expression of IL-6 was induced in response to both gpK8.1 and KSHV virions, indicating that virus-cell interactions trigger the inductions of multiple cellular responses. Our results indicate that, during the early events of infection, KSHV possesses mechanisms to differentially modulate the expression of inflammatory cytokines.
MATERIALS AND METHODS
Cells and medium.
Normal human dermal fibroblasts (NHDF; Clonetics, San Diego, CA) were maintained in Dulbecco's modified Eagle medium supplemented with 10% fetal bovine serum (FBS; HyClone), 1% glutamine, and 1% penicillin-streptomycin-Fungizone (PSF; Gibco-BRL). BCBL-1 cells (obtained through the AIDS Research and Reference Reagent Program) and green fluorescent protein (GFP)-BCBL-1 cells (a gift from J. Vieira) (57) were maintained in RPMI supplemented with 10% FBS, 1% PSF, and β-mercaptoethanol. Trichoplusia ni (BTI-TN-5B1-4) insect cells were maintained in Express Five SFM (Gibco-BRL) supplemented with 5% FBS and 1% PSF.
Construction and purification of recombinant gpK8.1 protein.
Hydropathy analysis of gpK8.1 revealed that the likely membrane-spanning domain lies from amino acids (aa) 197 to 215. Therefore, the extracellular domain of gpK8.1 containing amino acids 1 to 196 was PCR amplified from a plasmid encoding the full-length gpK8.1A cDNA (gift from J. Jung, Harvard Medical School) (29) by using the forward primer 5′-GGATCCGCCACCATGAGTTCCACACAGATTCG, with a BamHI site, and the reverse primer 5′-AAGCTTATTAATGATGATGATGATGATGGGTCC GTATTTC, with a six-His tag and a HindIII site. The resulting fragment was cloned into a modified version of pTriEx1.1 (Novagen) that had the NcoI site deleted to eliminate an additional start codon. To generate recombinant baculovirus, pTriEx-gpK8.1-S was cotransfected with BaculoGold DNA (BD Pharmingen) into BTI-TN-5B1-4 insect cells. Clones were selected by monitoring gpK8.1 protein expression via Western blot analysis with an anti-gpK8.1 rabbit polyclonal antibody (gift from J. Jung) and a goat anti-rabbit horseradish peroxidase (HRP) secondary antibody.
To produce soluble gpK8.1 protein, BTI-TN-5B1-4 cells were infected at a multiplicity of infection of 0.1 PFU/cell. At 24 h postinfection, cells were washed twice with phosphate-buffered saline (PBS) (10 mM sodium phosphate, 150 mM sodium chloride, pH 7.2) and covered with serum-free medium. The supernatant was collected at 72 h and dialyzed for 16 h against PBS using dialysis tubing (SpectraPor) with a molecular weight cutoff of 12,000 to 14,000. To purify six-His-tagged soluble gpK8.1, imidazole was added to the dialyzed culture medium to a final concentration of 10 mM. Nickel-nitrilotriacetic acid agarose beads (QIAGEN) were added to the medium and incubated for approximately 16 h at 4°C. The slurry was placed in a column and washed sequentially with 10 bed volumes of low-pH wash buffer (50 mM NaPO4, 10% glycerol, pH 6.0), followed by imidazole wash buffer (50 mM NaPO4, 0.5 M NaCl, 10% glycerol, 20 mM imidazole, pH 7.0). The protein was eluted with elution buffer (20 mM NaPO4, 0.5 M NaCl, 10% glycerol, 500 mM imidazole, pH 7.5) and dialyzed against PBS by using dialysis tubing with a molecular weight cutoff of 12,000 to 14,000. Glycerol was added to a final concentration of 10% prior to storage at −80°C. The protein concentrations of stocks of a truncated “soluble” version of gpK8.1 (gpK8.1-S) were determined by a Bio-Rad protein assay (Bio-Rad, Hercules, CA).
Virus production.
To produce cell-free KSHV virions, BCBL-1 or GFP-BCBL-1 cells were grown to a density of 2.5 × 105 cells/ml and induced with 20 ng/ml tetradecanoyl phorbol acetate. After 18 h, cells were pelleted and suspended in medium without tetradecanoyl phorbol acetate. At 5 days postinduction, cells were pelleted and the supernatant was filtered through a 0.45-μm filter. Virions were pelleted at 29,000 × g for 2 h and suspended in 1:100 of the original induction volume in serum-free endothelial basal medium (Gibco). A fluorescence-based PCR assay (TaqMan-based system) was used to calculate the number of virions present in KSHV stocks based upon genome copy number, using primers specific for ORF25 and methods described previously (54). The KSHV stocks used ranged in concentration from 8.0 × 108 to 1.2 × 109 genomes per ml. The AD169 strain of HCMV was propagated, and titers were determined as described previously (13). Unless otherwise indicated, experiments were performed with KSHV and HCMV virions UV inactivated as previously described (14). Briefly, virus was placed in a 24-well dish and irradiated in a Stratagene 2400 UV cross-linker for 4 min (9.9 × 105 μJ). This dose reduced viral gene expression 200-fold, as monitored by GFP fluorescence in 293T cells infected with UV-inactivated KSHV-GFP virions (57).
Dot blot analysis of gpK8.1 in KSVH virions.
To measure the amounts of gpK8.1 protein contained in virions and soluble preparations, serial dilutions of KSHV and gpK8.1-S stocks were aspirated in triplicate onto nitrocellulose membranes by using a 96-well format apparatus in the presence of lysis buffer (50 mM Tris, pH 7.4, 250 mM NaCl, 0.1% NP-40, 50 mM NaF, 5 mM EDTA). The resulting blots were probed with monoclonal anti-gpK8.1A/B antibody (Advanced Biotechnologies, Columbia, MD), followed by goat anti-mouse HRP secondary antibody (Pierce), enhanced chemiluminescence (PerkinElmer), and exposure to film. Samples were quantified with ImageQuant (Molecular Dynamics), and gpK8.1 content of KSHV dilutions was determined by plotting along a gpK8.1-S standard curve. From this analysis, we estimated that the amount of KSHV stock that contained 4.7 × 108 genome-positive particles contained approximately the same amount of immunoreactive gpK8.1 as did 1.7 μg of our gpK8.1-S preparation.
Immunoblot analysis of IRF-3.
To detect the various phosphorylated forms of IRF-3, NHDF cells were treated with either UV-inactivated KSHV (6.7 × 108 genomes/ml) or gpK8.1-S (10 μg/ml) for 6 h at 37°C in the presence of cycloheximide (100 μg/ml), washed in PBS, and lysed in harvest buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 30 mM NaF, 5 mM EDTA, 10% glycerol, 40 mM β-glycerophosphate, 1 mM Na2VO3, 0.1 mM phenylmethylsulfonyl fluoride, 1% NP-40). Proteins were resolved by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, transferred to a nitrocellulose membrane filter, and blotted with anti-IRF-3 antibody (Santa Cruz Biotechnology; catalog no. sc-9082), followed by goat anti-rabbit HRP secondary antibody (Pierce). Proteins bound with antibody were visualized using enhanced chemiluminescence (PerkinElmer).
Indirect immunofluorescence of IRF-3.
To examine IRF-3 nuclear translocation, NHDF cells were seeded on glass coverslips and incubated with either UV-inactivated KSHV (4 × 108 genomes/ml) or gpK8.1-S (5 μg/ml) for 6 h at 37°C in the presence of cycloheximide (100 μg/ml). Cells were fixed in 3% paraformaldehyde for 30 min, permeabilized in 0.1% Triton X-100 for 10 min, and blocked in 20% purified goat serum (Pierce) for 1 h. Cells were washed and incubated with IRF-3 antibody (1:50), followed by Alexa Fluor 594 goat anti-rabbit secondary antibody (Molecular Probes). Nuclei were counterstained with Hoechst dye, washed, and viewed under a fluorescence microscope.
RT-PCR analysis.
NHDF cells were serum starved for 18 h and then incubated with either UV-inactivated KSHV (6.7 × 108 genomes/ml) or gpK8.1-S (10 μg/ml), and total cellular RNA was harvested 6 h posttreatment with RNA-STAT60 (Tel-Test “B,” Inc., Friendswood, TX) as recommended by the manufacturer. Briefly, cells were lysed by the addition of phenol-guanidinium thiocyanate and chloroform extracted, and RNA was isopropanol precipitated. Reverse transcription-PCR (RT-PCR) was performed on 100 ng of recovered total cellular RNA using rTth DNA polymerase (Applied Biosystems). The primer pairs used were as follows: for glyceraldehyde-3-phosphate dehydrogenase (GADPH}, sense was 5′-GAGCCAAAAGGGTCATC and antisense was 5′-GTGGTCATGAGTCCTTC; for beta interferon, sense was 5′-CACTACAGCTCTTTCCATGA and antisense was 5′-AGGATTTCCACTCTGACTATGGTCC (35 cycles); for IFN-stimulated gene 56 (ISG56), sense was 5′-CATCAGGTCAAGGATAGTCTGGAGC and antisense was 5′-GGATTCAGGGTTTTCAGGGTCC (35 cycles); for IL-6, sense was 5′-TGTGTGAAAGCAGCAAAGAGGC and antisense was 5′-TTGGGTCAGGGGTGGTTATTG (35 cycles). 2′-5′ oligoadenylate synthetase (OAS) and ISG54 primer sets were described previously (5).
The specificity of gpK8.1-S effects was tested by preincubating gpK8.1-S (10 μg/ml) with soluble heparin (10 μg/ml, 25 μg/ml, 50 μg/ml, and 100 μg/ml; Sigma) or polymyxin B (50 μg/ml and 10 μg/ml; Sigma) for 30 min at 20°C. Poly(I:C) (50 μg/ml; Amersham) was incubated with or without heparin (100 μg/ml). NHDF cells were incubated with ligand-heparin mixes for 6 h, and RNA was harvested as described above.
Cytokine ELISAs.
NHDF cells were mock infected, treated with increasing concentrations of UV-inactivated KSHV (4.7 × 107 to 4.7 × 109 genomes/ml), or treated with increasing concentrations of soluble gpK8.1 (0.4 to 40 μg/ml) in 0.25 ml total volume. At 18 h postinfection, medium samples were collected and levels of IFN-β were determined by enzyme-linked immunosorbent assay (ELISA) (PBL Biomedical Laboratories, Piscataway, NJ) according to the manufacturer's instructions. IL-6 levels were determined by ELISA (BD Biosciences, San Diego, CA) according to the manufacturer's instructions.
Antiviral assay.
NHDF cells were treated with 100 units/ml recombinant IFN-α/β mixture (BioSource International), UV-inactivated KSHV (4.7 × 108, 4.7 × 107, and 4.7 × 106 genomes/ml), or soluble glycoprotein gpK8.1 for 6 h. The cells were washed twice in PBS and challenged with 100 PFU/well of vesicular stomatitis virus (VSV) for 1 h at 37°C. Cells were then washed and overlaid with a 60:40 mixture of 2× Eagle's minimal essential medium (BioWhittaker; Walkersville, MD) and 1% agarose. Plaques were visualized by crystal violet staining at 48 h posttreatment.
RESULTS
Construction and synthesis of soluble gpK8.1.
Glycoprotein gpK8.1 is one of at least eight virally encoded glycoproteins associated with KSHV virions (63). To examine the individual contribution of glycoprotein gpK8.1 to cellular signaling responses during infection, a truncated “soluble” version of gpK8.1 (gpK8.1-S), composed solely of the ectodomain (aa 1 to 196), was constructed by deleting the transmembrane and cytoplasmic domains (Fig. 1A). When expressed in 293T cells, gpK8.1-S was noticeably smaller than full-length gpK8.1, as detected by immunoblotting (Fig. 1B). The multiple bands observed for both forms are likely due to differential glycosylation, as gpK8.1 possesses both N-linked and O-linked carbohydrate chains (65), and the mobility of gpK8.1 on SDS-PAGE is sensitive to inhibitors of N-linked glycosylation (29). gpK8.1-S was expressed in insect cells by using recombinant baculovirus and purified by nickel affinity chromatography (Fig. 1C). This approach yielded an estimated 8 mg of protein per liter of culture, with an approximate purity of 80 to 90% as determined by Coomassie blue staining.
FIG. 1.
Expression of soluble gpK8.1 protein. (A) Full-length gpK8.1 is 228 aa long, including a signal sequence and a transmembrane domain. gpK8.1-S contains the N-terminal 196 aa of gpK8.1 and six-His at the C terminus. (B) 293T cells were transfected with pTriEx plasmid encoding either full-length gpK8.1 (lane 1) or soluble gpK8.1 lacking the transmembrane domain (lane 2). gpK8.1 was detected by Western blotting with an anti-gpK8.1 rabbit polyclonal antibody (a gift from J. Jung). (C) Fractions were collected during the purification of gpK8.1-S and analyzed by SDS-PAGE and Coomassie blue staining. Lane 1, supernatant; lane 2, column flowthrough; lane 3, imidazole wash; lane 4, elution. *, gpK8.1-S.
Glycoprotein gpK8.1 induces IFN-β and ISG expression.
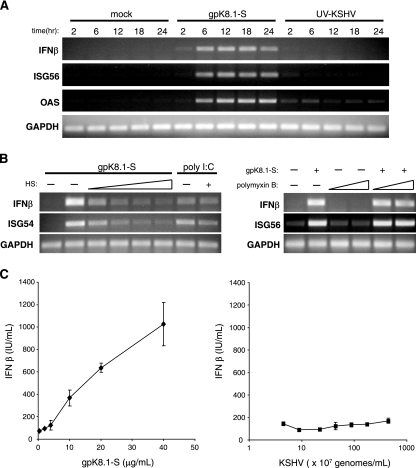
The regulation of ISG expression occurs at the level of transcription in response to either IFN-α/β signaling or IRF-3 activation, which serves as a regulator of both IFN-β and a subset of ISGs. The modulation of IFN-related gene expression has been reported both during KSHV lytic infection of endothelial cells (45) and during the early events of KSHV infection (41). To test whether soluble gpK8.1 affects the expression of antiviral genes, the accumulation of RNAs encoding IFN-β and two IFN-responsive genes (OAS and ISG56) was monitored by RT-PCR analysis. KSHV gene transcripts have been detected as early as 2 h postinfection of endothelial cells, indicating that viral gene expression occurs quickly after KSHV entry (25). To prevent any effects of de novo viral gene expression, KSHV virions were UV inactivated (UV-KSHV). It has been previously demonstrated that UV inactivation of KSHV particles does not reduce its ability to both bind and enter cells, and virions retain their abilities to activate signaling pathways (51, 61). In fibroblasts treated with gpK8.1-S, the accumulation of RNAs for all three antiviral genes was readily detected at 6 h and continued to 24 h posttreatment (Fig. 2A). However, UV-KSHV virions failed to induce detectable accumulation of IFN-β or ISG56 RNAs at any of the time points tested and the detected accumulation of OAS mRNA was significantly less than that observed for gpK8.1-S. Because the accumulation of RNA encoding IFN-β was observed to be robust at 6 h posttreatment with gpK8.1-S, subsequent experiments examined gpK8.1-mediated cellular effects at this time point.
FIG. 2.
KSHV induces ISG transcription via gpK8.1. (A) NHDF cells were mock treated or treated with gpK8.1-S (10 μg/ml) or UV-KSHV (6.7 × 108 genomes/ml). Total RNA was harvested at 2-h, 6-h, 12-h, 18-h, and 24-h time points, and RT-PCR analysis was performed with primers specific for ISG56, OAS, IFN-β, and GAPDH. PCR products were analyzed on a 1% agarose gel and visualized by ethidium bromide staining. (B) NHDF cells were mock treated or treated with gpK8.1-S (10 μg/ml) in the presence (+) of increasing amounts of soluble heparin sulfate (10 μg/ml, 25 μg/ml, 50 μg/ml, and 100 μg/ml) or polymyxin B (50 μg/ml and 10 μg/ml) or treated with poly(I:C) (50 μg/ml) in the presence or absence (−) of 100 μg/ml soluble heparin. Samples were harvested and analyzed as described above. (C) NHDF cells were mock infected and treated with increasing concentrations of UV-inactivated KSHV or soluble gpK8.1 as indicated. At 18 h posttreatment, the medium was analyzed for secreted IFN-β by ELISA. The results shown are representative of at least three independent RT-PCR experiments. Error bars indicate standard deviations.
gpK8.1 binds to cell surface heparan sulfate, and this interaction can be inhibited by soluble heparin (58). As a control for specificity, the effect of gpK8.1-S on the transcription of IFN-β and ISG54 in NHDF cells was examined in the presence of increasing amounts of soluble heparin sulfate. ISG54 is another antiviral gene whose transcription is regulated in a manner similar to that of ISG56. The ability of gpK8.1-S to induce RNA accumulation of these genes decreased with increasing heparin concentrations, indicating that blocking gpK8.1 attachment to the cell surface reduced its capacity to activate antiviral gene expression. The synthetic double-stranded RNA poly(I:C), which activates the expression of ISGs through interaction with Toll-like receptor 3, was unaffected by the addition of soluble heparin.
The endotoxin lipopolysaccharide (LPS) activates strong IFN-α/β responses, including ISG gene expression, through its interaction with Toll-like receptor 4. To exclude the possibility of endotoxin contamination as a contributor to ISG induction in response to our ligand, gpK8.1-S was treated with polymyxin B, a potent neutralizer of the biological effects of LPS, including the activation of IFN-α/β responses (Fig. 2B). The addition of polymyxin B had no effect upon the ability of gpK8.1-S to induce IFN-β or ISG56 RNA accumulation, indicating that gpK8.1-S and not LPS contamination is responsible for the observed antiviral response to gpK8.1-S.
To confirm that RNA accumulation of IFN-β correlated with its protein expression, the presence of IFN-β was measured by ELISA. At 18 h posttreatment, the concentration of IFN-β in the medium increased proportionally with the concentration of gpK8.1-S. However, UV-KSHV did not stimulate cells to produce detectable IFN-β at any of the concentrations tested (Fig. 2C). Taken together, these results indicate that although antiviral genes, including IFN-β, are induced by glycoprotein gpK8.1 and are readily detected at 6 h, the same response is not elicited by KSHV virions.
KSHV virions and gpK8.1 induce IL-6 expression.
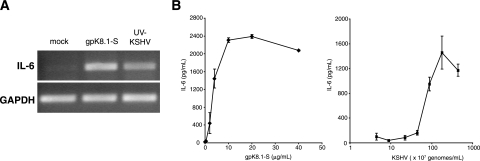
In addition to IFN-α/β, proinflammatory cytokines are another class of molecules that are often secreted in response to viral infection. Cellular IL-6 is a pleiotropic growth factor that is important in the pathogenesis of KSHV-mediated disorders, as evidenced by the elevated levels of IL-6 in patients with KS neoplasms and the dependence of PEL cells upon IL-6 for growth and survival (3, 16). The upregulation of IL-6 transcription has been observed during the early events of infection (41, 61), consistent with viral glycoproteins participating in its induction. In support of this hypothesis, RNA encoding IL-6 was increased in NHDF cells treated with either gpK8.1-S or UV-KSHV virions (Fig. 3A). The RT-PCR results were confirmed by the detection of cellular IL-6 in the medium at 18 h posttreatment in response to a range of concentrations of both gpK8.1-S and UV-KSHV (Fig. 3B). These data indicate that the regulation of the expression of IL-6 in response to UV-KSHV virions differs from the regulation of components of the IFN pathway, such as ISG56 and IFN-β.
FIG. 3.
(A) IL-6 is upregulated in response to KSHV and glycoprotein gpK8.1. NHDF cells were mock treated or treated with gpK8.1-S (10 μg/ml) or UV-KSHV (6.7 × 108 genomes/ml). After 6 h of incubation, total RNA was harvested and RT-PCR analysis was performed with primers specific for IL-6. (B) NHDF cells were mock infected and treated with increasing concentrations of UV-inactivated KSHV or soluble gpK8.1 as indicated. At 18 h posttreatment, the medium was analyzed for secreted IL-6 by ELISA. Error bars indicate standard deviations.
KSHV virions inhibit gpK8.1-mediated antiviral gene transcription.
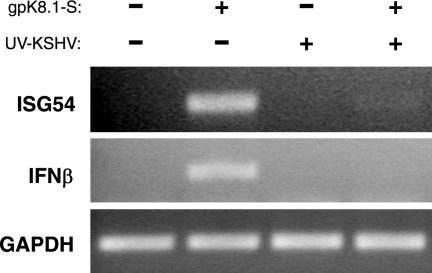
Cells respond to gpK8.1-S stimulation by inducing antiviral genes. However, UV-KSHV virions which possess glycoprotein gpK8.1 on the viral envelope do not induce a similar response. Thus KSHV virions may contain a factor that downregulates the IFN response. To test this possibility, we performed competition experiments to determine whether virions can inhibit gpK8.1-mediated ISG transcription. Fibroblasts treated simultaneously with UV-KSHV virions and gpK8.1-S had undetectable levels of ISG54 and IFN-β transcription, similar to what is seen in cells treated with UV-KSHV virions alone (Fig. 4).
FIG. 4.
KSHV inhibits gpK8.1-mediated ISG transcription. NHDF cells were mock treated, treated with gpK8.1-S (10 μg/ml), UV-KSHV (6.7 × 108 genomes/ml), or gpK8.1-S and KSHV together. After 6 h incubation, total RNA was harvested and RT-PCR analysis was performed with primers specific for ISG54, IFN-β, and GAPDH. −, absence of; +, presence of.
Glycoprotein gpK8.1 induces IRF-3 activation.
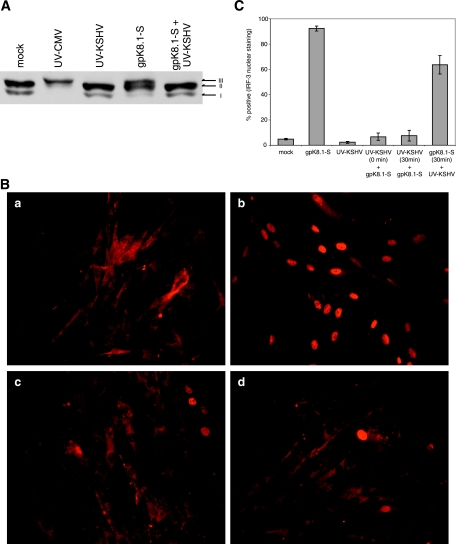
The promoters of IFN-β, ISG54, and ISG56 contain regulatory elements that are dependent upon IRF-3 for initial induction, and the activation of cytoplasmic IRF-3 is regulated by phosphorylation after virus infection. We examined the effects of either gpK8.1-S or UV-KSHV virions on the phosphorylation state of IRF-3 in human fibroblasts. Two hypophosphorylated forms of IRF-3 (forms I and II), which represent the transcriptionally inactive IRF-3 located predominantly in the cytoplasm, were detected in mock-treated cells (Fig. 5A). UV-inactivated HCMV, which is known to activate IRF-3, was included as a positive control (4, 46). Following treatment with gpK8.1-S, the more slowly migrating, phosphorylated form of IRF-3 was detected (designated form III). In contrast to gpK8.1-S, only the hypophosphorylated forms of IRF-3 were observed in cells treated with UV-KSHV virions. Consistent with the RT-PCR data, only the inactive hypophosphorylated forms of IRF-3 were observed in cells treated simultaneously with UV-KSHV and gpK8.1-S, indicating that KSHV virions downregulate the antiviral response at a point in the signaling pathway upstream of IRF-3 activation.
FIG. 5.
IRF-3 is activated in response to glycoprotein gpK8.1. (A) NHDF cells were mock treated, infected with UV-inactivated HCMV (multiplicity of infection of 1), or treated with gpK8.1-S (10 μg/ml) or UV-KSHV (6.7 × 108 genomes/ml) in the presence of cycloheximide (100 μg/ml). At 6 h posttreatment, whole-cell extracts were prepared and analyzed by immunoblotting with an anti-IRF-3 antibody. (B) IRF-3 translocates to the nucleus in response to glycoprotein gpK8.1. NHDF cells were (a) mock treated, (b) treated with gpK8.1-S (5 μg/ml), (c) infected with UV-KSHV (4 × 108 genomes/ml), or (d) treated with both gpK8.1 and KSHV in the presence of cycloheximide (100 μg/ml). At 6 h posttreatment, cells were fixed and cellular localization of IRF-3 was determined by indirect immunofluorescence. (C) NHDF cells were treated with gpK8.1-S or UV-KSHV as described above. Mixed samples were treated either by adding gpK8.1 and UV-KSHV simultaneously or by adding UV-KSHV for 30 min pre- or postaddition of gpK8.1 as indicated. IRF-3 was detected by indirect immunofluorescence, and nuclei were scored for IRF-3 staining in multiple fields of view (n > 100 nuclei) for each sample. Error bars indicate standard deviations.
Following C-terminal phosphorylation, IRF-3 dimerizes and rapidly translocates into the nucleus (31). Consistent with the phosphorylation results, gpK8.1-S induced nuclear localization of IRF-3, whereas IRF-3 remained in the cytoplasm of both cells treated with UV-KSHV virions or treated with gpK8.1-S and UV-KSHV virions simultaneously (Fig. 5B). To quantify the effects of KSHV on gpK8.1-mediated nuclear localization, fluorescently stained cells were scored for nuclear IRF-3. To determine the kinetics of their competition, UV-KSHV virions were added to cells either simultaneously or 30 min pre- or postaddition of gpK8.1-S (Fig. 5C). UV-KSHV virions inhibited IRF-3 nuclear localization almost completely when added prior to or simultaneously with gpK8.1-S. Interestingly, UV-KSHV virions had a slight inhibitory effect on cells treated with gpK8.1-S prior to KSHV treatment. Taken together, these results indicate that although gpK8.1 induces the activation of IRF-3, this activation event is suppressed when cells are exposed to gpK8.1 in the context of the virion, suggesting that KSHV possesses the ability to control the IFN-α/β response during infection.
KSHV abrogates the gpK8.1-mediated antiviral response.
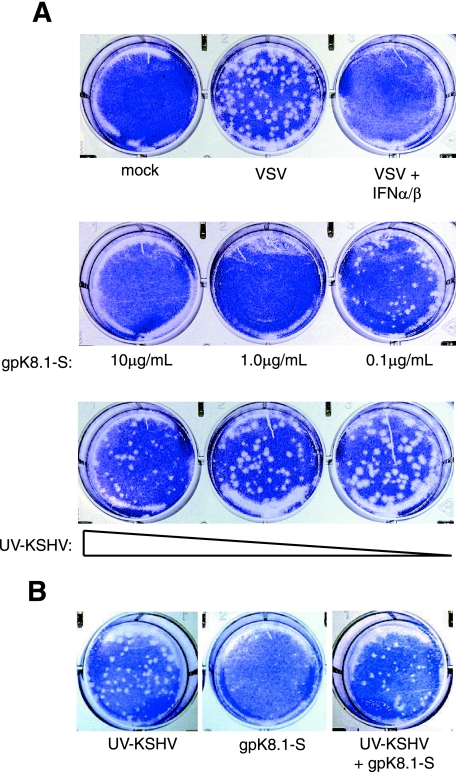
The consequence of IFN-α/β and ISG expression is the creation of an environment hostile to viruses (21). VSV is a negative-strand RNA virus whose growth is sensitive to the effects of the IFN pathway and can be used to assess the antiviral status of the cell. Cells treated with soluble IFN-α/β for 6 h were resistant to VSV infection (Fig. 6A). Similarly, cells treated with gpK8.1-S were refractory to VSV replication. Only at the lowest concentration tested (0.1 μg/ml) were VSV plaques observed, and these plaques were smaller and fewer in number. UV-KSHV virions failed to elicit an antiviral response in fibroblasts as predicted by the inability of KSHV to activate detectable expression of IFN-β or ISGs (Fig. 6A).
FIG. 6.
Cells establish an antiviral state in response to gpK8.1. (A) NHDF cells were treated with gpK8.1 at the concentrations indicated and UV-KSHV (4.7 × 108, 4.7 × 107, 4.7 × 106 genomes/ml) or IFN-α/β (100 U/ml; Biosource). After 6 h of incubation, the cells were washed and challenged with VSV (100 PFU/well) for 1 h. At 36 h post-VSV challenge, plaques were visualized with crystal violet. The mock-infected well was not challenged with VSV. (B) NHDF cells were mock treated, treated with gpK8.1-S (5 μg/ml), or treated with both UV-KSHV (4.7 × 108 genomes/ml) and gpK8.1-S and challenged with VSV as described above.
To examine the effect of KSHV virions on the soluble gpK8.1-mediated antiviral response, competition experiments were performed. To allow the delivery of KSHV structural components, such as tegument proteins, fibroblasts were treated with UV-KSHV virions for 30 min prior to the addition of gpK8.1-S. In contrast to cells treated with gpK8.1-S alone, cells pretreated with UV-KSHV virions allowed the replication of VSV in spite of subsequent treatment with gpK8.1-S (Fig. 6B). These results indicate that the cellular response to glycoprotein gpK8.1 is the initiation of a functional antiviral state, but exposure to gpK8.1 in the context of KSHV virions does not generate this response. These data suggest that KSHV virions contain a factor which inhibits the gpK8.1-induced antiviral response.
DISCUSSION
We have found that KSHV envelope glycoprotein gpK8.1 induces the expression of IFN-β, while the virus itself does not. Based on our data, we propose that gpK8.1 initiates the cellular IFN pathway via its interactions with cell-surface components. In vitro transcriptional profiling studies demonstrate that gene products involved in the antiviral response (ISG54, ISG56, and OAS) are upregulated when latently infected microvascular endothelial cells are induced into lytic gene expression (45). Soluble gpK8.1 triggers the transcription of some of the same ISGs reported in these studies. In cells undergoing lytic replication, KSHV expresses vIRFs which act as negative regulators of cellular IRF activity (20, 30, 32). It is therefore likely that the ISG expression measured in the microarray study resulted from adjacent cells exposed to either newly produced virions or cell-surface glycoprotein gpK8.1. More recently, microarray analysis performed during the first 4 h of infection in vitro revealed the upregulation of a similar subset of antiviral genes in both fibroblasts and endothelial cells (41). Our data suggest that gpK8.1 activates signal transduction pathways during virus binding or entry that contribute to this cellular antiviral response.
Activation of the IFN pathway leads to the stimulation of antiviral genes that have profound consequences for viral replication. IFN-α inhibits KSHV reactivation in both PEL cell lines and peripheral blood mononuclear cells isolated from KS patients (9, 35). Cells treated with gpK8.1-S activate the IFN-α/β pathway, as measured by the expression of both IFN-β and several ISGs. The expression of these genes results in a functional antiviral state since fibroblasts treated with gpK8.1-S are refractory to VSV replication. In addition, medium transferred from cells treated with gpK8.1-S for 6 h was unable to inhibit VSV replication (data not shown), indicating that although IFN-β is detected in the medium at 18 h posttreatment (Fig. 2C), it is not responsible for the antiviral effect observed at 6 h. Together, these data suggest that the functional antiviral state observed in these experiments is primarily generated by antiviral genes induced by gpK8.1.
KSHV virions failed to elicit an IFN response in fibroblasts, a distinction from other human herpesviruses, specifically HCMV and HSV-1, which induce and sustain high levels of IFN-α/β and ISGs in fibroblasts when viral gene expression is inhibited (4, 38, 42, 46). This result was intriguing because gpK8.1 is a component of the viral envelope, and our results suggest that gpK8.1 alone is sufficient to generate a cellular IFN response. Furthermore, when cells are treated simultaneously with UV-KSHV and soluble gpK8.1, virions suppress gpK8.1-mediated IRF-3 activation and ISG induction and dramatically inhibit the antiviral response. There are several possible explanations for these observations, but we favor a scenario where KSHV virions contain one or more structural components that inhibit the antiviral response. The ability of KSHV to suppress an IFN response is similar to what has been observed with RhCMV, which also fails to induce antiviral genes in host cells in either the presence or the absence of viral gene expression (15). Additionally, coinfection experiments with RhCMV and HCMV in rhesus fibroblasts demonstrated that RhCMV infection inhibits HCMV-mediated activation IRF-3, suggesting that RhCMV particles are not simply undetected by host cells, but contain structural components that potently suppress the host antiviral response. Similarly, the ability of KSHV virions to limit the antiviral response in the presence of exogenously added gpK8.1 may account for the observation that gpK8.1 present in virions does not elicit a robust response. An alternative explanation is that virion-mediated inhibition is occurring through transcriptional repression since KSHV infection rapidly activates several signaling pathways (51) and could potentially inhibit the expression of antiviral genes through some negative transcriptional regulation. However, we observed that both IRF-3 phosphorylation and nuclear localization is inhibited by KSHV, indicating that the block occurs upstream of IRF-3 activation rather than at the level of transcription. Another possibility is that KSHV virions fail to elicit an antiviral response altogether, and the suppression of the soluble gpK8.1-mediated antiviral response is due to competition for binding sites between viral envelope gpK8.1 and soluble gpK8.1-S. Testing this possibility experimentally is complicated by the fact that both K8.1 and KSHV virions bind cell surface heparan sulfate. In addition, the inability of KSHV to elicit an IFN response seems unlikely given work by Collins et al. (12) that suggests that most enveloped viruses activate IRF-3-mediated antiviral responses during the entry process, unless they possess inhibitors to disarm these responses. Therefore, our hypothesis that KSHV utilizes structural components to disarm the IFN pathway is consistent with other reports in the literature.
The mechanism by which KSHV virions downregulate the IFN-α/β response is unknown. Our data support a model where one or more virion components delivered to the cell during infection disrupt the activation of the IFN-α/β pathway upstream of IRF-3 activation. During KSHV entry, tegument and capsid proteins are delivered to the cytoplasm after fusion of the viral envelope with the cellular membrane. IRF-3 and its activating kinases are also localized in the cytoplasm, and this overlap may provide the opportunity for viral proteins to interact with cellular signaling components. Recent structural analysis of KSHV virions by mass spectrometry has identified viral proteins packaged into virus particles, including envelope, tegument, and capsid proteins (63). Although several of the virion components are uncharacterized, ORF45 has been identified as a tegument protein that is antagonistic to IFN responses. When overexpressed in 293T cells, ORF45 binds IRF-7, inhibits its phosphorylation, and blocks the activation of both IFN-α/β promoters in response to Sendai virus infection (64). However, the ORF45-IRF-7 interaction may not fully explain our observation that KSHV virions mediate IFN pathway inhibition. Our data indicate that IRF-3 activation is inhibited by virions, and the inhibition or IRF-3 phosphorylation correlated with both a decrease in the accumulation of RNA encoding IFN-β and increased VSV viral replication in fibroblasts, suggesting that IRF-3 plays a key role in these responses. Furthermore, transcription levels of ORF45 remain undetectable early after primary infection of both fibroblasts and endothelial cells (25), while cellular IRF-7 transcription is strongly upregulated by 2 h (41), suggesting that the amount of IRF-7 may quickly surpass that of virion-delivered ORF45. This raises the possibility that the virion contains components in addition to ORF45 that are capable of inhibiting antiviral responses.
The upregulation of IRF-7 following infection appears contradictory to KSHV inhibiting the antiviral response. However, recent work has suggested that nascent IRF-7 may prevent Rta-mediated induction of viral genes, thus negatively influencing viral entry into the lytic cycle early during infection (59). This commandeering of IRF-7 activity, combined with blocking activation of IRF-3, suggests that KSHV does not simply block the IFN response but selectively modulates the function of its components to regulate its own replication cycle.
In this study, not all of the ISGs examined were modulated in the same manner. Similar to ISG54, OAS contains an IFN response element in its promoter and is considered a typical IFN-inducible antiviral gene (40). Although KSHV virions downregulated gpK8.1-mediated induction of ISG54 and IFN-β, OAS was upregulated in response to both soluble gpK8.1 and KSHV virions. The detection of OAS transcripts corresponds to a previous report where OAS, but not ISG54, was detected following KSHV infection in fibroblasts and endothelial cells (41). This variation in antiviral gene induction may reflect the differences in promoter regulation between the ISGs. It is interesting to note that the expression of OAS is not upregulated in response to constitutively active IRF-3 (22) and requires the activation of the IFN-α/β receptor (IFNAR1) (40), yet we did not detect IFN-β in the medium of KSHV-treated cells.
Cellular IL-6 is a pleiotropic cytokine that has a critical role in KSHV pathogenesis and functions as an autocrine growth factor for both KS and PEL cells (19, 33). The upregulation of IL-6 in response to primary infection of KSHV in vitro has been detected as early as 2 h by microarray analysis in human fibroblasts (41). We observed that IL-6 is upregulated in response to both gpK8.1-S and transcriptionally inert KSHV, suggesting that this response is initially mediated by virus-cell interactions and gpK8.1 is a contributor to this response. Several viral gene products expressed immediately after infection (vFLIP, latency-associated nuclear antigen, Rta, and vIL-6) also upregulate IL-6 expression, suggesting that KSHV employs multiple and possibly redundant mechanisms to ensure that IL-6 is rapidly expressed. The different expression patterns between IL-6 and IFN-β genes in response to KSHV virions reflect the intrinsic ability of KSHV to selectively regulate cellular genes that have either beneficial or deleterious effects upon KSHV infection.
Our findings help illustrate the complexity of KSHV interactions with host cells. Throughout its life cycle, the virus encodes immunomodulatory molecules to alter host immune responses. Here we demonstrate that, before viral gene expression is established, virion structural components can modulate cellular innate defenses, presumably to the advantage of the virus. Although IFN-α/β is detrimental to KSHV, the modulation of the IFN response may serve to influence the expression of other factors that can contribute to inflammation, a key component of KSHV-associated pathology. Ultimately, KSHV appears capable of balancing the activation and repression of host cell innate defenses to promote its own survival.
Acknowledgments
We thank J. Jung for the gpK8.1 expression plasmid pFJ-35/37 and anti-gpK8.1 antibody and J. Vieira for GFP-modified BCBL-1 (T8.1C) cells. BCBL-1 cells were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH (from Michael McGrath and Don Ganem).
This work was supported by NIH grant CA022443. S.T.P. was supported by NIH training grant T32 CA009135-29.
Footnotes
Published ahead of print on 6 September 2006.
REFERENCES
- 1.Ankel, H., M. R. Capobianchi, C. Castilletti, and F. Dianzani. 1994. Interferon induction by HIV glycoprotein 120: role of the V3 loop. Virology 205:34-43. [DOI] [PubMed] [Google Scholar]
- 2.Ankel, H., D. F. Westra, S. Welling-Wester, and P. Lebon. 1998. Induction of interferon-alpha by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology 251:317-326. [DOI] [PubMed] [Google Scholar]
- 3.Asou, H., J. W. Said, R. Yang, R. Munker, D. J. Park, N. Kamada, and H. P. Koeffler. 1998. Mechanisms of growth control of Kaposi's sarcoma-associated herpes virus-associated primary effusion lymphoma cells. Blood 91:2475-2481. [PubMed] [Google Scholar]
- 4.Boehme, K. W., J. Singh, S. T. Perry, and T. Compton. 2004. Human cytomegalovirus elicits a coordinated cellular antiviral response via envelope glycoprotein B. J. Virol. 78:1202-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capobianchi, M. R., H. Ankel, F. Ameglio, R. Paganelli, P. M. Pizzoli, and F. Dianzani. 1992. Recombinant glycoprotein 120 of human immunodeficiency virus is a potent interferon inducer. AIDS Res. Hum. Retrovir. 8:575-579. [DOI] [PubMed] [Google Scholar]
- 8.Chandran, B., C. Bloomer, S. R. Chan, L. Zhu, E. Goldstein, and R. Horvat. 1998. Human herpesvirus-8 ORF K8.1 gene encodes immunogenic glycoproteins generated by spliced transcripts. Virology 249:140-149. [DOI] [PubMed] [Google Scholar]
- 9.Chang, J., R. Renne, D. Dittmer, and D. Ganem. 2000. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology 266:17-25. [DOI] [PubMed] [Google Scholar]
- 10.Chin, K. C., and P. Cresswell. 2001. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomegalovirus. Proc. Natl. Acad. Sci. USA 98:15125-15130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Choi, J., R. E. Means, B. Damania, and J. U. Jung. 2001. Molecular piracy of Kaposi's sarcoma associated herpesvirus. Cytokine Growth Factor Rev. 12:245-257. [DOI] [PubMed] [Google Scholar]
- 12.Collins, S. E., R. S. Noyce, and K. L. Mossman. 2004. Innate cellular response to virus particle entry requires IRF3 but not virus replication. J. Virol. 78:1706-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Compton, T. 1993. An immortalized human fibroblast cell line is permissive for human cytomegalovirus infection. J. Virol. 67:3644-3648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Compton, T., E. A. Kurt-Jones, K. W. Boehme, J. Belko, E. Latz, D. T. Golenbock, and R. W. Finberg. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588-4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeFilippis, V., and K. Fruh. 2005. Rhesus cytomegalovirus particles prevent activation of interferon regulatory factor 3. J. Virol. 79:6419-6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensoli, B., C. Sgadari, G. Barillari, M. C. Sirianni, M. Sturzl, and P. Monini. 2001. Biology of Kaposi's sarcoma. Eur. J. Cancer 37:1251-1269. [DOI] [PubMed] [Google Scholar]
- 17.Ensoli, B., and M. Sturzl. 1998. Kaposi's sarcoma: a result of the interplay among inflammatory cytokines, angiogenic factors and viral agents. Cytokine Growth Factor Rev. 9:63-83. [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald, K. A., S. M. McWhirter, K. L. Faia, D. C. Rowe, E. Latz, D. T. Golenbock, A. J. Coyle, S. M. Liao, and T. Maniatis. 2003. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 4:491-496. [DOI] [PubMed] [Google Scholar]
- 19.Foussat, A., J. Wijdenes, L. Bouchet, G. Gaidano, F. Neipel, K. Balabanian, P. Galanaud, J. Couderc, and D. Emilie. 1999. Human interleukin-6 is in vivo an autocrine growth factor for human herpesvirus-8-infected malignant B lymphocytes. Eur. Cytokine Netw. 10:501-508. [PubMed] [Google Scholar]
- 20.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 21.Goodbourn, S., L. Didcock, and R. E. Randall. 2000. Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. J. Gen. Virol. 81:2341-2364. [DOI] [PubMed] [Google Scholar]
- 22.Grandvaux, N., M. J. Servant, B. tenOever, G. C. Sen, S. Balachandran, G. N. Barber, R. Lin, and J. Hiscott. 2002. Transcriptional profiling of interferon regulatory factor 3 target genes: direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76:5532-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda, K., H. Yanai, H. Negishi, M. Asagiri, M. Sato, T. Mizutani, N. Shimada, Y. Ohba, A. Takaoka, N. Yoshida, and T. Taniguchi. 2005. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434:772-777. [DOI] [PubMed] [Google Scholar]
- 24.Honda, K., H. Yanai, A. Takaoka, and T. Taniguchi. 2005. Regulation of the type I IFN induction: a current view. Int. Immunol. 17:1367-1378. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan, H. H., P. P. Naranatt, M. S. Smith, L. Zeng, C. Bloomer, and B. Chandran. 2004. Concurrent expression of latent and a limited number of lytic genes with immune modulation and antiapoptotic function by Kaposi's sarcoma-associated herpesvirus early during infection of primary endothelial and fibroblast cells and subsequent decline of lytic gene expression. J. Virol. 78:3601-3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laude, H., J. Gelfi, L. Lavenant, and B. Charley. 1992. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J. Virol. 66:743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 28.Leger-Ravet, M. B., M. Peuchmaur, O. Devergne, J. Audouin, M. Raphael, J. Van Damme, P. Galanaud, J. Diebold, and D. Emilie. 1991. Interleukin-6 gene expression in Castleman's disease. Blood 78:2923-2930. [PubMed] [Google Scholar]
- 29.Li, M., J. MacKey, S. C. Czajak, R. C. Desrosiers, A. A. Lackner, and J. U. Jung. 1999. Identification and characterization of Kaposi's sarcoma-associated herpesvirus K8.1 virion glycoprotein. J. Virol. 73:1341-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, R., P. Genin, Y. Mamane, M. Sgarbanti, A. Battistini, W. J. Harrington, Jr., G. N. Barber, and J. Hiscott. 2001. HHV-8 encoded vIRF-1 represses the interferon antiviral response by blocking IRF-3 recruitment of the CBP/p300 coactivators. Oncogene 20:800-811. [DOI] [PubMed] [Google Scholar]
- 31.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miles, S. A., A. R. Rezai, J. F. Salazar-Gonzalez, M. Vander Meyden, R. H. Stevens, D. M. Logan, R. T. Mitsuyasu, T. Taga, T. Hirano, T. Kishimoto, et al. 1990. AIDS Kaposi sarcoma-derived cells produce and respond to interleukin 6. Proc. Natl. Acad. Sci. USA 87:4068-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mittnacht, S., P. Straub, H. Kirchner, and H. Jacobsen. 1988. Interferon treatment inhibits onset of herpes simplex virus immediate-early transcription. Virology 164:201-210. [DOI] [PubMed] [Google Scholar]
- 35.Monini, P., F. Carlini, M. Sturzl, P. Rimessi, F. Superti, M. Franco, G. Melucci-Vigo, A. Cafaro, D. Goletti, C. Sgadari, S. Butto, P. Leone, C. Chiozzini, C. Barresi, A. Tinari, A. Bonaccorsi, M. R. Capobianchi, M. Giuliani, A. di Carlo, M. Andreoni, G. Rezza, and B. Ensoli. 1999. Alpha interferon inhibits human herpesvirus 8 (HHV-8) reactivation in primary effusion lymphoma cells and reduces HHV-8 load in cultured peripheral blood mononuclear cells. J. Virol. 73:4029-4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monini, P., S. Colombini, M. Sturzl, D. Goletti, A. Cafaro, C. Sgadari, S. Butto, M. Franco, P. Leone, S. Fais, G. Melucci-Vigo, C. Chiozzini, F. Carlini, G. Ascherl, E. Cornali, C. Zietz, E. Ramazzotti, F. Ensoli, M. Andreoni, P. Pezzotti, G. Rezza, R. Yarchoan, R. C. Gallo, and B. Ensoli. 1999. Reactivation and persistence of human herpesvirus-8 infection in B cells and monocytes by Th-1 cytokines increased in Kaposi's sarcoma. Blood 93:4044-4058. [PubMed] [Google Scholar]
- 37.Moore, P. S., and Y. Chang. 2001. Molecular virology of Kaposi's sarcoma-associated herpesvirus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 356:499-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mossman, K. L., P. F. Macgregor, J. J. Rozmus, A. B. Goryachev, A. M. Edwards, and J. R. Smiley. 2001. Herpes simplex virus triggers and then disarms a host antiviral response. J. Virol. 75:750-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mossman, K. L., H. A. Saffran, and J. R. Smiley. 2000. Herpes simplex virus ICP0 mutants are hypersensitive to interferon. J. Virol. 74:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakaya, T., M. Sato, N. Hata, M. Asagiri, H. Suemori, S. Noguchi, N. Tanaka, and T. Taniguchi. 2001. Gene induction pathways mediated by distinct IRFs during viral infection. Biochem. Biophys. Res. Commun. 283:1150-1156. [DOI] [PubMed] [Google Scholar]
- 41.Naranatt, P. P., H. H. Krishnan, S. R. Svojanovsky, C. Bloomer, S. Mathur, and B. Chandran. 2004. Host gene induction and transcriptional reprogramming in Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8)-infected endothelial, fibroblast, and B cells: insights into modulation events early during infection. Cancer Res. 64:72-84. [DOI] [PubMed] [Google Scholar]
- 42.Navarro, L., K. Mowen, S. Rodems, B. Weaver, N. Reich, D. Spector, and M. David. 1998. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol. Cell. Biol. 18:3796-3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nemerow, G. R., R. A. Houghten, M. D. Moore, and N. R. Cooper. 1989. Identification of an epitope in the major envelope protein of Epstein-Barr virus that mediates viral binding to the B lymphocyte EBV receptor (CR2). Cell 56:369-377. [DOI] [PubMed] [Google Scholar]
- 44.Oberman, F., and A. Panet. 1988. Inhibition of transcription of herpes simplex virus immediate early genes in interferon-treated human cells. J. Gen. Virol. 69:1167-1177. [DOI] [PubMed] [Google Scholar]
- 45.Poole, L. J., Y. Yu, P. S. Kim, Q. Z. Zheng, J. Pevsner, and G. S. Hayward. 2002. Altered patterns of cellular gene expression in dermal microvascular endothelial cells infected with Kaposi's sarcoma-associated herpesvirus. J. Virol. 76:3395-3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Preston, C. M., A. N. Harman, and M. J. Nicholl. 2001. Activation of interferon response factor-3 in human cells infected with herpes simplex virus type 1 or human cytomegalovirus. J. Virol. 75:8909-8916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raab, M. S., J. C. Albrecht, A. Birkmann, S. Yaguboglu, D. Lang, B. Fleckenstein, and F. Neipel. 1998. The immunogenic glycoprotein gp35-37 of human herpesvirus 8 is encoded by open reading frame K8.1. J. Virol. 72:6725-6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sainz, B., Jr., H. L. Lamarca, R. F. Garry, and C. A. Morris. 2005. Synergistic inhibition of human cytomegalovirus replication by interferon-alpha/beta and interferon-gamma. Virol. J. 2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samuel, C. E. 2001. Antiviral actions of interferons. Clin. Microbiol. Rev. 14:778-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma, S., B. R. tenOever, N. Grandvaux, G. P. Zhou, R. Lin, and J. Hiscott. 2003. Triggering the interferon antiviral response through an IKK-related pathway. Science 300:1148-1151. [DOI] [PubMed] [Google Scholar]
- 51.Sharma-Walia, N., H. H. Krishnan, P. P. Naranatt, L. Zeng, M. S. Smith, and B. Chandran. 2005. ERK1/2 and MEK1/2 induced by Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) early during infection of target cells are essential for expression of viral genes and for establishment of infection. J. Virol. 79:10308-10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharma-Walia, N., P. P. Naranatt, H. H. Krishnan, L. Zeng, and B. Chandran. 2004. Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 envelope glycoprotein gB induces the integrin-dependent focal adhesion kinase-Src-phosphatidylinositol 3-kinase-rho GTPase signal pathways and cytoskeletal rearrangements. J. Virol. 78:4207-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stamey, F. R., M. M. Patel, B. P. Holloway, and P. E. Pellett. 2001. Quantitative, fluorogenic probe PCR assay for detection of human herpesvirus 8 DNA in clinical specimens. J. Clin. Microbiol. 39:3537-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stinski, M. F., D. R. Thomsen, and J. E. Rodriguez. 1982. Synthesis of human cytomegalovirus-specified RNA and protein in interferon-treated cells at early times after infection. J. Gen. Virol. 60:261-270. [DOI] [PubMed] [Google Scholar]
- 56.Taylor, R. T., and W. A. Bresnahan. 2005. Human cytomegalovirus immediate-early 2 gene expression blocks virus-induced beta interferon production. J. Virol. 79:3873-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vieira, J., P. O'Hearn, L. Kimball, B. Chandran, and L. Corey. 2001. Activation of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) lytic replication by human cytomegalovirus. J. Virol. 75:1378-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang, F. Z., S. M. Akula, N. P. Pramod, L. Zeng, and B. Chandran. 2001. Human herpesvirus 8 envelope glycoprotein K8.1A interaction with the target cells involves heparan sulfate. J. Virol. 75:7517-7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, J., J. Zhang, L. Zhang, W. Harrington, Jr., J. T. West, and C. Wood. 2005. Modulation of human herpesvirus 8/Kaposi's sarcoma-associated herpesvirus replication and transcription activator transactivation by interferon regulatory factor 7. J. Virol. 79:2420-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Weaver, B. K., K. P. Kumar, and N. C. Reich. 1998. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell. Biol. 18:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie, J., H. Pan, S. Yoo, and S. J. Gao. 2005. Kaposi's sarcoma-associated herpesvirus induction of AP-1 and interleukin 6 during primary infection mediated by multiple mitogen-activated protein kinase pathways. J. Virol. 79:15027-15037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoneyama, M., W. Suhara, and T. Fujita. 2002. Control of IRF-3 activation by phosphorylation. J. Interferon Cytokine Res. 22:73-76. [DOI] [PubMed] [Google Scholar]
- 63.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79:800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu, F. X., S. M. King, E. J. Smith, D. E. Levy, and Y. Yuan. 2002. A Kaposi's sarcoma-associated herpesviral protein inhibits virus-mediated induction of type I interferon by blocking IRF-7 phosphorylation and nuclear accumulation. Proc. Natl. Acad. Sci. USA 99:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhu, L., V. Puri, and B. Chandran. 1999. Characterization of human herpesvirus-8 K8.1A/B glycoproteins by monoclonal antibodies. Virology 262:237-249. [DOI] [PubMed] [Google Scholar]