Abstract
In contrast to humans, adult but not infant small animals are resistant to rotavirus diarrhea. The pathophysiological mechanism behind this age-restricted diarrhea is currently unresolved, and this question was investigated by studying the secretory state of the small intestines of adult mice infected with rotavirus. Immunohistochemistry and histological examinations revealed that rotavirus (strain EDIM) infects all parts of the small intestines of adult mice, with significant numbers of infected cells in the ilea at 2 and 4 days postinfection. Furthermore, quantitative PCR revealed that 100-fold more viral RNA was produced in the ilea than in the jejuna or duodena of adult mice. In vitro perfusion experiments of the small intestine did not reveal any significant changes in net fluid secretion among mice infected for 3 days or 4 days or in those that were noninfected (37 ± 9 μl · h−1 · cm−1, 22 ± 13 μl · h−1 · cm−1, and 33 ± 6 μl · h−1 · cm−1, respectively) or in transmucosal potential difference (4.0 ± 0.3 mV versus 3.9 ± 0.4 mV), a marker for active chloride secretion, between control and rotavirus-infected mice. In vivo experiments also did not show any differences in potential difference between uninfected and infected small intestines. Furthermore, no significant differences in weight between infected and uninfected small intestines were found, nor were any differences in fecal output observed between infected and control mice. Altogether, these data suggest that rotavirus infection is not sufficient to stimulate chloride and water secretion from the small intestines of adult mice.
Rotavirus (RV) is an important cause of acute gastroenteritis in young children and is also an important pathogen in several animal species. In contrast to the situation in humans, where symptomatic reinfections do occur in adults (1, 23, 30), most adult small animals show an age-dependent resistance to symptomatic RV infections (4, 5, 12, 38). The pathophysiological mechanisms behind this age-dependent resistance are currently unresolved, but it has been proposed that they are due to compensatory fluid absorption by the larger colons of older animals. It has also been suggested that the low expression of proteolytic enzymes in newborn mice is a possible mechanism (13). Furthermore, an interesting observation is that the age-dependent resistance in adult mice correlates with the lack of chemokine responses (28). We have previously shown that in infant mice, rotavirus infection gives rise to an increased fluid secretion and an increased transmucosal potential difference (PD), a marker for active chloride secretion (16). The aim of this study was to investigate the secretory state of the small intestines of adult mice. Using established (16, 32, 33) in vivo and in vitro methods, we measured net fluid transport and PD and found that in spite of virus infection, there was no evidence for chloride or water secretion from the small intestines of murine rotavirus-infected adult mice. This suggests that rotavirus infection in adult mice, in contrast to newborn mice (16), is not sufficient to stimulate water secretion from the small intestine.
MATERIALS AND METHODS
Rotavirus infection of adult mice.
Rotavirus-naïve adult BALB/c mice (B & K Laboratories, Sollentuna, Sweden) were kept under standardized environmental conditions. Adult mice 6 weeks of age were orally inoculated with (40 50% shedding doses) wild-type murine (strain EDIM) rotavirus, kindly provided by Marie Rippenhoff-Talty, Buffalo, NY. Before the experiment, the animals were fasted overnight, with free access to water. At the end of the experiment, they were killed under full anesthesia by cutting of the heart. The protocol was approved by the ethical committee in Stockholm, Sweden (registration no. N38/01).
Enzyme-linked immunosorbent assay.
In this study, the primary determinant of infection was the analysis of rotavirus shedding in fecal output as described previously (11, 37 ). Stool samples were collected from each animal from day 0 to day 9 and stored at −70°C. Two fecal pellets collected from each individual mouse were mixed with cold phosphate-buffered saline containing 0.1% Tween 20 and 0.5% bovine serum albumin (BSA) and then centrifuged to remove fecal solids. An enzyme-linked immunosorbent assay was used to detect rotavirus in stool samples as previously described (10).
Histopathology.
Specimens (2 cm) were taken from the duodenum, the middle of the jejunum, and the ileum 5 cm proximal to the ileocecal junction 3 days postinfection (p.i.). Each specimen was placed on a micropore filter (pore size, 0.2 μm; Schleicher & Schuell, Dassel, Germany) and cut open along its longitudinal axis to obtain access to crypts and villi. The specimens were fixed for 3 h in Carnoy's solution (60% methanol, 30% chloroform, 10% acetic acid) and for 20 h in 70% ethanol and then embedded in paraffin. From each specimen, six 3-μm-thick sections were cut at 100-μm intervals and stained with hematoxylin and eosin (2).
Immunohistochemistry.
For immunohistochemistry, paraffin-embedded specimens were cut into thin sections. Briefly, tissues were hydrated for 5 min in xylene (twice) (Histolab, Sweden), followed by 100% ethanol (twice), 95% ethanol (once), and 70% ethanol (once) and finally in washing buffer (0.5 M Tris-HCl-0.9% NaCl, pH 7.6). Slides were then incubated for 10 min in washing buffer containing 0.3% H2O2 to remove endogenous peroxidase, followed by three washes with washing buffer. Each specimen was then incubated in 1% BSA (Sigma-Aldrich)-washing buffer for 30 min at room temperature in a humid chamber. A rabbit polyclonal rotavirus antiserum (K224) diluted 1:100 in 1% BSA-washing buffer (50 μl/sample) was then added to each specimen for 1 h at 37°C in a humid chamber. The slides were washed again, 50 μl of horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (Dako Cytomation, Denmark) diluted 1/1,000 in 1% BSA-washing buffer was added, and the slides were incubated at 37°C for 1 h in a humid chamber. After the slides were washed three times, diaminobenzidine staining substrate (1 ml diaminobenzidine solution plus 50 ml washing buffer plus 10 μl H2O2 [30%]) (Saveen Werner AB, Stockholm, Sweden) was added for 3 to 5 min, followed by three washes in distilled water. Finally, the slides were stained with hematoxylin.
Measurement of fluid secretion.
Fluid production by the intestine was measured by four different methods: (i) in vitro perfusion experiments; (ii) in vivo PD measurement; (iii) weight measurement; and (iv) fecal output.
(i) In vitro perfusion experiments.
The in vitro perfusion method has been described elsewhere. Briefly, noninfected mice and mice at 3 and 4 days postinfection were anesthetized with tribromethanol, at 125 mg/kg of body weight, intraperitoneally. The abdomen of each mouse was opened, and a 3- to 5-cm ileal segment was flushed with isotonic saline and then transferred to a perfusion chamber containing a modified Krebs-Henseleit solution that was continuously oxygenated with 5% CO2 in oxygen at 37°C. The intestinal segment was provided with plastic tubing at both ends and perfused at a constant rate (3 ml/h). The perfusion solution consisted of the modified Krebs-Henseleit solution containing mannitol instead of glucose (122 mM NaCl, 25 mM NaHCO3, 3.5 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgCl2, 2 mM CaCl2, 30 mM mannitol) and a radioactively labeled nonabsorbable marker, [14C]polyethylene glycol 4000 (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom), at a concentration of 0.07 μCi/ml. The tracer and the plastic tubing were immersed in a solution containing [14C]polyethylene glycol 4000 (2 g/l) for 24 h before use to prevent any absorbance of the tracer into the plastic tubing. After a calibration time of 30 min, samples were collected every 30 min at the outflow site of the perfusion system for 1.5 h. The radioactivity of triplicate samples was measured from the inflow and outflow solutions. The sample was mixed with 3.0 ml of scintillation fluid (Ultima Gold XR, Packard), and the radioactivity was measured. Net fluid transport was estimated from the rate of perfusion and the radioactivity of the solutions entering and leaving the intestine. Transmucosal PD was also monitored during the perfusion experiment as previously described (16). One electrode was connected by a T tube to the inlet of the intestine, and the other was in contact with the solution in the organ bath. Each agar bridge was immersed in a beaker with a saturated KCl solution which also contained a calomel half-cell (model 402; Radiometer AB, Brönshöj, Denmark). The PD was continuously recorded on a polygraph, which was displayed on a chart recorder. Experiments lasted for 2 h.
(ii) PD measurements in vivo.
In the second type of experiment, the PD was measured in vivo (34). The experiments were performed with noninfected mice and mice at 3 days postinfection. The mice were anesthetized with continuous isoflurane gas. Each animal was placed on a heating pad, and its temperature was continuously monitored. The abdomen was opened at the midline, and a 3-cm-long jejunal segment with sufficient blood supply from an artery from the mesentery root was chosen. The segment was carefully rinsed with isotonic saline, and the proximal and distal segments were cannulated with polyethylene tubing for fluid administration. The proximal end was connected to a catheter with saline agar for PD recording. The other agar bridge was placed in the saline-soaked abdominal cavity. PD was measured as mentioned above after a calibration time of 30 min. The segment was soaked with isotonic saline and covered with a thin plastic film to prevent evaporation. Experiments lasted for 2 h.
(iii) Intestinal weight comparison.
The intestinal weight experiment included weight comparisons of the small intestines of infected and noninfected mice. The entire small intestine of each mouse from the duodenum to the cecum was ligated and removed 3 and 5 days postinfection and was immediately weighed to avoid dehydration. Its length was also determined.
(iv) Fecal output.
The numbers of pellets from EDIM-infected and control mice were measured over 1 h on the third day of infection (n = 8). Fecal output per 30 min was calculated.
Quantitative reverse transcription-PCR assay for rotavirus.
Rotavirus and hypoxanthine-guanine phosphoribosyltransferase (HPRT) RNA from freshly extracted duodenal, jejunal, and ileal tissues were obtained from adult and infant mice 4 days after infection and quantified by real-time PCR. Equal amounts of tissue from the jejuna, ilea, and duodena of adult and infant mice were homogenized, and RNA was extracted using an RNeasy Protect minikit (QIAGEN, Hilden, Germany).
Total RNA was extracted and transcribed into cDNA as described previously (27, 29). The real-time PCR was performed with duplicate 25-μl reaction mixtures containing Platinum SYBR green qPCR Supermix-UDG (Invitrogen), 150 nM concentrations of forward and reverse primers, and 0.5 μl of cDNA on an ABI Prism 7500 sequence detection system (Applied Biosystems). The following rotavirus-specific, gene 6-specific primers were used: 5′-TTCCACCAGGAATGAATTGGAC-3′ (sense) and 5′-GGTCCTCACTTTACCAGCATG-3′ (antisense). For HPRT, the primers 5′ CCC AGC GTC GTG ATT AGC 3′ (sense) and 5′ GGA ATA AAC ACT TTT TCC AAA TCC 3′ (antisense) were used.
Serialfold dilutions of a cDNA sample were amplified to control amplification efficiency for each primer pair. Thereafter, the cycle threshold (CT) values for all cDNA samples were obtained. The HPRT gene was used as a control gene to calculate the ΔCT values for individual samples. The relative amounts of RV/HPRT transcripts were calculated using the 2−ΔΔCT method as described previously (15). These values were then used to calculate the relative expression levels of RV RNA in infected tissues. Tissues from noninfected mice showed no RV amplification but did show normal HPRT mRNA levels.
RT-PCR.
Equal amounts of feces from RV-infected adult and infant mice were collected 2 and 4 days after infection, and RNA was extracted using a QIAmp viral RNA minikit (QIAGEN, Hilden, Germany), according to the manufacturer's instructions.
Five microliters of RNA was mixed with 1 μl of a 10-pmol/μl concentration of gene 6-specific primers (U65988.1). The forward primer (5′-TTC CAC CAG GAA TGA ATT GGAC-3′) and reverse primer (5′-GGT CCT CAC TTT ACC AGC ATG-3′) were denatured at 97°C for 5 min and quickly chilled on ice for 2 min, followed by the addition of one RT-PCR bead (Amersham Biosciences, United Kingdom) and RNase-free water to a final volume of 50 μl. The reverse transcription reaction was carried out for 30 min at 42°C to produce cDNA, followed by 5 min at 95°C to inactivate the reverse transcriptase enzyme. The PCR consisted of 35 cycles of 94°C for 15 seconds, 54°C for 30 seconds, and 72°C for 30 seconds, followed by a final extension of 72°C for 10 min. The amplicons were analyzed by gel electrophoresis using 2% agarose gel and ethidium bromide staining.
Statistical analysis.
Results are expressed as means ± standard errors of the means (SEM). Statistical analyses of all data were performed using repeated-measures analysis of variance and, if appropriate, Student's t test. A P value of <0.05 was considered significant.
RESULTS
Murine rotavirus-infected duodenal, jejunal, and ileal tissues of adult mice.
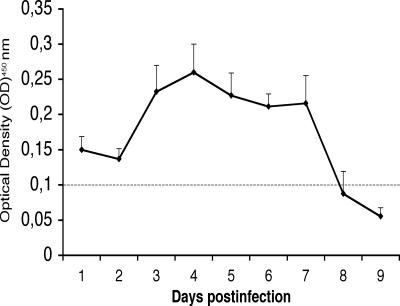
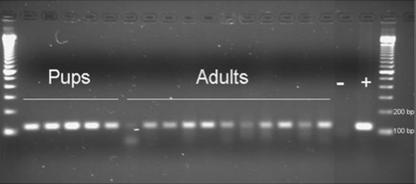
All adult mice (n = 8) infected with wild-type EDIM were found to shed virus for up to 7 days, with a peak at day 4 (Fig. 1). To obtain additional information about viral shedding, a PCR was used to detect viral RNA (gene 6) at 2 and 4 days p.i. As illustrated in Fig. 2, all adult infected mice shed viral RNA at 2 days p.i., although with quantitative variations. The same pattern was observed when samples were examined at 4 days p.i. (data not shown), implying that all adult mice excreted virus (RNA). To obtain quantitative information about the viral shedding, infant mice were also infected, and the amount of excreted RNA was compared to that of adult mice. Figure 2 shows that more viral RNA was shed from infant mice than from adult mice.
FIG. 1.
Shedding of rotavirus following infection with EDIM (n = 8). Values are means ± SEM. The horizontal line shows the optical density cutoff at 450 nm of 0.1.
FIG. 2.
Rotavirus PCR of stool samples collected 2 days p.i. from mouse pups (n = 5) and adult mice (n = 11) infected with EDIM rotavirus. Gene 6-specific primers were used to amplify a 118-bp PCR fragment. The amplicons were visualized with ethidium bromide on a 2% agarose gel. −, negative sample; +, positive sample.
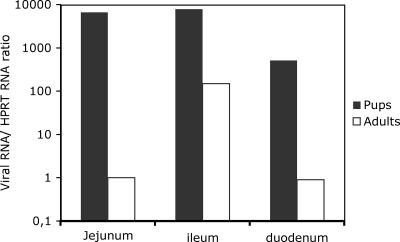
The shedding of virus from adult mice raised the questions of whether all segments of the small intestine become infected and, if infected, how extensive is the infection. To address these questions, viral RNA was quantified and immunohistochemistry was performed with different segments of the small intestines of infant and adult mice. To obtain quantitative information about viral synthesis, adult and infant mice were infected with EDIM, and the amounts of viral RNA in different intestine segments were quantified 4 days p.i. Figure 3 shows not only that viral RNA synthesis occurred in the small intestines of adult mice 4 days p.i. but also that the ileum was the segment with the most-pronounced viral RNA synthesis. The viral synthesis was approximately 100-fold higher in the ileum than in the jejunum or the duodenum, which contrasts with results for infant mice, for which no such difference was observed.
FIG. 3.
Quantitative determination of rotavirus (EDIM) RNA in the intestines of infant and adult mice 4 days p.i., measured as the n-fold increase in viral RNA concentration compared to HPRT mRNA. The figure shows that the synthesis of viral RNA is higher in infant than in adult mice, relative to mRNA HPRT. The numbers presented are mean values (n = 3).
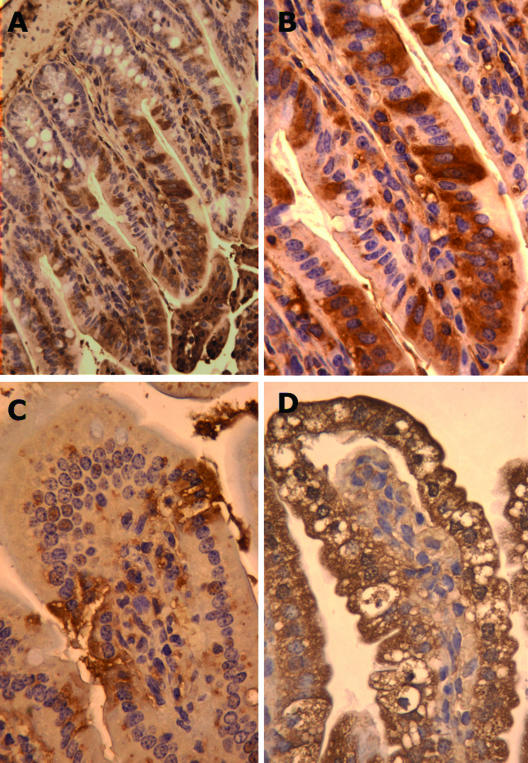
A possible reason for the absence of diarrhea in adult mice could be the presence of no or few infected cells in the small intestine. To investigate this in more detail, adult and infant mice were infected and the duodena, jejuna, and ilea were processed for immunohistochemistry 2 and 4 days p.i. Immunostaining with a polyclonal rotavirus antiserum revealed that the villi of all segments of the small intestines of adult mice were infected, with the highest number of infected cells found in the ileum. The numbers of infected cells in the ilea of adult mice were significant at 2 days p.i. (Fig. 4A and B) and occasionally resulted in histological lesions (Fig. 4C). No obvious differences in the numbers of infected cells or locations were found between 2 and 4 days p.i. Figure 4D shows extensive immunostaining and lesions from the ilea of infant mice 2 days p.i. It should be noted that a distinct feature of the infected villi of infant mice was vacuolization (Fig. 4D), a feature not observed in adult mice (Fig. 4A to C).
FIG. 4.
Immunohistochemistry of ileal tissue from infant and adult mice at 2 days p.i. Rotavirus-infected cells were identified with a rabbit polyclonal antiserum that recognizes predominantly vp6. (A) Immunoperoxidase staining of an infected ileum from an adult mouse. (B) A larger magnification of the tissue shown in panel A illustrating in more detail the infection of individual cells. (C) Immunoperoxidase staining of an ileal lesion from an adult mouse. Note the severe lesions proximate to the infected cells. (D) Immunoperoxidase staining of ileal tissue from an infected infant mouse with a large number of infected cells and severe vacuolization. Vacuoles in villous cells were a unique feature of infant mice.
Rotavirus infection in adult mice does not result in salt (PD) or net fluid secretion from the small intestine.
It has previously been shown that rotavirus infection in adult mice results in virus shedding without concomitant diarrhea (37). To investigate possible mechanisms behind this age-dependent resistance, adult mice were infected with EDIM and the transmucosal PD and the net fluid transport in control mice and in mice infected for 3 and 4 days were monitored.
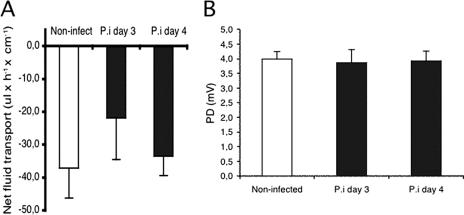
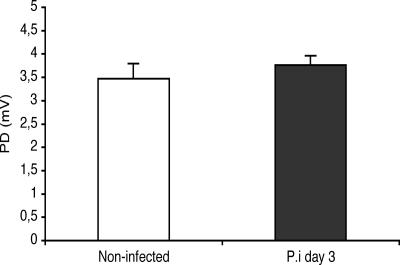
The perfusion experiments revealed that EDIM infection in adult mice did not lead to increased PD (Fig. 5), presumably chloride secretion, compared to that in control mice (4.0 ± 0.3 mV [control] versus 3.9 ± 0.4 mV [4 days p.i.]), nor were there any statistically significant differences in net fluid transport (37 ± 9 μl · h−1 · cm−1, 22 ± 13 μl · h−1 · cm−1, and 33 ± 6 μl · h−1 · cm−1, respectively) among mice infected for 3 days (n = 8) or 4 days (n = 8) or in noninfected mice (n = 8) (Fig. 5). To extend these in vitro observations, a series of in vivo perfusion experiments was also performed. In agreement with the in vitro observations, there were no significant differences in PD between control and infected mice at 3 days postinfection (Fig. 6), suggesting no differences in chloride transport between infected and noninfected mice.
FIG. 5.
In vitro perfusion experiments recording net fluid transport (A) and PD (B) in control segments and in infected segments at days 3 and 4 (n = 8 in each group). Values are means ± SEM.
FIG. 6.
In vivo recording of jejunal potential differences in control and rotavirus (EDIM)-infected mice at day 3 (n = 8 in each group). Values are means ± SEM.
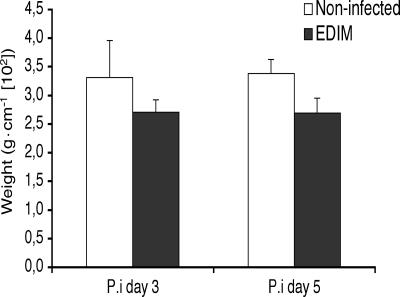
Rotavirus infection in adult mice does not result in increased weight of the small intestine.
An increased fluid secretion and/or tissue edema should presumably lead to an increase in the weight of the small intestine, when ligated in situ. We therefore removed the entire small intestine at 3 and 5 days postinfection and determined the weight. As shown in Fig. 7, there were no significant differences in small intestine weight between infected and control mice. Furthermore, direct visual inspection of infected and uninfected duodena and jejuna at 3 days postinfection did not reveal any apparent differences in color, size, or shape (data not shown).
FIG. 7.
Comparison of the small intestinal weights of control and rotavirus (EDIM)-infected mice at days 3 and 5 (n = 4 in each group). Values are means ± SEM.
Increased intestinal motility has been recognized as an important factor for the genesis of diarrhea. To crudely test whether rotavirus infection leads to increased intestinal motility, albeit in the absence of diarrhea, the numbers of pellets from EDIM-infected mice and control mice were measured during 1 h on the third day of infection (n = 8). Fecal outputs per 30 min were calculated. In infected animals, the fecal output was 8.7 pellets/30 min, compared to 8.3 pellets/30 min in control animals, thus suggesting that rotavirus infection in adult mice does not result in increased intestinal motility, as judged from their fecal output.
DISCUSSION
Despite proof of viral infection, and replication particularly in the ileum, we found no evidence of fluid secretion, chloride secretion, fluid accumulation, or increased fecal output for adult mice infected with rotavirus. Using established in vivo and in vitro methods (16, 32, 33), we measured net fluid transport and transmucosal PD and found no evidence of chloride or water secretion from the small intestine.
These observations are in strong contrast to a previous study, where we used the same methodology to show that infant mice infected with RV respond with salt and water secretion from the small intestine (16). Not only do these two observations illustrate that the pathophysiology of rotavirus disease is complex and most likely involves several mechanisms, of which some are age dependent, but also, and more important, this study shows for the first time that the absence of clinical diarrhea in adult mice is not solely a consequence of increased absorption of water in the colon, as has been previously suggested without experimental support.
While several mechanisms have been discussed to explain the age-dependent secretory response to rotavirus, there is yet a limited amount of experimental data available. The most simplistic explanation for the absence of diarrhea in adult mice is that no or few cells in the small intestine are infected compared to those in infant mice. To investigate this, immunohistochemistry, quantitative PCR, and histological examination of all intestinal segments of the small intestine were performed. The studies revealed that rotavirus infects all parts of the small intestines of adult mice but preferentially the ilea. Quantitative PCR revealed that 100-fold more viral RNA was produced in the ileum than in the jejunum or duodenum. This observation was supported by immunohistochemistry showing that more villous cells in the ileum were infected than in the jejunum or the duodenum, an observation also supported by other studies of mice (4, 21). Thus, the ileum appears to be the intestinal segment most susceptible to rotavirus infection, regardless of whether the infection takes place in infant or adult mice. It has previously been speculated that the age-related resistance in mice would be receptor dependent (11, 37). Our observations do not fully support this, as all intestinal segments in both adult and infant mice could be infected, but the fact that the ileum was more susceptible to infection than the jejunum and duodenum could also be due to segment-specific differences in receptor concentration.
A critical part of this study was to estimate the extent of infection in cells and relate this to infant mice and other animal models. Immunohistochemistry revealed that more cells were infected in infant mice than in adults, but more important, the numbers of infected cells in the ilea of adult mice (Fig. 4) were significantly higher than the small percentages of cells in the ilea previously shown to be sufficient to cause diarrhea in infant mice (24). Thus, the number of infected cells is not a limiting factor in the cause of diarrhea in mice. However, the possibility that the restricted number of enterocytes infected in adults compared to that in infant mice is a contributing factor to the age-restricted diarrhea cannot be excluded.
In several animal species, profuse diarrhea occurs prior to the detection of histological changes in the intestine, including villous blunting (9, 18, 19, 35, 36). Furthermore, some animals exhibit diarrhea in the absence of clear histopathological changes, such as infant mice infected with heterologous rotavirus strains (3), whereas rotavirus-infected rabbits show typical histological changes in the intestine in the absence of diarrhea (5). Moreover, oral administration of epidermal growth factor to rotavirus-infected piglets can help to restore enzyme activities and intestinal mucosa but not resolve diarrhea (39); thus, a clear relationship among infection, mucosal lesions, and diarrhea is lacking.
While mucosal injury was significant in infant mice, it was limited in frequency and severity in adult mice. In none of the animal models were crypt cells found to be infected, an observation reported earlier (4, 33). The most-pronounced histological difference between adult and infant infected intestines was the extensive vacuolation found in infant mice (Fig. 4). A possible role of vacuolation in diarrhea has been proposed from studies of rats. Ciarlet et al. (4) found that rhesus RV-inoculated 5-day-old rats exhibited diarrhea without inflammation. No effect on villous height or width or other histological lesions were seen, but large vacuoles were observed. Extensive vacuolation in infant mice has been reported previously (25, 33) but has not been highlighted as a major feature of rotavirus-induced pathology in calves with diarrhea (20), lambs (31), or piglets (35). One might propose that cells with significant vacuolation should contain viral particles, something that was not investigated in this study but has been examined elsewhere. Osborne et al. (25) and Coelho et al. (6) found no clear correlation between the severity of enterocyte vacuolation and the presence of virus particles. Furthermore, Ciarlet et al. (4) found no virus particles in vacuoles of infected rats.
Another explanation for the different intestinal responses to rotavirus implies an impaired absorptive capacity of the villous cells in infant mice but not in adult mice. It is possible to reveal such a mechanism by measurements of transmural PD. Since PD represents a net measurement of electrogenic epithelial transport, differences in rates of glucose and/or amino acid absorption by sodium-coupled symports could be reflected in the PD. Such measurements in secreting infant mice revealed a higher PD in the rotavirus-infected gut than in the noninfected gut (14, 16), whereas no such difference was seen in the adult mice of the present study. The increased PD in infant mice can be explained in terms of an increased lumen-tissue flux of positive ions and concomitant glucose/amino acid absorption. Hence, the PD measurements are not compatible with decreased rates of glucose/amino acid absorption in infant mice. However, an attenuated absorptive capacity of nonelectrogenic transport mechanisms in infant mice may contribute to the secretory response to rotavirus. Collins and coworkers (7, 8) studied the intestinal enzyme profiles of rotavirus diarrhea in infant mice and found that while lactose malabsorption could be one contributing factor to the fluid loss at the peak of diarrhea (72 h p.i.), carbohydrate malabsorption could not be a key factor leading to diarrhea in infant mice.
The activation of a secretory reflex(es) in the enteric nervous system represents another proposed mechanism for rotavirus fluid secretion. This hypothesis implies that nerve afferent fibers, located just underneath the intestinal epithelium, are activated by amines/peptides secreted from the enteroendocrine cells and/or by chemokines, prostaglandins, or nitric oxide released from enterocytes exposed to microorganisms (17, 21, 26). In line with this, Rodriguez-Diaz et al. (27) found not only that NSP4 and rotavirus stimulated the release of NO from HT-29 cells but also that NO2/NO3 occurred in increased amounts in the urine of rotavirus-infected mice and young children. It is in this interesting context that Rollo et al. (28) reported that rotavirus evokes the release of chemokines only from epithelial cells obtained from mice less than 2 weeks old. Thus, one possible mechanism for the lack of effect of RV in adult mice may be that RV does not cause a release of chemokines to activate an enteric nervous system secretory reflex(es).
As the number of infected cells and histological lesions, including vacuoles, cannot entirely explain fluid secretion, it is tempting to speculate about impaired age-dependent cellular receptor signaling. Apart from the cytokines and NO discussed above, NSP4 should be considered. Morris et al. (22) found that NSP4 induces diarrhea in mice in a dose- and age-dependent manner. The same group has proposed that age-restricted diarrhea is not due to an age-dependent receptor for NSP4 but rather to an age-dependent event downstream in the signaling pathway from Ca2+ mobilization (22).
Acknowledgments
This study was supported by the Swedish Research Council (grant 8266).
Claudia Istrate, a student of the Gulbenkian Ph.D. Program in Biomedicine, Portugal, had a grant from Fundação para a Ciência e Tecnologia (grant SFRH/BD/9616/2002).
We thank Beatrice Karlsson and Johan Nordgren for valuable technical support.
Footnotes
Published ahead of print on 30 August 2006.
REFERENCES
- 1.Anderson, E. J., and S. G. Weber. 2004. Rotavirus infection in adults. Lancet Infect. Dis. 4:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banasaz, M., E. Norin, R. Holma, and T. Midtvedt. 2002. Increased enterocyte production in gnotobiotic rats mono-associated with Lactobacillus rhamnosus GG. Appl. Environ. Microbiol. 68:3031-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burns, J. W., A. A. Krishnaney, P. T. Vo, R. V. Rouse, L. J. Anderson, and H. B. Greenberg. 1995. Analyses of homologous rotavirus infection in the mouse model. Virology 207:143-153. [DOI] [PubMed] [Google Scholar]
- 4.Ciarlet, M., M. E. Conner, M. J. Finegold, and M. K. Estes. 2002. Group A rotavirus infection and age-dependent diarrheal disease in rats: a new animal model to study the pathophysiology of rotavirus infection. J. Virol. 76:41-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ciarlet, M., M. A. Gilger, C. Barone, M. McArthur, M. K. Estes, and M. E. Conner. 1998. Rotavirus disease, but not infection and development of intestinal histopathological lesions, is age restricted in rabbits. Virology 251:343-360. [DOI] [PubMed] [Google Scholar]
- 6.Coelho, K. I., A. S. Bryden, C. Hall, and T. H. Flewett. 1981. Pathology of rotavirus infection in suckling mice: a study by conventional histology, immunofluorescence, ultrathin sections, and scanning electron microscopy. Ultrastruct. Pathol. 2:59-80. [DOI] [PubMed] [Google Scholar]
- 7.Collins, J., D. C. Candy, W. G. Starkey, A. J. Spencer, M. P. Osborne, and J. Stephen. 1990. Disaccharidase activities in small intestine of rotavirus-infected suckling mice: a histochemical study. J. Pediatr. Gastroenterol. Nutr. 11:395-403. [DOI] [PubMed] [Google Scholar]
- 8.Collins, J., W. G. Starkey, T. S. Wallis, G. J. Clarke, K. J. Worton, A. J. Spencer, S. J. Haddon, M. P. Osborne, D. C. Candy, and J. Stephen. 1988. Intestinal enzyme profiles in normal and rotavirus-infected mice. J. Pediatr. Gastroenterol. Nutr. 7:264-272. [DOI] [PubMed] [Google Scholar]
- 9.Collins, J. E., D. A. Benfield, and J. R. Duimstra. 1989. Comparative virulence of two porcine group-A rotavirus isolates in gnotobiotic pigs. Am. J. Vet. Res. 50:827-835. [PubMed] [Google Scholar]
- 10.Espinoza, F., M. Paniagua, H. Hallander, K. O. Hedlund, and L. Svensson. 1997. Prevalence and characteristics of severe rotavirus infections in Nicaraguan children. Ann. Trop. Paediatr. 17:25-32. [DOI] [PubMed] [Google Scholar]
- 11.Feng, N., M. A. Franco, and H. B. Greenberg. 1997. Murine model of rotavirus infection. Adv. Exp. Med. Biol. 412:233-240. [DOI] [PubMed] [Google Scholar]
- 12.Gelberg, H. B. 1992. Studies on the age resistance of swine to group A rotavirus infection. Vet. Pathol. 29:161-168. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg, H. B., H. F. Clark, and P. A. Offit. 1994. Rotavirus pathology and pathophysiology. Curr. Top. Microbiol. Immunol. 185:255-283. [DOI] [PubMed] [Google Scholar]
- 14.Kordasti, S., H. Sjovall, O. Lundgren, and L. Svensson. 2004. Serotonin and vasoactive intestinal peptide antagonists attenuate rotavirus diarrhoea. Gut 53:952-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods Companion Methods Enzymol. 25:402-408. [DOI] [PubMed] [Google Scholar]
- 16.Lundgren, O., A. T. Peregrin, K. Persson, S. Kordasti, I. Uhnoo, and L. Svensson. 2000. Role of the enteric nervous system in the fluid and electrolyte secretion of rotavirus diarrhea. Science 287:491-495. [DOI] [PubMed] [Google Scholar]
- 17.Lundgren, O., and L. Svensson. 2001. Pathogenesis of rotavirus diarrhea. Microbes Infect. 3:1145-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdaragh, J. P., M. E. Bergeland, R. C. Meyer, M. W. Johnshoy, I. J. Stotz, D. A. Benfield, and R. Hammer. 1980. Pathogenesis of rotaviral enteritis in gnotobiotic pigs: a microscopic study. Am. J. Vet. Res. 41:1572-1581. [PubMed] [Google Scholar]
- 19.Mebus, C. A. 1976. Reovirus-like calf enteritis. Am. J. Dig. Dis. 21:592-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mebus, C. A., and L. E. Newman. 1977. Scanning electron, light, and immunofluorescent microscopy of intestine of gnotobiotic calf infected with reovirus-like agent. Am. J. Vet. Res. 38:553-558. [PubMed] [Google Scholar]
- 21.Michelangeli, F., and M. C. Ruiz. 2003. Physiology and pathophysiology of the gut in relation to viral diarrhea, p. 23-50. In U. Desselberger and J. Gray (ed.), Viral gastroenteritis. Elsevier, Amsterdam, The Netherlands. [DOI] [PMC free article] [PubMed]
- 22.Morris, A. P., J. K. Scott, J. M. Ball, C. Q. Zeng, W. K. O'Neal, and M. K. Estes. 1999. NSP4 elicits age-dependent diarrhea and Ca(2+)mediated I(-) influx into intestinal crypts of CF mice. Am. J. Physiol. 277:G431-G444. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson, M., B. Svenungsson, K. O. Hedlund, I. Uhnoo, A. Lagergren, T. Akre, and L. Svensson. 2000. Incidence and genetic diversity of group C rotavirus among adults. J. Infect. Dis. 182:678-684. [DOI] [PubMed] [Google Scholar]
- 24.Offit, P. A., H. F. Clark, M. J. Kornstein, and S. A. Plotkin. 1984. A murine model for oral infection with a primate rotavirus (simian SA11). J. Virol. 51:233-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osborne, M. P., S. J. Haddon, A. J. Spencer, J. Collins, W. G. Starkey, T. S. Wallis, G. J. Clarke, K. J. Worton, D. C. Candy, and J. Stephen. 1988. An electron microscopic investigation of time-related changes in the intestine of neonatal mice infected with murine rotavirus. J. Pediatr. Gastroenterol. Nutr. 7:236-248. [DOI] [PubMed] [Google Scholar]
- 26.Ramig, R. F. 2004. Pathogenesis of intestinal and systemic rotavirus infection. J. Virol. 78:10213-10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Diaz, J., M. Banasaz, C. Istrate, J. Buesa, O. Lundgren, F. Espinoza, T. Sundqvist, M. Rottenberg, and L. Svensson. 2006. Role of nitric oxide during rotavirus infection. J. Med. Virol. 78:979-985. [DOI] [PubMed] [Google Scholar]
- 28.Rollo, E. E., K. P. Kumar, N. C. Reich, J. Cohen, J. Angel, H. B. Greenberg, R. Sheth, J. Anderson, B. Oh, S. J. Hempson, E. R. Mackow, and R. D. Shaw. 1999. The epithelial cell response to rotavirus infection. J. Immunol. 163:4442-4452. [PubMed] [Google Scholar]
- 29.Rottenberg, M. E., E. Castanos-Velez, R. de Mesquita, O. G. Laguardia, P. Biberfeld, and A. Orn. 1996. Intracellular colocalization of Trypanosoma cruzi and inducible nitric oxide synthase (iNOS): evidence for dual pathway of iNOS induction. Eur. J. Immunol. 26:3203-3213. [DOI] [PubMed] [Google Scholar]
- 30.Rubilar-Abreu, E., K.-O. Hedlund, L. Svensson, and C. Mittelholzer. 2005. Serotype G9 rotavirus infections in adults in Sweden. J. Clin. Microbiol. 43:1374-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snodgrass, D. R., K. W. Angus, and E. W. Gray. 1977. Rotavirus infection in lambs: pathogenesis and pathology. Arch. Virol. 55:263-274. [DOI] [PubMed] [Google Scholar]
- 32.Starkey, W. G., D. C. Candy, D. Thornber, J. Collins, A. J. Spencer, M. P. Osborne, and J. Stephen. 1990. An in vitro model to study aspects of the pathophysiology of murine rotavirus-induced diarrhoea. J. Pediatr. Gastroenterol. Nutr. 10:361-370. [DOI] [PubMed] [Google Scholar]
- 33.Starkey, W. G., J. Collins, T. S. Wallis, G. J. Clarke, A. J. Spencer, S. J. Haddon, M. P. Osborne, D. C. Candy, and J. Stephen. 1986. Kinetics, tissue specificity and pathological changes in murine rotavirus infection of mice. J. Gen. Virol. 67:2625-2634. [DOI] [PubMed] [Google Scholar]
- 34.Sun, Y., B. M. Fihn, M. Jodal, and H. Sjovall. 2000. Effects of neural blocking agents on motor activity and secretion in the proximal and distal rat colon: evidence of marked segmental differences in nicotinic receptor activity. Scand. J. Gastroenterol. 35:380-388. [DOI] [PubMed] [Google Scholar]
- 35.Theil, K. W., E. H. Bohl, R. F. Cross, E. M. Kohler, and A. G. Agnes. 1978. Pathogenesis of porcine rotaviral infection in experimentally inoculated gnotobiotic pigs. Am. J. Vet. Res. 39:213-220. [PubMed] [Google Scholar]
- 36.Ward, R. L., B. B. Mason, D. I. Bernstein, D. S. Sander, V. E. Smith, G. A. Zandle, and R. S. Rappaport. 1997. Attenuation of a human rotavirus vaccine candidate did not correlate with mutations in the NSP4 protein gene. J. Virol. 71:6267-6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ward, R. L., M. M. McNeal, and J. F. Sheridan. 1990. Development of an adult mouse model for studies on protection against rotavirus. J. Virol. 64:5070-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf, J. L., G. Cukor, N. R. Blacklow, R. Dambrauskas, and J. S. Trier. 1981. Susceptibility of mice to rotavirus infection: effects of age and administration of corticosteroids. Infect. Immun. 33:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zijlstra, R. T., J. Odle, W. F. Hall, B. W. Petschow, H. B. Gelberg, and R. E. Litov. 1994. Effect of orally administered epidermal growth factor on intestinal recovery of neonatal pigs infected with rotavirus. J. Pediatr. Gastroenterol. Nutr. 19:382-390. [DOI] [PubMed] [Google Scholar]